
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Weiqiang Liu | -- | 2780 | 2022-04-14 08:02:56 | | | |
| 2 | Dean Liu | + 1 word(s) | 2781 | 2022-04-18 03:31:17 | | | | |
| 3 | Dean Liu | -5 word(s) | 2776 | 2022-04-18 07:53:54 | | | | |
| 4 | Dean Liu | -3 word(s) | 2773 | 2022-04-22 07:35:02 | | |
Video Upload Options
Creep responses of intervertebral discs (IVDs) are essential for spinal biomechanics clarification. Yet, there still lacks a well-recognized investigation protocol for this phenomenon. Researchers aim at providing an overview of the in vitro creep tests reported by previous studies, specifically specimen species, testing environment, loading regimes and major results, based on which a preliminary consensus that may guide future creep studies is proposed. Specimens used in creep studies can be simplified as a “bone–disc–bone” structure where three mathematical models can be adopted for describing IVDs’ responses. The preload of 10–50 N for 30 min or three cycles followed by 4 h-creep under constant compression is recommended for ex vivo simulation of physiological condition of long-time sitting or lying. It is worth noticing that species of specimens, environment temperature and humidity all have influences on biomechanical behaviors, and thus are summarized and compared. All factors should be carefully set according to a guideline before tests are conducted to urge comparable results across studies. To this end, researchers also provide a guideline, as mentioned before, and specific steps that might facilitate the community of biomechanics to obtain more repeatable and comparable results from both natural specimens and novel biomaterials.
1. Introduction
Creep is a time-dependent response of IVD and is a typical feature of viscoelastic materials. In 1982, Twomey et al. [1] defined creep as the progressive deformation of a structure under the constant load when the materials are stressed below their fracture thresholds. One of the most intuitive phenomena, which suggests the formation of creep is that the human body has a height change of 1–2 cm per day [2][3]. To date, several studies described the creep behaviors of the spine under axial compression [4][5][6][7][8][9][10][11][12][13][14][15][16][17]. These investigations highlighted the non-linear and time-dependent