
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Khaled O. Sebakhy | -- | 1612 | 2022-04-13 11:59:59 | | | |
| 2 | Lindsay Dong | Meta information modification | 1612 | 2022-04-15 04:47:18 | | |
Video Upload Options
Solid state nuclear magnetic resonance (ssNMR) is a powerful and attractive characterization method for obtaining insights into the chemical structure and dynamics of a wide range of materials. Current interest in cellulose-based materials, as sustainable and renewable natural polymer products, requires deep investigation and analysis of the chemical structure, molecular packing, end chain motion, functional modification, and solvent–matrix interactions, which strongly dictate the final product properties and tailor their end applications. In comparison to other spectroscopic techniques, on an atomic level, ssNMR is considered more advanced, especially in the structural analysis of cellulose-based materials
1. Introduction
2. Cellulose Structure Investigated via Solid State NMR Spectroscopy
2.1. Pure Cellulose with 13C CP MAS and Different Crystal Phases
Different cellulose materials obtained from pulp (cellulose I) [25][26][27], regenerated cellulose fibers (cellulose II) [28], cellulose-based shells [29], and bacterial cellulose [30][31] were characterized by 13C CP/MAS solid state NMR [32]. The 13C CP MAS NMR spectrum was extended over a chemical shift from 57 to 108 ppm and divided into four separate regions, resolved to a different extent, including less resolved regions C6 (57–67 ppm), C1 (102–108 ppm) and C2,3,5 in the cellulosic ring (70–80 ppm), and well resolved region C4 (80–92 ppm) [33]. Ultra-structural information regarding crystallinity could be extracted from the C4 peak in the isolated cellulose spectrum, which is resolved into one crystalline and another amorphous region. When spectral deconvolution was applied to the C4 region of untreated sugarcane bagasse, several signals were recorded that were a sum of four signals and were assigned to the crystalline region possessing narrow fitting and sharpness, corresponding to α (90 ppm), β (86 ppm), a mixture of α + β cellulose (89 ppm) crystal structures and paracrystalline cellulose (88 ppm). To the right, the signal for inaccessible fibril surface appears at (83 ppm) and two other signals (84 and 82 ppm) are assigned to accessible fibril surface [33]. The cellulose amorphous region consists of two characteristic doublets at 84 and 85 ppm, corresponding to the accessible fibril surface and identified as located on top of the two different crystal planes, with a broad signal for deeply located inaccessible fibril surface at round 85.4 ppm [34]. The spatial distribution of the cellulose morphologies within the cellulose microfibril suggests a core-shell structure, consisting of an outer amorphous shell composed mainly of accessible cellulose microfibril surfaces [35].
2.2. Cellulose Polymorphism in Plant Primary Cell Walls
Another way to visualize cellulose polymorphism in plant cell walls is by using the 2D 13C-13C INADEQUATE spectrum to collect spectrograms of unlabeled stems of wild-type rice using the DNP system. At first, this 2D experiment was considered impossible to achieve, because there is a low natural abundance of 13C (1.1%) and a lower probability of detecting a cross peak between two carbons. However, now this experiment can be completed using DNP system within 35 h [37].
