
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Haoran Xing | -- | 3410 | 2022-04-14 03:00:36 | | | |
| 2 | Conner Chen | -7 word(s) | 3403 | 2022-04-14 03:40:44 | | | | |
| 3 | Conner Chen | -1 word(s) | 3402 | 2022-04-14 03:42:56 | | | | |
| 4 | Conner Chen | -6 word(s) | 3396 | 2022-04-18 08:03:19 | | | | |
| 5 | Conner Chen | Meta information modification | 3396 | 2022-04-18 08:04:46 | | |
Video Upload Options
In order to cope with the problem that no gas fire suppressant can be used in the future, perfluoro-2-methyl-3-pentanone (C6F12O), also known as FK-5112, Novec 1230 or Novec 649, with its environmentally friendly performance, zero ODP, GWP of approximately one and atmospheric lifetime of up to two weeks (as shown in Table 1), has been considered as the next generation of halon alternatives. C6F12O belongs to fluorinated ketones which is different to HFCs. Its nontoxicity, noncombustibility, excellent insulation properties and fire suppression efficiency have attracted worldwide attention. The thermophysical parameters, safety and environmental issues and other properties such as the dispersion characteristics of C6F12O are important indexes to evaluate whether it is appropriate to replace the halon. Meanwhile, these properties and parameters are critical to determine the application scenes of fire suppressant, the selection of fire extinguishment facilities and the engineering calculation of the fire extinguishment system.
1. Thermophysical Parameters
| Properties | Data |
|---|---|
| Boiling point (1 atm) | 49.2 °C |
| Freezing point | −108.0 °C |
| Critical temperature | 168.7 °C |
| Critical pressure | 18.65 bar |
| Critical density | 639.1 kg/m3 |
| Density, sat. Liquid | 1.6 g/ml |
| Density, gas (1 atm) | 0.0136 g/mL |
| Specific volume, gas (1 atm) | 0.0733 m3/kg |
| Specific heat, liquid | 1.103 kJ/(kg °C) |