behaviors of natural IVDs, showing a rapid decrease in axial height after early compression followed by a slow decrease until reaching equilibrium. Nevertheless, a normalized loading protocol is not yet available to which every study adheres, thus resulting in a significant challenge in comparing mechanical results across studies and hampering the development and testing of biomaterials used for spinal implants [18][19].
2. Factors That Can Influence the Mechanical Properties of IVDs
2.1. Species
2.1.1. Difference in Geometry
2.1.2. Difference in Glycosaminoglycan (GAG) and Water Content
2.1.3. Difference in Axial Compressive Mechanics
2.2. Specimen Harvesting and Storage

2.3. Testing Environment
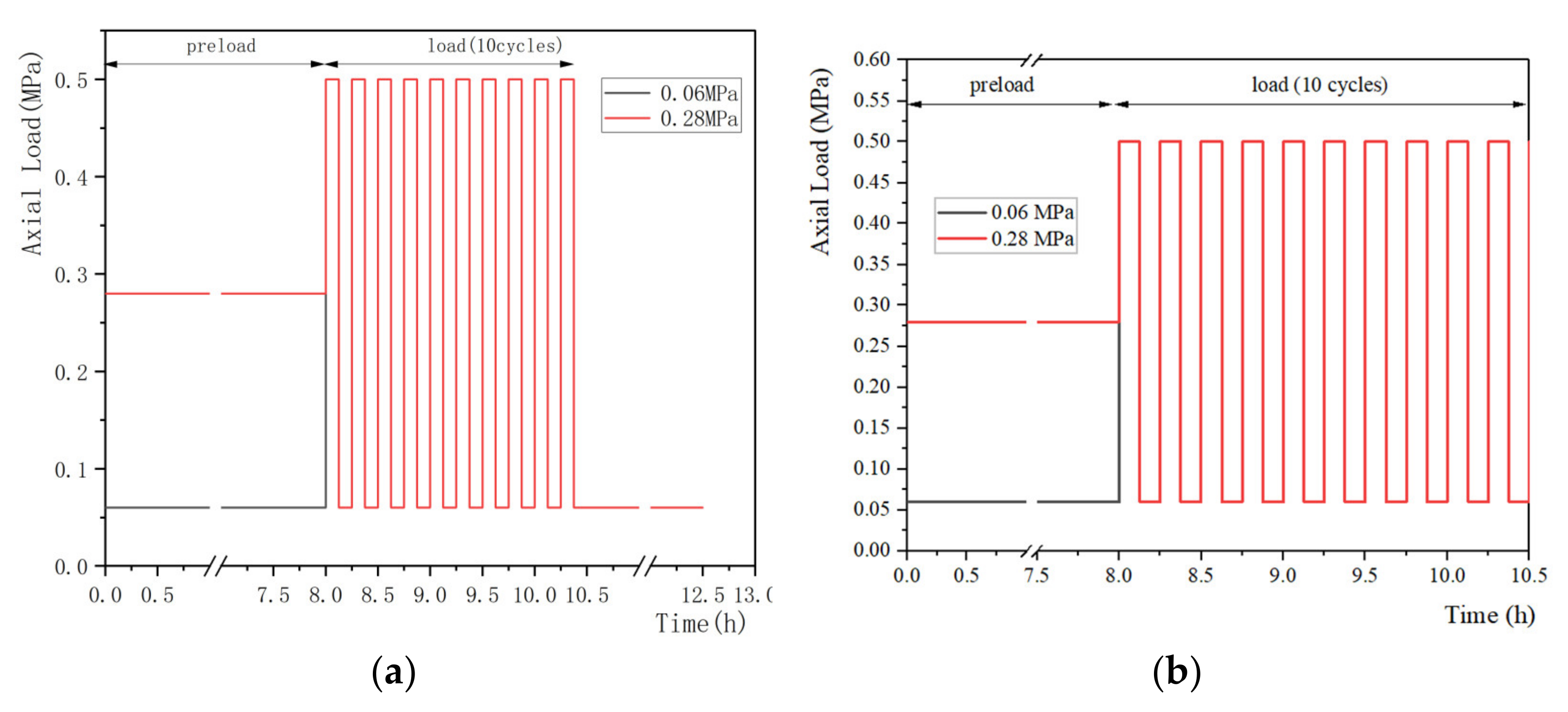
2.4. Preload, Load Magnitudes and Duration
2.4.1. Preload
2.4.2. Load Magnitude and Duration


3. Selection of Loading Regime during Creep
3.1. Static Load
3.2. Quasi-Static Load
3.3. Dynamic Load
References
- Twomey, L.; Taylor, J. Flexion Creep Deformation and Hysteresis in the Lumbar Vertebral Column. Spine 1982, 7, 116–122.
- Koeller, W.; Funke, F.; Hartmann, F.; Copley, A.L.; Witte, S. Biomechanical behavior of human intervertebral discs subjected to log lasting axial loading. Biorheology 1984, 21, 675–686.
- Leatt, P.; Reilly, T.; Troup, J.G. Spinal loading during circuit weight-training and running. Br. J. Sports Med. 1986, 20, 119–124.
- Pollintine, P.; Tunen, M.V.; Luo, J.; Brown, M.D.; Dolan, P.; Adams, M.A. Time-dependent Compressive Deformation of the Ageing Spine: Relevance to Spinal Stenosis. Spine 2010, 35, 386–394.
- Adams, M.; Hutton, W.C. The Effect of Posture on the Fluid Content of Lumbar Intervertebral Discs. Spine 1983, 8, 665–671.
- Adams, M.A.; Dolan, P. Time-dependent changes in the lumbar spine’s resistance to bending. Clin. Biomech. 1996, 11, 194–200.
- Brown, T.; Hansen, R.J.; Yorra, A.J. Some mechanical tests on the lumbosacral spine with particular reference to the intervertebral discs: A preliminary report. J. Bone Jt. Surg. Am. 1957, 39-A, 1135–1164.
- Burns, M.L.; Kaleps, I.; Kazarian, L.E. Analysis of compressive creep behavior of the vertebral unit subjected to a uniform axial loading using exact parametric solution equations of Kelvin-solid models—Part I. Human intervertebral joints. J. Biomech. 1984, 17, 113–130.