3. Lignocellulosic Biomass Structure Interpretation
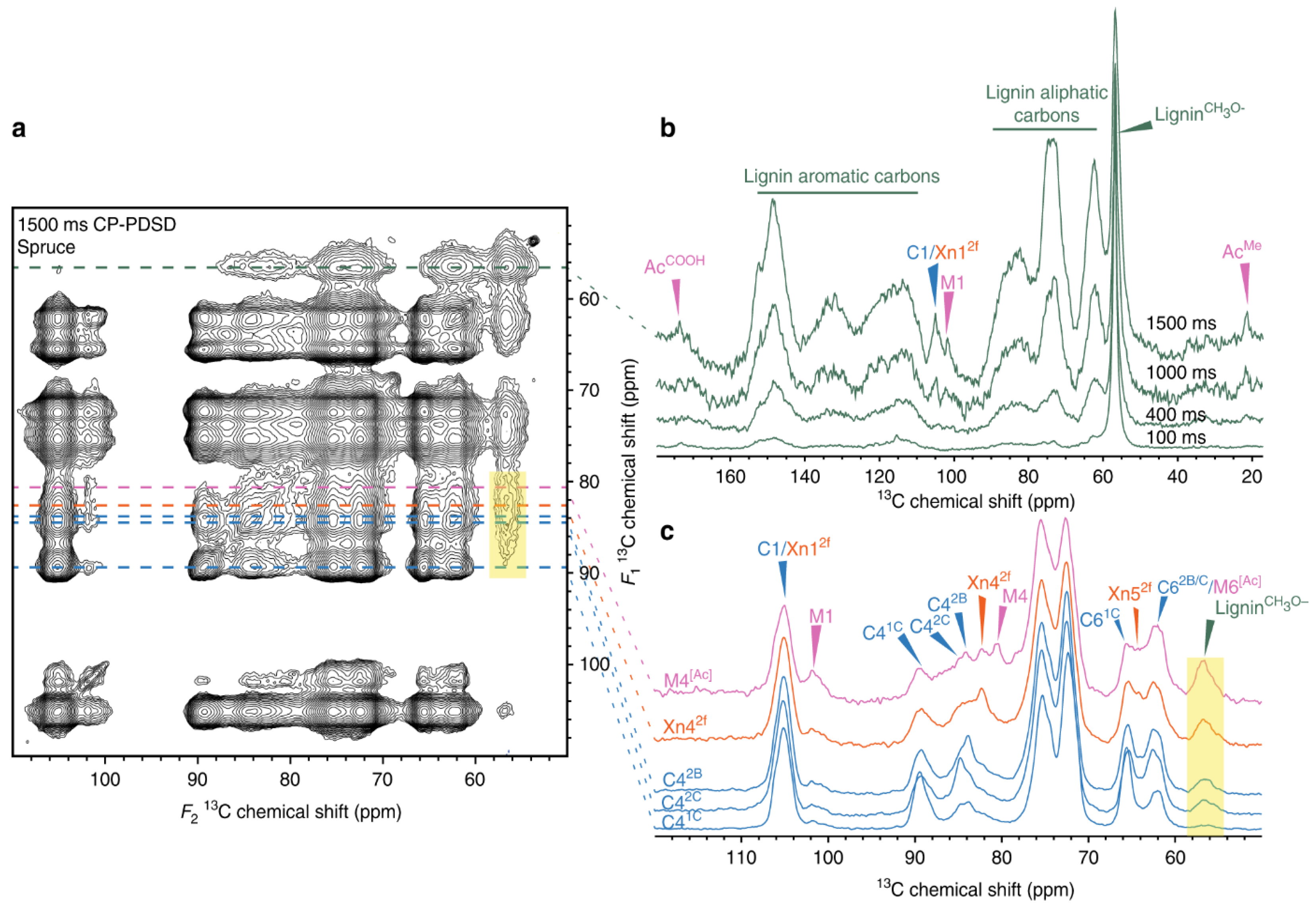
4. Effect of Oxidation on Pulp and Viscous Cellulose
5. Production of Crystalline Nanocelluloses via Oxidation of Microcrystalline Cellulose
References
- Baghaei, B.; Skrifvars, M. All-Cellulose Composites: A Review of Recent Studies on Structure, Properties and Applications. Molecules 2020, 25, 2836.
- Esen, E.; Meier, M.A.R. Sustainable Functionalization of 2,3-Dialdehyde Cellulose via the Passerini Three-Component Reaction. ACS Sustain. Chem. Eng. 2020, 8, 15755–15760.
- Khandelwal, M.; Alan, H.W. Hierarchical Organisation in the Most Abundant Biopolymer–Cellulose. MRS Online Proc. Libr. 2013, 1504, mrsf12-1504-v02-03.
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393.
- Kumar Gupta, P.; Sai Raghunath, S.; Venkatesh Prasanna, D.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An Update on Overview of Cellulose, Its Structure and Applications. In Cellulose; Rodríguez Pascual, A.E., Eugenio Martín, M., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-83968-056-4.
- Li, Y.-Y.; Wang, B.; Ma, M.-G.; Wang, B. Review of Recent Development on Preparation, Properties, and Applications of Cellulose-Based Functional Materials. Int. J. Polym. Sci. 2018, 2018, 8973643.
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly Efficient and Sustainable Preparation of Carboxylic and Thermostable Cellulose Nanocrystals via FeCl3-Catalyzed Innocuous Citric Acid Hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700.