| Specific heat, vapor (1atm) | 0.891 kJ/(kg °C) |
| Heat of vaporization (boiling point) | 88 kJ/kg |
| Liquid viscosity (0 °C/25 °C) | 0.56/0.39 centistokes |
| Relative dielectric strength, (1atm, n2 = 1.0) | 2.3 |
2. Safety
2.1. Toxicity
| Fire Suppressant |
LC50/ALC (%) | NOAEL (%) | LOAEL (%) | MEC (%) |
|---|---|---|---|---|
| C6F12O | >10 | 10 | >10 | 3.5–6.4 |
| HFC 227ea | >80 | 9 | 10.5 | 5.8–6.6 |
| HFC 125 | >70 | 7.5 | 10 | 8.1–9.4 |
| Halon1301 | >80 | 5 | 7.5 | 2.9–4.0 |
2.2. Corrosion
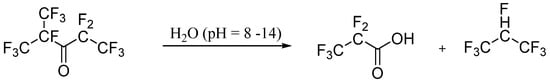
3. Environment
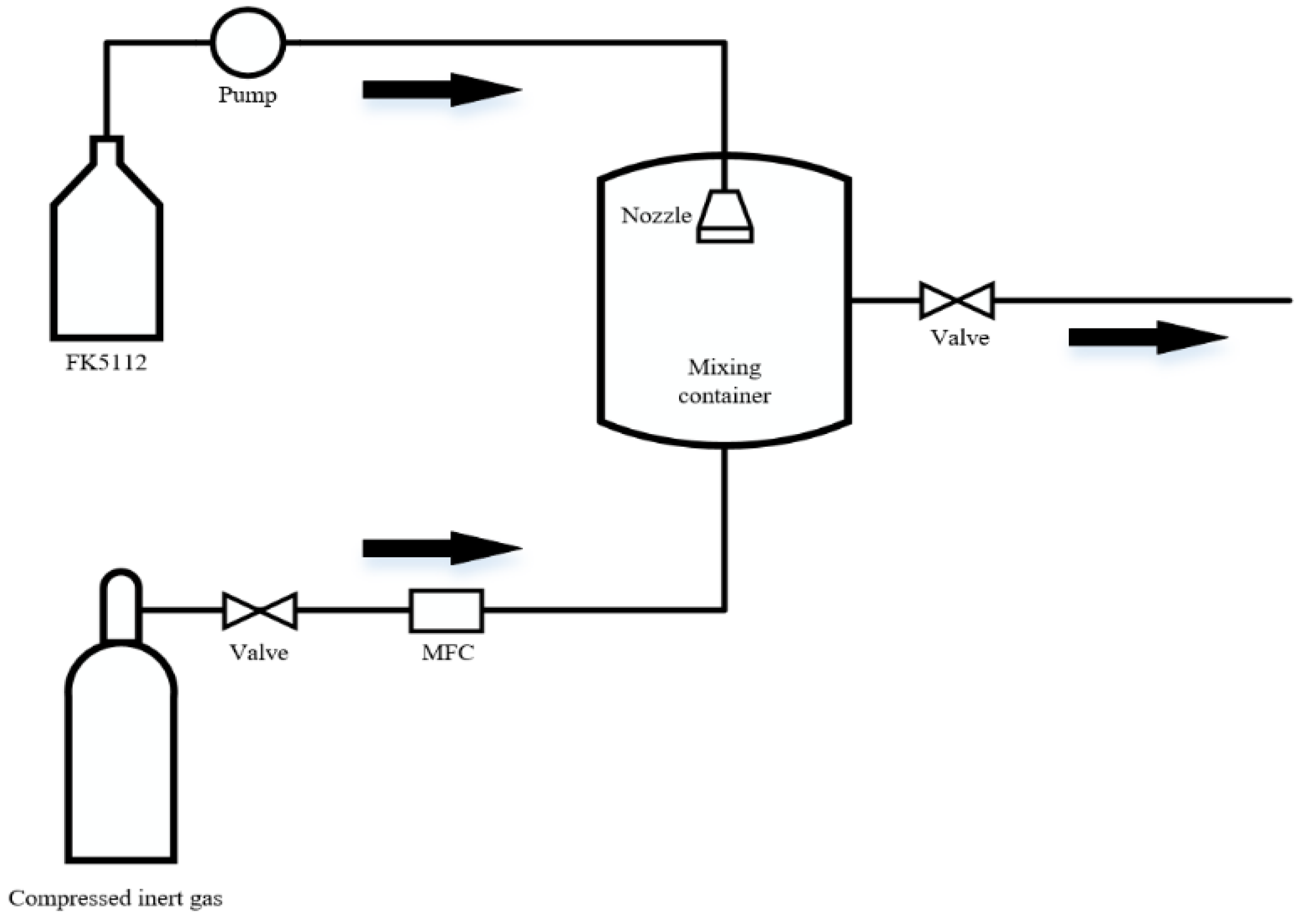
4. Dispersion Performance
| Fire Suppressants | Molecular Weight | Boiling Point (°C, 1 atm) | Vapor Pressure (MPa, 25 °C) | Diffusion Coefficient (10−6 m2/s, 1 atm, 0 °C) |
|---|---|---|---|---|
| Halon 1301 | 149 | −57.9 | 1.62 | 7.49 |
| HFC 125 | 120 | −48.5 | 1.37 | 6.81 |
| HFC 227ea | 170 | −16.4 | 0.458 | 5.68 |
| C6F12O | 316 | 49.2 | 0.04 | 4.17 |
References
- Mclinden, M.O.; RPerkins, A.; Lemmon, E.W.; Fortin, T.J. Thermodynamic Properties of 1,1,1,2,2,4,5,5,5-Nonafluoro-4-(trifluoromethyl)-3-pentanone: Vapor Pressure, (p, ρ, T) Behavior, and Speed of Sound Measurements, and an Equation of State. J. Chem. Eng. Data 2015, 60, 150930083719001.
- Tanaka, K. Measurement of pρT Properties of 1,1,1,2,2,4,5,5,5-Nonafluoro-4-(trifluoromethyl)-3-pentanone in the Near-Critical and Supercritical Regions. J. Chem. Eng. Data 2016, 61, 3958–3961.
- Wen, C.; Meng, X.; Huber, M.L.; Wu, J. Measurement and Correlation of the Viscosity of 1,1,1,2,2,4,5,5,5-Nonafluoro-4-(trifluoromethyl)-3-pentanone. J. Chem. Eng. Data 2017, 62, 3603–3609.
- Cui, J.; Yan, S.; Bi, S.; Wu, J. Saturated Liquid Dynamic Viscosity and Surface Tension of trans-1-Chloro-3,3,3-trifluoropropene and Dodecafluoro-2-methylpentan-3-one. J. Chem. Eng. Data 2018, 63, 751–756.
