
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ruben De la Torre | + 2241 word(s) | 2241 | 2020-09-23 10:28:20 | | | |
| 2 | Peter Tang | -3 word(s) | 2238 | 2020-09-25 05:31:10 | | | | |
| 3 | Peter Tang | Meta information modification | 2238 | 2020-10-26 02:46:17 | | |
Video Upload Options
Spasticity is a motor disorder that causes stiffness or tightness of the muscles and can interfere with normal movement, speech, and gait. Traditionally, the spasticity assessment is carried out by clinicians using standardized procedures for objective evaluation. However, these procedures are manually performed and, thereby, they could be influenced by the clinician’s subjectivity or expertise. The automation of such traditional methods for spasticity evaluation is an interesting and emerging field in neurorehabilitation. One of the most promising approaches is the use of robot-aided systems.
1. Introduction
Over the past 40 years, the research in the upper motor neuron syndrome has focused on spasticity [1]. Lance defined spasticity as a motor disorder characterized by an increase in tonic stretch reflexes with exaggerated tendon jerks, resulting from hyper-excitability of the stretch reflex [2]. Most of the scenarios where we commonly find this type of syndrome are after a stroke, a spinal cord injury, or another neurological disorder affecting the central nervous system (CNS). Consequently, the phenomenon of spasticity is complex due to the heterogeneity of symptoms and the nature of motor control. The management of spasticity primarily involves two perspectives: ameliorating and assessing the degree of spasticity.On one side, pharmacological and non-pharmacological approach therapies haven been employed as a treatment for reducing the spasticity effects [3]. Regarding pharmacological treatment, Baclofen is considered one of the first-line countermeasures, but Bont-A Toxin is the most common medication used to mitigate the effects of spasticity [4]. An injection reduces muscle tone up to 3 months and improves the upper limb capacities [5]. Non-pharmacological treatments mainly involve physical interventions aiming to minimize changes in the viscoelastic properties of connective tissue, muscles and joints, and changing patterns of spams [6]. Furthermore, recent studies have confirmed that the combined use of Bont-A with physical rehabilitation shortens the patient recovery time [7]; however, more clinical trials are needed to support the analysis considering previous studies and reviews [8][9]. One the other side, the understanding and diagnosis of spasticity have evolved exponentially in the last decades [10], and the assessment procedure has been attempted with a variety of methods. At the very beginning, some scales were created to evaluate the level of the disorder [11], such as the modified Ashworth scale (MAS), the Tardieu scale, the Spam severity scale, among others [10]. Currently, these scales are still the golden standard in clinical practice despite novel approaches having been proposed [12]. However, the existing scales are based on the perception of the clinician that evaluates the patient's spasticity through their perception, experience, and training over the years.This approach might not be appropriate because it establishes a subjective magnitude based on human impression. Not all clinicians have the same background and finally, the evaluation could depend on multiple imperceptible details [13]. Furthermore, this interpretation of the patient's spasticity could extend or reduce the rehabilitation process and modify the movements or the specified therapy used in the sessions [14]. Consequently, an objective measurement of spasticity is required, and for that purpose, the development of robot-based systems can help clinicians to objectify the assessment of different components of the syndrome.
2. Spasticity Management: Assessment and Treatments
A variety of sensorimotor and cognitive limitations can appear following an upper motor neuron (UMN) lesion. In the case of sensorimotor problems, they can be sorted in ‘positive' and ‘negative' features [15][16]. The positive features involve abnormal reflex responses, spasticity, spasms, clonus, and dis-synergic movement patterns. The negative features include muscle weakness, loss of dexterity, and fatigue. The combination of these positive and negative features leads to the loss of functionality and, consequently, the UMN syndrome must be understood as a complex picture where spasticity is only one component [17]. However, a particular focus on spasticity is considered under the premise that spasticity affects functional recovery and results in secondary complications like contractures, weakness, and pain.In this way, spasticity can be considered globally as a disorder or disruption on voluntary control of muscles and stretch reflexes caused by damage in the central nervous system. The specific pathophysiology of spasticity remains unclear, but several theories have been suggested to explain the causes of this phenomenon [18][19]. On the one hand, spasticity can appear as a result of an imbalance of neurotransmitters involved with the alpha motor neurons after damage to the nervous system and related muscles. This imbalance affects the inhibitory and excitatory signals sent to the muscles, causing them to lock in place.On the other hand, an alternative theory points to the formation of lesions in the upper motor neurons. Once again, the hypothesis is that the flow of muscle contraction signals can be impaired and produce spasticity. The effects on muscles and joints depend on the type of neurological damage. A further description of the causes of spasticity is out the scope of this paper; however, some published papers on the specific topic may help to understand the pathophysiology and underlying mechanism of spasticity [16][17][20].On account of the above, the formal definition of spasticity has been redefined over the years [10][21]. Originally, it was associated with “a soft yielding resistance that appeared only towards the end of a passive stretch and increased amplitude stretch reflex” [22]. In 1980, Lance suggested defining spasticity as “a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (muscle tone) and increased tendon jerks resulting from disinhibition of the stretch reflex, as one component of an upper motor neuron lesion” [2]. Later, other characteristics of upper motor neuron syndrome were added to this definition [10]. More recently, according to the Spasticity Study Group “SPASM” (Support Programme for Assembly of a database for Spasticity Measurement), a more practical definition could be “a sensorimotor control disorder that emerges as a result of upper motor neuron syndrome and in the form of muscles' involuntary intermittent or permanent activation” [23]. The continuous updates in spasticity definition and the poorly understood of the underlying mechanism highlight the fact that the impact of spasticity is extremely variable, making its management difficult. Hence, the following section presents a brief overview of principal aspects that involve spasticity management. This includes the most commonly used scales to quantify the degree of spasticity and the existing treatments to reduce its aftermaths.