- Hirsch, C. The Reaction of Intervertebral Discs to Compression Forces. J. Bone Jt. Surg. 1955, 37, 1188–1196.
- Hirsch, C.; Nachemson, A. New observations on the mechanical behavior oflumbar discs. Acta Orthop. Scand. 1994, 23, 254–283.
- Kazarian, L.E. Creep Characteristics of the Human Spinal Column. Orthop. Clin. N. Am. 1975, 6, 3–18.
- Koeller, W.; Meier, W.; Hartmann, F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression. A comparison of lumbar and thoracic discs. Spine 1984, 9, 725–733.
- Kulak, R.F.; Schultz, A.B.; Belytschko, T.; Galante, J. Biomechanical characteristics of vertebral motion segments and inter-vertebral disks. Orthop. Clin. N. Am. 1975, 6, 121–133.
- Lin, H.; Lui, Y.K.; Ray, G.; Nikravesh, P. Systems identification for material properties of the intervertebral joint. J. Biomech. 1978, 11, 1–14.
- Lin, L.-C.; Hedman, T.P.; Wang, S.-J.; Huoh, M.; Chuang, S.-Y. The Analysis of Axisymmetric Viscoelasticity, Time-Dependent Recovery, and Hydration in Rat Tail Intervertebral Discs by Radial Compression Test. J. Appl. Biomech. 2009, 25, 133–139.
- Markolf, K.L. Deformation of the thoracolumbar intervertebral joints in response to external loads: A biomechanical study using autopsy material. J. Bone Jt. Surg. 1972, 54, 511–533.
- Virgin, W.J. Experimental investigations into the physical properties of the intervertebral disc. J. Bone Jt. Surg. Br. 1951, 33, 607–611.
- Schmidt, H.; Reitmaier, S.; Graichen, F.; Shirazi-Adl, A. Review of the fluid flow within intervertebral discs—How could in vitro measurements replicate in vivo? J. Biomech. 2016, 49, 3133–3146.
- Newell, N.; Little, J.P.; Christou, A.; Adams, M.A.; Adam, C.J.; Masouros, S.D. Biomechanics of the Human Intervertebral Disc: A Review of Testing Techniques and Results. J. Mech. Behav. Biomed. Mater. 2017, 69, 420–434.
- O’Connell, G.D.; Vresilovic, E.J.; Elliott, D.M. Comparison of animals used in disc research to human lumbar disc geometry. Spine 2007, 32, 328–333.
- Kuo, Y.-W.; Wang, J.-L. Rheology of Intervertebral Disc. Spine 2010, 35, E743–E752.
- Ishihara, H.; Tsuji, H.; Hirano, N.; Ohshirna, H.; Terahata, N. Biorheological responses of the intact and nucleotomized in-tervertebral discs to compressive, tensile, and vibratory stresses. Clin. Biomech. 1993, 8, 250–254.
- Beckstein, J.C.; Sen, S.; Schaer, T.P.; Vresilovic, E.J.; Elliott, D.M. Comparison of animal discs used in disc research to human lumbar disc: Axial compression mechanics and glycosaminoglycan content. Spine 2008, 33, E166–E173.
- Gooyers, C.E.; McMillan, R.D.; Howarth, S.J.; Callaghan, J.P. The Impact of Posture and Prolonged Cyclic Compressive Loading on Vertebral Joint Mechanics. Spine 2012, 37, E1023–E1029.
- Iatridis, J.C.; Laible, J.P.; Krag, M.H. Influence of Fixed Charge Density Magnitude and Distribution on the Intervertebral Disc: Applications of a Poroelastic and Chemical Electric (PEACE) Model. J. Biomech. Eng. 2003, 125, 12–24.
- Boxberger, J.I.; Sen, S.; Yerramalli, C.S.; Elliott, D.M. Nucleus pulposus glycosaminoglycan content is correlated with axial mechanics in rat lumbar motion segments. J. Orthop. Res. 2006, 24, 1906–1915.
- Johannessen, W.; Elliott, D.M. Effects of degeneration on the biphasic material properties of human nucleus pulposus in con-fined compression. Spine 2005, 30, E724–E729.