- Onwukamike, K.N.; Grelier, S.; Grau, E.; Cramail, H.; Meier, M.A.R. Critical Review on Sustainable Homogeneous Cellulose Modification: Why Renewability Is Not Enough. ACS Sustain. Chem. Eng. 2019, 7, 1826–1840.
- Rahmatika, A.M.; Toyoda, Y.; Nguyen, T.T.; Goi, Y.; Kitamura, T.; Morita, Y.; Kume, K.; Ogi, T. Cellulose Nanofiber and Magnetic Nanoparticles as Building Blocks Constructing Biomass-Based Porous Structured Particles and Their Protein Adsorption Performance. ACS Sustain. Chem. Eng. 2020, 8, 18686–18695.
- Ummartyotin, S.; Manuspiya, H. A Critical Review on Cellulose: From Fundamental to an Approach on Sensor Technology. Renew. Sustain. Energy Rev. 2015, 41, 402–412.
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and Valorization of Cellulose, Lignin and Lignocellulose Using Ionic Liquids. J. Bioresour. Bioprod. 2020, 5, 79–95.
- Updegraff, D.M. Semimicro Determination of Cellulose Inbiological Materials. Anal. Biochem. 1969, 32, 420–424.
- Holtzapple, M.T. CELLULOSE. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 998–1007. ISBN 978-0-12-227055-0.
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Chapter 4—Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 67–89. ISBN 978-0-12-396983-5.
- Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. Chapter 23—Biodegradable Polymers. In Principles of Tissue Engineering, 4th ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 441–473. ISBN 978-0-12-398358-9.
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Egorov, Y.E.; Kulichikhin, V.G.; Mikhailov, Y.M. New Hydrated Cellulose Fiber Based on Flax Cellulose. Russ. J. Gen. Chem. 2021, 91, 1807–1815.
- Mettler, M.S.; Paulsen, A.D.; Vlachos, D.G.; Dauenhauer, P.J. Pyrolytic Conversion of Cellulose to Fuels: Levoglucosan Deoxygenation via Elimination and Cyclization within Molten Biomass. Energy Environ. Sci. 2012, 5, 7864–7868.
- Jiang, X.; Bai, Y.; Chen, X.; Liu, W. A Review on Raw Materials, Commercial Production and Properties of Lyocell Fiber. J. Bioresour. Bioprod. 2020, 5, 16–25.
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2012, 49, 255–266.
- Courtenay, J.C.; Johns, M.A.; Galembeck, F.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Scott, J.L.; Sharma, R.I. Surface Modified Cellulose Scaffolds for Tissue Engineering. Cellulose 2017, 24, 253–267.
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B.; Ribeiro, S.J.L. A Multipurpose Natural and Renewable Polymer in Medical Applications: Bacterial Cellulose. Carbohydr. Polym. 2016, 153, 406–420.
- George, J.; Sabapathi, S.N. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54.
- Mohamed, M.A.; Abd Mutalib, M.; Mohd Hir, Z.A.; Zain, M.F.M.; Mohamad, A.B.; Minggu, L.J.; Awang, N.A.; Salleh, W.N. An Overview on Cellulose-Based Material in Tailoring Bio-Hybrid Nanostructured Photocatalysts for Water Treatment and Renewable Energy Applications. Int. J. Biol. Macromol. 2017, 103, 1232–1256.
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose Nanocomposites: Fabrication and Biomedical Applications. J. Bioresour. Bioprod. 2020, 5, 223–237.
- Liitiä, T.; Maunu, S.L.; Hortling, B.; Tamminen, T.; Pekkala, O.; Varhimo, A. Cellulose Crystallinity and Ordering of Hemicelluloses in Pine and Birch Pulps as Revealed by Solid-State NMR Spectroscopic Methods. Cellulose 2003, 10, 307–316.
- Liitiä, T.; Maunu, S.L.; Hortling, B. Solid State NMR Studies on Inhomogeneous Structure of Fibre Wall in Kraft Pulp. Holzforschung 2001, 55, 503–510.
- Siller, M.; Amer, H.; Bacher, M.; Roggenstein, W.; Rosenau, T.; Potthast, A. Effects of Periodate Oxidation on Cellulose Polymorphs. Cellulose 2015, 22, 2245–2261.