- Perkins, R.A.; Huber, M.L.; Assael, M.J. Measurement and Correlation of the Thermal Conductivity of 1,1,1,2,2,4,5,5,5-Nonafluoro-4-(trifluoromethyl)-3-pentanone. J. Chem. Eng. Data 2018, 63, 2783–2789.
- 3M Company. 3MTM Novec1230TM Fire Protection Fluid; 3M Company: Saint Paul, MN, USA, 2020.
- Burch, I.A.; Kennett, S.R.; Fletcher, L.E. A Risk Assessment Approach for Selecting a Replacement for Halon 1301 Fire Suppressant; Defence Science and Technology Organisation: Melbourne, Australia, 2001.
- Su, J.Z.; Kim, A.K.; Mawhinney, J.R. Review of total flooding gaseous agents as Halon 1301 substitutes. J. Fire Prot. Eng. 1996, 8, 45–63.
- Rivers, P.E. Advancement in Sustainable Fire Suppression Development C, F-Ketone: A Novel New Halon Replacement Alternative to HFCs and PFCs. In Proceedings of the 2001 Halon Options Technical Working Conference, Albuquerque, NM, USA, 24–26 April 2001.
- Xu, X.; Jiang, L.; Xu, L.; Chen, X. Acute inhalation toxicity of fire extinguishing agents Novec1230. J. Environ. Health 2015, 32, 742–743.
- Zeng, F.P.; Lei, Z.C.; Miao, Y.L.; Yao, Q.; Tang, J. Reaction Thermodynamics of Overthermal Decomposition of C6F12O; Springer International Publishing: Cham, Switzerland, 2020; pp. 43–51.
- Zhang, X.X.; Tian, S.S.; Xiao, S.; Deng, Z.T.; Li, Y.; Tang, J. Insulation Strength and Decomposition Characteristics of a C6F12O and N-2 Gas Mixture. Energies 2017, 10, 1170.
- Li, Y.; Zhang, X.; Tian, S.; Xiao, S.; Li, Y.; Chen, D. Insight into the decomposition mechanism of C6F12O-CO2 gas mixture. Chem. Eng. J. 2019, 360, 929–940.
- Christophorou, L.G.; Olthoff, J.K. Gaseous Dielectrics VIII; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998; 639p.
- Zhang, X.; Li, Y.; Xiao, S.; Tang, J.; Tian, S.; Deng, Z. Decomposition Mechanism of C5F10O: An Environmentally Friendly Insulation Medium. Environ. Sci. Technol. 2017, 51, 10127–10136.