- Demers, C.N.; Antoniou, J.; Mwale, F. Value and Limitations of Using the Bovine Tail as a Model for the Human Lumbar Spine. Spine 2004, 29, 2793–2799.
- Antoniou, J.; Steffen, T.; Nelson, F.; Winterbottom, N.; Hollander, A.P.; Poole, R.A.; Aebi, M.; Alini, M. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 1996, 98, 996–1003.
- Urban, J.P.; McMullin, J.F. Swelling pressure of the intervertebral disc: Influence of proteoglycan and collagen contents. Biorheology 1985, 22, 145–157.
- Yates, J.P.; Giangregorio, L.; McGill, S.M. The Influence of Intervertebral Disc Shape on the Pathway of Posterior/Posterolateral Partial Herniation. Spine 2010, 35, 734–739.
- Van Heeswijk, V.M.; Thambyah, A.; Robertson, P.A.; Broom, N.D. Posterolateral disc prolapse in flexion initiated by lateral inner annular failure: An investigation of the herniation pathway. Spine 2017, 42, 1604–1613.
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2007, 17, 2–19.
- Reitmaier, S.; Schmidt, H.; Ihler, R.; Kocak, T.; Graf, N.; Ignatius, A.; Wilke, H.-J. Preliminary Investigations on Intradiscal Pressures during Daily Activities: An In Vivo Study Using the Merino Sheep. PLoS ONE 2013, 8, e69610.
- Schmidt, H.; Shirazi-Adl, A.; Schilling, C.; Dreischarf, M. Preload substantially influences the intervertebral disc stiffness in loading–unloading cycles of compression. J. Biomech. 2016, 49, 1926–1932.
- Schmidt, H.; Schilling, C.; Reyna, A.L.P.; Shirazi-Adl, A.; Dreischarf, M. Fluid-flow dependent response of intervertebral discs under cyclic loading: On the role of specimen preparation and preconditioning. J. Biomech. 2016, 49, 846–856.
- Oravec, D.; Kim, W.; Flynn, M.J.; Yeni, Y.N. The relationship of whole human vertebral body creep to geometric, microstructural, and material properties. J. Biomech. 2018, 73, 92–98.
- McMillan, D.W.; Garbutt, G.; Adams, M.A. Effect of sustained loading on the water content of intervertebral discs: Implications for disc metabolism. Ann. Rheum. Dis. 1996, 55, 880–887.
- Heuer, F.; Schmidt, H.; Klezl, Z.; Claes, L.; Wilke, H.-J. Stepwise reduction of functional spinal structures increase range of motion and change lordosis angle. J. Biomech. 2007, 40, 271–280.
- Dhillon, N.; Bass, E.C.; Lotz, J.C. Effect of Frozen Storage on the Creep Behavior of Human Intervertebral Discs. Spine 2001, 26, 883–888.
- Panjabi, M.M.; Krag, M.; Summers, D.; Videman, T. Biomechanical time-tolerance of fresh cadaveric human spine specimens. J. Orthop. Res. 1985, 3, 292–300.
- Smeathers, J.E.; Joanes, D.N. Dynamic compressive properties of human lumbar intervertebral joints: A comparison between fresh and thawed specimens. J. Biomech. 1988, 21, 425–433.
- Galante, J.O. Tensile properties of the human lumbar annulus fibrosus. Acta Orthop. Scand. Suppl. 1967, 100, 1–91.
- Tan, J.S.; Uppuganti, S. Cumulative multiple freeze-thaw cycles and testing does not affect subsequent within-day variation in intervertebral flexibility of human cadaveric lumbosacral spine. Spine 2012, 37, E1238–E1242.
- Callaghan, J.P.; McGill, S.M. Frozen storage increases the ultimate compressive load of porcine vertebrae. J. Orthop. Res. 1995, 13, 809–812.
- Sunni, N.; Askin, G.N.; Labrom, R.D.; Izatt, M.T.; Pearcy, M.J.; Adam, C.J. The effect of repeated loading and freeze-thaw cycling on immature bovine thoracic motion segment stiffness. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1100–1107.