- Zuckerstätter, G.; Terinte, N.; Sixta, H.; Schuster, K.C. Novel Insight into Cellulose Supramolecular Structure through 13C CP-MAS NMR Spectroscopy and Paramagnetic Relaxation Enhancement. Carbohydr. Polym. 2013, 93, 122–128.
- Modica, A.; Rosselli, S.; Catinella, G.; Sottile, F.; Catania, C.A.; Cavallaro, G.; Lazzara, G.; Botta, L.; Spinella, A.; Bruno, M. Solid State 13C-NMR Methodology for the Cellulose Composition Studies of the Shells of Prunus Dulcis and Their Derived Cellulosic Materials. Carbohydr. Polym. 2020, 240, 116290.
- Casaburi, A.; Montoya Rojo, Ú.; Cerrutti, P.; Vázquez, A.; Foresti, M.L. Carboxymethyl Cellulose with Tailored Degree of Substitution Obtained from Bacterial Cellulose. Food Hydrocoll. 2018, 75, 147–156.
- Meza-Contreras, J.C.; Manriquez-Gonzalez, R.; Gutiérrez-Ortega, J.A.; Gonzalez-Garcia, Y. XRD and Solid State 13C-NMR Evaluation of the Crystallinity Enhancement of 13C-Labeled Bacterial Cellulose Biosynthesized by Komagataeibacter Xylinus under Different Stimuli: A Comparative Strategy of Analyses. Carbohydr. Res. 2018, 461, 51–59.
- Foston, M.B.; Hubbell, C.A.; Ragauskas, A.J. Cellulose Isolation Methodology for NMR Analysis of Cellulose Ultrastructure. Materials 2011, 4, 1985–2002.
- Ghosh, M.; Prajapati, B.P.; Suryawanshi, R.K.; Kishor Dey, K.; Kango, N. Study of the Effect of Enzymatic Deconstruction on Natural Cellulose by NMR Measurements. Chem. Phys. Lett. 2019, 727, 105–115.
- Palme, A.; Idström, A.; Nordstierna, L.; Brelid, H. Chemical and Ultrastructural Changes in Cotton Cellulose Induced by Laundering and Textile Use. Cellulose 2014, 21, 4681–4691.
- Foston, M. Advances in Solid-State NMR of Cellulose. Curr. Opin. Biotechnol. 2014, 27, 176–184.
- Wang, T.; Yang, H.; Kubicki, J.D.; Hong, M. Cellulose Structural Polymorphism in Plant Primary Cell Walls Investigated by High-Field 2D Solid-State NMR Spectroscopy and Density Functional Theory Calculations. Biomacromolecules 2016, 17, 2210–2222.
- Zhao, W.; Kirui, A.; Deligey, F.; Mentink-Vigier, F.; Zhou, Y.; Zhang, B.; Wang, T. Solid-State NMR of Unlabeled Plant Cell Walls: High-Resolution Structural Analysis without Isotopic Enrichment. Biotechnol. Biofuels 2021, 14, 14.
- Fu, L.; McCallum, S.A.; Miao, J.; Hart, C.; Tudryn, G.J.; Zhang, F.; Linhardt, R.J. Rapid and Accurate Determination of the Lignin Content of Lignocellulosic Biomass by Solid-State NMR. Fuel 2015, 141, 39–45.
- Kang, X.; Kirui, A.; Dickwella Widanage, M.C.; Mentink-Vigier, F.; Cosgrove, D.J.; Wang, T. Lignin-Polysaccharide Interactions in Plant Secondary Cell Walls Revealed by Solid-State NMR. Nat. Commun. 2019, 10, 347.
- Terrett, O.M.; Lyczakowski, J.J.; Yu, L.; Iuga, D.; Franks, W.T.; Brown, S.P.; Dupree, R.; Dupree, P. Molecular Architecture of Softwood Revealed by Solid-State NMR. Nat. Commun. 2019, 10, 4978.
- Coseri, S.; Biliuta, G.; Simionescu, B.C.; Stana-Kleinschek, K.; Ribitsch, V.; Harabagiu, V. Oxidized Cellulose—Survey of the Most Recent Achievements. Carbohydr. Polym. 2013, 93, 207–215.