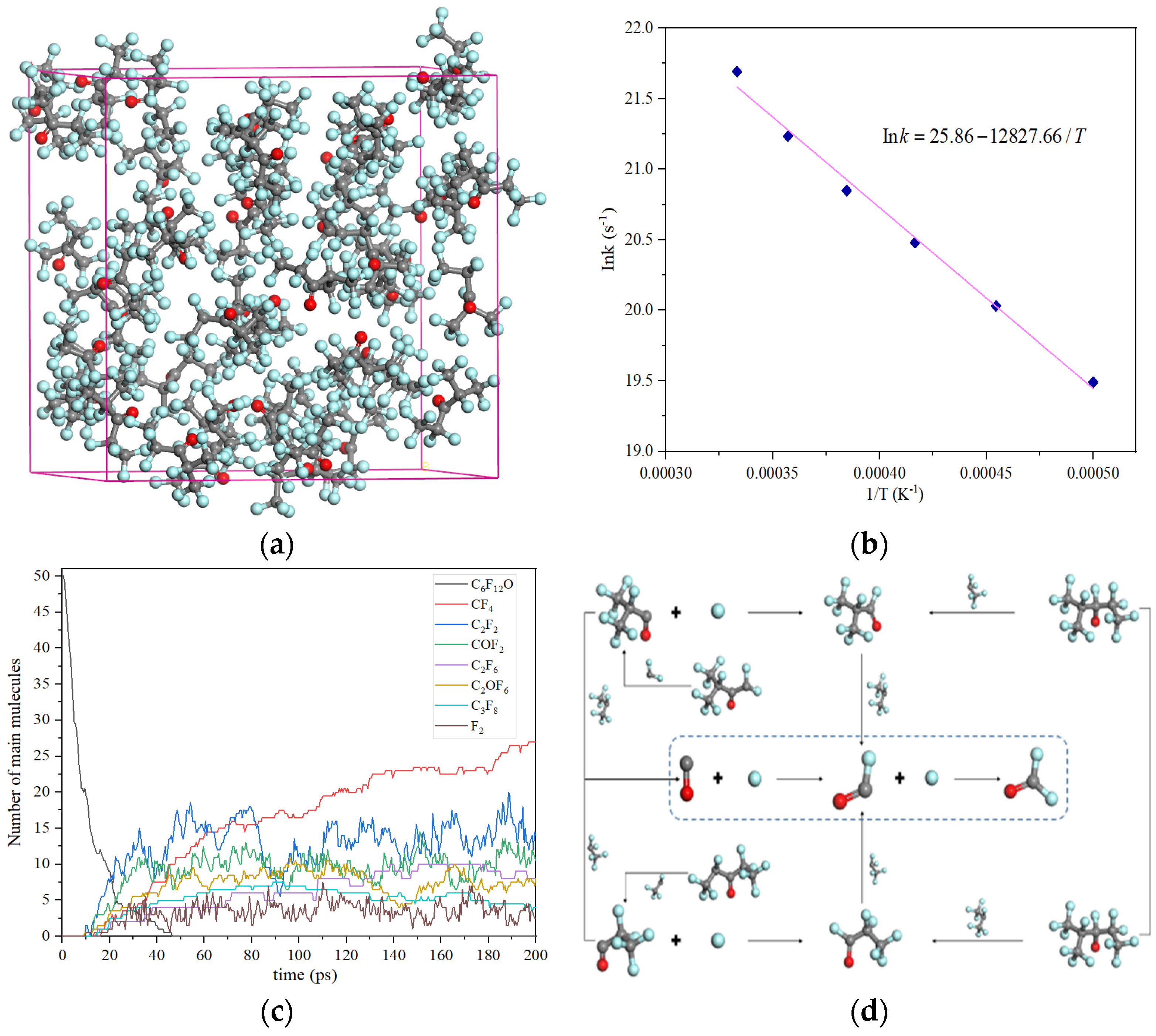
- Xing, H.; Cheng, Y.; Lu, S.; Tao, N.; Zhang, H. A reactive molecular dynamics study of the pyrolysis mechanism of C6F12O. Mol. Phys. 2021, 119, e1976425.
- Cui, F.; Qin, K.; Shi, L.; Zhang, P.; Pan, R. Study on Thermal Pyrolysis of C6 F-ketone Fire Extinguishant at High Temperatures. Explos. Mater. 2015, 44, 5–8.
- Ditch, B.D. Thermal Decomposition Products Testing With 1,1,1,2,2,4,5,5,5 nonafluoro-4-trifluoromethyl pentan-3-one (C6 F-ketone) during Fire Extinguishing. Master’s Thesis, Worcester Polytechnic University, Worcester, MA, USA, 2003.
- Andersson, B.; Blomqvist, P. Experimental study of thermal breakdown products from halogenated extinguishing agents. Fire Saf. J. 2011, 46, 104–115.
- Tuma, P.E. Fluoroketone C2F5C(O)CF(CF3)(2) as a heat transfer fluid for passive and pumped 2-phase applications. In Proceedings of the 2008 Twenty-Fourth Annual IEEE Semiconductor Thermal Measurement and Management Symposium, San Jose, CA, USA, 16–20 March 2008; pp. 174–181.
- Li, Y.; Zhang, X.; Tian, S.; Xiao, S.; Chen, Q.; Chen, D.; Cui, Z.; Tang, J. Insight into the Compatibility Between C6F12O and Metal Materials: Experiment and Theory. IEEE Access 2018, 6, 58154–58160.
- Zhang, X.; Wang, Y.; Li, Y.; Li, Y.; Ye, F.; Tian, S.; Chen, D.; Xiao, S.; Tang, J. Thermal compatibility properties of C6F12O-air gas mixture with metal materials. AIP Adv. 2019, 9, 125024.
- Zhuo, R.; Chen, Q.; Wang, D.; Fu, M.; Tang, J.; Hu, J.; Jiang, Y. Compatibility between C6F12O–N2 Gas Mixture and Metal Used in Medium-Voltage Switchgears. Energies 2019, 12, 4639.
- Zhang, X.; Lan, J.; Tian, S.; Rao, X.; Li, X.; Yuan, Z.; Jin, X.; Gao, S.; Zhang, X. Study of compatibility between eco-friendly insulating medium C6F12O and sealing material EPDM. J. Mol. Struct. 2021, 1244, 130949.