- O’Connell, G.D.; Jacobs, N.T.; Sen, S.; Vresilovic, E.J.; Elliott, D.M. Axial creep loading and unloaded recovery of the human intervertebral disc and the effect of degeneration. J. Mech. Behav. Biomed. Mater. 2011, 4, 933–942.
- Paul, C.P.L.; Schoorl, T.; Zuiderbaan, H.A.; ZandiehDoulabi, B.; van der Veen, A.J.; van de Ven, P.M.; Smit, T.H.; van Royen, B.J.; Helder, M.N.; Mullender, M.G. Dynamic and Static Overloading Induce Early Degenerative Processes in Caprine Lumbar Intervertebral Discs. PLoS ONE 2013, 8, e62411.
- Paul, C.P.L.; Zuiderbaan, H.A.; ZandiehDoulabi, B.; van der Veen, A.J.; van de Ven, P.M.; Smit, T.H.; Helder, M.N.; van Royen, B.J.; Mullender, M.G. Simulated-physiological loading conditions preserve biological and mechanical properties of caprine lumbar intervertebral discs in ex vivo culture. PLoS ONE 2012, 7, e33147.
- Wilke, H.-J.; Wenger, K.; Claes, L. Testing criteria for spinal implants: Recommendations for the standardization of in vitro stability testing of spinal implants. Eur. Spine J. 1998, 7, 148–154.
- Vergroesen, P.; van der Veen, A.; Emanuel, K.S.; van Dieën, J.V.; Smit, T.H. The poro-elastic behaviour of the intervertebral disc: A new perspective on diurnal fluid flow. J. Biomech. 2016, 49, 857–863.
- Ingelmark, B.E.; Ekholm, R. The compressibility of intervertebral disks: An experimental investigation on the intervertebral disk between the third and fourth lumbar vertebrae in man. Acta Soc. Med. Ups. 1952, 57, 202–217.
- Brinckmann, P.; Horst, M. The influence of vertebral body fracture, intradiscal injection, and partial discectomy on the radial bulge and height of human lumbar discs. Spine 1985, 10, 138–145.
- Koeller, W.; Muehlhaus, S.; Meier, W.; Hartmann, F. Biomechanical properties of human intervertebral discs subjected to axial dynamic compression—Influence of age and degeneration. J. Biomech. 1986, 19, 807–816.
- Parkinson, R.J.; Durkin, J.L.; Callaghan, J.P. Estimating the Compressive Strength of the Porcine Cervical Spine: An Examination of the Utility of DXA. Spine 2005, 30, E492–E498.
- Janevic, J.; Ashton-Miller, J.A.; Schultz, A.B. Large compressive preloads decrease lumbar motion segment flexibility. J. Orthop. Res. 1991, 9, 228–236.
- Van der Veen, A.J.; Mullender, M.; Smit, T.H.; Kingma, I.; van Dieën, J.H. Flow-Related Mechanics of the Intervertebral Disc: The Validity of an In Vitro Model. Spine 2005, 30, E534–E539.
- Van der Veen, A.J.; Mullender, M.G.; Kingma, I.; van Dieen, J.H.; Smit, T.H. Contribution of vertebral bodies, endplates, and in-tervertebral discs to the compression creep of spinal motion segments. J. Biomech. 2008, 41, 1260–1268.
- Costi, J.; Heinze, K.; Lawless, I.; Stanley, R.; Freeman, B. Do combined compression, flexion and axial rotation place degenerated discs at risk of posterolateral herniation? Measurement of 3D lumbar intervertebral disc internal strains during repetitive loading. Bone Jt. J. Orthop. Proc. Suppl. 2014, 96-B, 219.
- Vergroesen, P.-P.A.; Van Der Veen, A.J.; Van Royen, B.J.; Kingma, I.; Smit, T.H. Intradiscal pressure depends on recent loading and correlates with disc height and compressive stiffness. Eur. Spine J. 2014, 23, 2359–2368.
- Wilke, H.; Neef, P.; Caimi, M.; Hoogland, T.; Claes, L.E. New In Vivo Measurements of Pressures in the Intervertebral Disc in Daily Life. Spine 1999, 24, 755–762.
- Brinckmann, P.; Grootenboer, H. Change of Disc Height, Radial Disc Bulge, and Intradiscal Pressure from Discectomy An in Vitro Investigation on Human Lumbar Discs. Spine 1991, 16, 641–646.