- Kim, U.-J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate Oxidation of Crystalline Cellulose. Biomacromolecules 2000, 1, 488–492.
- Eyholzer, C.; Bordeanu, N.; Lopez-Suevos, F.; Rentsch, D.; Zimmermann, T.; Oksman, K. Preparation and Characterization of Water-Redispersible Nanofibrillated Cellulose in Powder Form. Cellulose 2010, 17, 19–30.
- Kumar, A.; Durand, H.; Zeno, E.; Balsollier, C.; Watbled, B.; Sillard, C.; Fort, S.; Baussanne, I.; Belgacem, N.; Lee, D.; et al. The Surface Chemistry of a Nanocellulose Drug Carrier Unravelled by MAS-DNP. Chem. Sci. 2020, 11, 3868–3877.
- Sannigrahi, P.; Miller, S.J.; Ragauskas, A.J. Effects of Organosolv Pretreatment and Enzymatic Hydrolysis on Cellulose Structure and Crystallinity in Loblolly Pine. Carbohydr. Res. 2010, 345, 965–970.
- Stefanovic, B.; Rosenau, T.; Potthast, A. Effect of Sonochemical Treatments on the Integrity and Oxidation State of Cellulose. Carbohydr. Polym. 2013, 92, 921–927.
- Zhao, H.; Kwak, J.H.; Wang, Y.; Franz, J.A.; White, J.M.; Holladay, J.E. Effects of Crystallinity on Dilute Acid Hydrolysis of Cellulose by Cellulose Ball-Milling Study. Energy Fuels 2006, 20, 807–811.
- Sirvio, J.; Hyvakko, U.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Periodate Oxidation of Cellulose at Elevated Temperatures Using Metal Salts as Cellulose Activators. Carbohydr. Polym. 2011, 83, 1293–1297.
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10.
- Park, S.; Johnson, D.K.; Ishizawa, C.I.; Parilla, P.A.; Davis, M.F. Measuring the Crystallinity Index of Cellulose by Solid State 13C Nuclear Magnetic Resonance. Cellulose 2009, 16, 641–647.
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-Oxidized Cellulose Nanofibers. Nanoscale 2011, 3, 71–85.
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88.
- Dufresne, A. Nanocellulose Processing Properties and Potential Applications. Curr. For. Rep. 2019, 5, 76–89.
- Capadona, J.R.; Van Den Berg, O.; Capadona, L.A.; Schroeter, M.; Rowan, S.J.; Tyler, D.J.; Weder, C. A Versatile Approach for the Processing of Polymer Nanocomposites with Self-Assembled Nanofibre Templates. Nat. Nanotechnol. 2007, 2, 765–769.
- Rezayat, M.; Blundell, R.K.; Camp, J.E.; Walsh, D.A.; Thielemans, W. Green One-Step Synthesis of Catalytically Active Palladium Nanoparticles Supported on Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2014, 2, 1241–1250.
- Idström, A.; Brelid, H.; Nydén, M.; Nordstierna, L. CP/MAS 13C NMR Study of Pulp Hornification Using Nanocrystalline Cellulose as a Model System. Carbohydr. Polym. 2013, 92, 881–884.
- Lemke, C.H.; Dong, R.Y.; Michal, C.A.; Hamad, W.Y. New Insights into Nano-Crystalline Cellulose Structure and Morphology Based on Solid-State NMR. Cellulose 2012, 19, 1619–1629.
- Liu, J.; Plog, A.; Groszewicz, P.; Zhao, L.; Xu, Y.; Breitzke, H.; Stark, A.; Hoffmann, R.; Gutmann, T.; Zhang, K.; et al. Design of a Heterogeneous Catalyst Based on Cellulose Nanocrystals for Cyclopropanation: Synthesis and Solid-State NMR Characterization. Chem. Eur. J. 2015, 21, 12414–12420.
- Gutmann, T.; Liu, J.; Rothermel, N.; Xu, Y.; Jaumann, E.; Werner, M.; Breitzke, H.; Sigurdsson, S.T.; Buntkowsky, G. Natural Abundance 15N NMR by Dynamic Nuclear Polarization: Fast Analysis of Binding Sites of a Novel Amine-Carboxyl-Linked Immobilized Dirhodium Catalyst. Chem. Eur. J. 2015, 21, 3798–3805.