- Saloutina, L.V.; Filyakova, T.I.; Zapevalov, A.Y.; Kodess, M.I.; Kolenko, I.P. Synthesis and Some Reactions of “2-X-Perfluoro-2-Methyl-3-Pentanones. Bull. Acad. Sci. Ussr Div. Chem. Sci. 1982, 31, 1685–1687.
- Jackson, D.A.; Young, C.J.; Hurley, M.D.; Wallington, T.J.; Mabury, S.A. Atmospheric Degradation of Perfluoro-2-methyl-3-pentanone: Photolysis, Hydrolysis and Hydration. Environ. Sci. Technol. 2011, 45, 8030–8036.
- Ke, W.; Yang, W.; Zhou, B.; Wang, K.; Sun, J.; Sun, X.; Xu, M.; Chen, Q.; Qiu, B.; Wang, W.; et al. The color change analysis of historic wooden remains after fire-suppression by fluorinated chemical gases. Herit. Sci. 2021, 9, 93.
- Robin, M.L. Fire protection in telecommunication facilities. Process Saf. Prog. 2010, 19, 107–111.
- Hirschler, M.M. Discussion of smoke corrosivity test methods: Analysis of existing tests and of their results. Fire Mater. 1993, 17, 231–247.
- Lunt, M.F.; Rigby, M.; Ganesan, A.L.; Manning, A.J.; Prinn, R.G.; O’Doherty, S.; Muehle, J.; Harth, C.M.; Salameh, P.K.; Arnold, T.; et al. Reconciling reported and unreported HFC emissions with atmospheric observations. Proc. Natl. Acad. Sci. USA 2015, 112, 5927–5931.
- Velders, G.J.M.; Fahey, D.W.; Daniel, J.S.; Andersen, S.O.; McFarland, M. Future atmospheric abundances and climate forcings from scenarios of global and regional hydrofluorocarbon (HFC) emissions. Atmos. Environ. 2015, 123, 200–209.
- Ravishankara, A.R.; Solomon, S.; Turnipseed, A.A.; Warren, R.F. Atmospheric Lifetimes of Long-Lived Halogenated Species. Science 1993, 259, 194–199.
- Mellouki, A.; Wallington, T.J.; Chen, J. Atmospheric Chemistry of Oxygenated Volatile Organic Compounds: Impacts on Air Quality and Climate. Chem. Rev. 2015, 115, 3984–4014.
- Taniguchi, N.; Wallington, T.J.; Hurley, M.D.; Guschin, A.G.; Molina, L.T.; Molina, M.J. Atmospheric chemistry of C2F5C(O)CF(CF3)(2): Photolysis and reaction with Cl atoms, OH radicals, and ozone. J. Phys. Chem. A 2003, 107, 2674–2679.
- D’Anna, B.; Sellevag, S.R.; Wirtz, K.; Nielsen, G.J. Photolysis Study of Perfluoro-2-methyl-3-pentanone under Natural Sunlight Conditions. Environ. Sci. Technol. 2005, 39, 8708–8711.
- Ren, Y.; Bernard, F.; Daele, V.; Mellouki, A. Atmospheric Fate and Impact of Perfluorinated Butanone and Pentanone. Environ. Sci. Technol. 2019, 53, 8862–8871.
- Fuller, E.N.; Schettler, P.D.J.; Giddings, J.C. New Method for Prediction of Binary Gas-Phase Diffusion Coefficients. Ind. Eng. Chem. 1966, 58, 18–27.
- Chen, W.W.; Zhou, X.M.; Liao, G.X. Experimental study of the storage behaviors of the Novec 1230-N_2 fire-extinguishing system. J. Saf. Environ. 2014, 14, 80–85.
- Yang, W.; Cong, X.; Zhao, L. Experimental study on application of temperature sensing self-starting fire extinguishing pipe system in confined space. J. Saf. Sci. Technol. 2019, 15, 18–23.
- Fan, W.; Gao, Z.; Wang, D.; Yang, Y.; Gao, Y. Study on the flow characteristics of fire extinguishing agent FK-5-1-12 in the pipeline release process. Fire Mater. 2022, 46, 376–387.
- Xing, H.; Lu, S.; Tao, N.; Liu, R. Study on Dispersion Characteristics of FK5112 Mixed with Inert Gas. In Proceedings of the 2019 9th International Conference on Fire Science and Fire Protection Engineering (ICFSFPE), Chengdu, China, 18–20 October 2019.