- Callaghan, J.P.; Gunning, J.L.; McGill, S.M. The relationship between lumbar spine load and muscle activity during extensor exercises. Phys. Ther. 1998, 78, 8–18.
- Han, K.-S.; Rohlmann, A.; Zander, T.; Taylor, W.R. Lumbar spinal loads vary with body height and weight. Med. Eng. Phys. 2013, 35, 969–977.
- Nachemson, A.L. Disc Pressure Measurements. Spine 1981, 6, 93–97.
- Nachemson, A.; Morris, J.M. In Vivo Measurements of Intradiscal Pressure. J. Bone Jt. Surg. 1964, 46, 1077–1092.
- Nachemson, A.; Morris, J. Lumbar discometry lumbar intradiscal pressure Page 37 of 55 measurements in vivo. Lancet 1963, 281, 1140–1142.
- Sato, K.; Kikuchi, S.; Yonezawa, T. In Vivo Intradiscal Pressure Measurement in Healthy Individuals and in Patients with Ongoing Back Problems. Spine 1999, 24, 2468–2474.
- Dreischarf, M.; Shirazi-Adl, A.; Arjmand, N.; Rohlmann, A.; Schmidt, H. Estimation of loads on human lumbar spine: A review of in vivo and computational model studies. J. Biomech. 2016, 49, 833–845.
- Ferguson, S.J.; Ito, K.; Nolte, L.-P. Fluid flow and convective transport of solutes within the intervertebral disc. J. Biomech. 2004, 37, 213–221.
- Bezci, S.E.; O’Connell, G.D. Osmotic Pressure Alters Time-dependent Recovery Behavior of the Intervertebral Disc. Spine 2018, 43, E334–E340.
- Vergroesen, P.-P.A.; Emanuel, K.S.; Peeters, M.; Kingma, I.; Smit, T.H. Are axial intervertebral disc biomechanics determined by osmosis? J. Biomech. 2018, 70, 4–9.
- Wilder, D.; Pope, M. Epidemiological and aetiological aspects of low back pain in vibration environments—An update. Clin. Biomech. 1996, 11, 61–73.
- Kumar, A.; Varghese, M.; Mohan, D.; Mahajan, P.; Gulati, P.; Kale, S. Effect of whole-body vibration on the low back. A study of tractor-driving farmers in north India. Spine 1999, 24, 2506–2515.
- Gullbrand, S.E.; Ashinsky, B.G.; Martin, J.T.; Pickup, S.; Smith, L.J.; Mauck, R.L.; Smith, H.E. Correlations between quantitative T 2 and T 1ρ MRI, mechanical properties and biochemical composition in a rabbit lumbar intervertebral disc degeneration model. J. Orthop. Res. 2016, 34, 1382–1388.
- Hedman, T.P.; Chen, W.-P.; Lin, L.-C.; Lin, H.-J.; Chuang, S.-Y. Effects of Collagen Crosslink Augmentation on Mechanism of Compressive Load Sharing in Intervertebral Discs. J. Med. Biol. Eng. 2017, 37, 94–101.
- Emanuel, K.S.; van der Veen, A.J.; Rustenburg, C.M.; Smit, T.H.; Kingma, I. Osmosis and viscoelasticity both contribute to time-dependent behaviour of the intervertebral disc under compressive load: A caprine in vitro study. J. Biomech. 2018, 70, 10–15.
- Bezci, S.E.; Lim, S.; O’Connell, G.D. Nonlinear stress-dependent recovery behavior of the intervertebral disc. J. Mech. Behav. Biomed. Mater. 2020, 110, 103881.
- Barrett, J.M.; Gooyers, C.E.; Karakolis, T.; Callaghan, J.P. The Impact of Posture on the Mechanical Properties of a Functional Spinal Unit During Cyclic Compressive Loading. J. Biomech. Eng. 2016, 138, 081007.
- Yang, X.; Cheng, X.; Luan, Y.; Liu, Q.; Zhang, C. Creep experimental study on the lumbar intervertebral disk under vibration compression load. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2019, 233, 858–867.