3. Current Status of Robot-Aided Spasticity Assessment Systems
The results of this literature review are quite revealing in several ways. First, we might have identified a growing tendency in the use of techniques for data processing. Initially, researchers did not include any data treatment, but from 2009, nearly all the new investigations are incorporating statistical software or predictive algorithms [24]. This result may be explained by the fact that we are accumulating a large amount of data, and during the last ten years, algorithms were created to manage this considerable volume and to find patterns in the big data [25]. Another important finding was that most of the developed devices matched their outcomes with the MAS scale. A possible explanation for this might be that the MAS is the most used scale among clinicians. The scale behaves better for upper limbs than for lower limbs, but even so, it is widely applied for measuring spasticity in the lower limb [26]. One interesting finding is that similar to data processing, therapist and patient interfaces have been evolving. Originally, no element between the patient and the device was present. Only a small computer with minor possibilities of customization was included for the therapist in some cases. Luckily, this relationship has been improved, new adapting tools for each patient have been added and new technologies as virtual reality (VR) have demonstrated their validity [27]. The device is not alone and additional inputs have been captured from external sensors [28]. Despite these promising advantages, further work is required to enhance the connection between machine, therapist, and patient. Closer inspection of the table could show a tendency in the rehabilitation utility. The devices do not only measure but also rehabilitate. This evidence is important because the patient will interact with only one device and the therapist will only handle one machine [29]. Nevertheless, the steady decline of arm modeling can thus be suggested. None of the last 14 studies focused their efforts on improving the actual arm model. This result is somewhat counterintuitive because a better understanding of arm behavior will guide to better research [30]. Additionally, the size of the patient samples in the studies is often small. This finding could be explained because of the strong regulation in the personal privacy data and more specifically in the clinical data.In general, it seems that a high number of robots have been designed in the last 15 years for supporting the clinician diagnosis, and the tendency seems to continue the following years with the Healthcare 4.0 concept, which extends the basis of Industry 4.0 in a scenario where patients and healthcare professionals are strictly correlated with the organization, the methodology and the technology [31]. Besides, real-time data will upload from the robot to the patient electronic file, and connectivity will enlarge the user experience, allowing the patients to follow their clinical status at any time from multiple devices. Thus, the therapist could conduct the complete process from a different place than the patient and the robot, adjusting the following sessions with the data acquired. A more precise evaluation will guide to better therapist planning. In order to reach this ideal panorama of smart rehabilitation, some concerns must be addressed.
4. Prospects for Improvement in Robot-Aided Upper Limb Spasticity Assessment
Rehabilitation technology and automation of manual processes have become an essential part of rehabilitation development. In this way, the traditional rehabilitation cycle is being transformed into a more autonomous process, denoted as the “automated rehabilitation cycle” [32]. Here, the clinicians are supported by several automated systems in daily activities. This automated rehab cycle is composed of automated assessment systems (AAS), decision support systems (DSS), and robotic rehabilitation systems (RRS). In this new holistic paradigm of rehabilitation, the AAS plays an essential role since they are used at the beginning of treatments to measure the level of functional impairment, and at the end of treatment to determine the effectiveness of therapy. In this context, the robot-aided systems for the assessment of upper limb spasticity fit into the AAS category.Intending to obtain an objective evaluation, Figure 1 illustrates the principal components that intervene in the robot-aided assessment process. The first component consists of capturing the relevant data from sensors from the robot or patients. The second component is related to the limb mobilization using the robotic device. Another relevant component is the modeling of arm behavior in order to quantify the degree of impairment. This reference model is created from the robot input, taking into consideration cut-off values from a healthy arm. The last component is related to provide the spasticity descriptor, which ideally might be reliable, robust, and with high resolution.
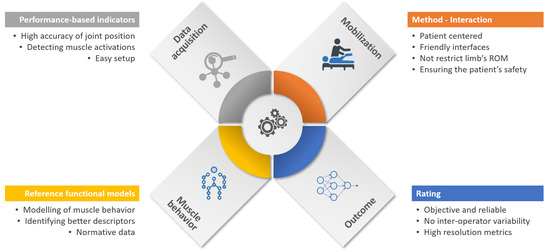
Figure 1. Essential components for robot-aided assessment of upper limb (UL) spasticity. Although it is clear that stages which participate in the process of evaluation, from data acquisition to outcome generation, new developments must consider some challenges and technical requirements in order to increase the level of automation and better use of gathered data. On account of the above, we encompass what we think future improvements are into three big fields: security, results' analysis, and a standard evaluation methodology.
5. Conclusions
Rehabilitation robotics comprises one of the fields that has grown steadily in recent decades. However, the main focus of research has been the development of systems to assist or improve intervention stages. Thus, a minor development of automatic assessment systems is identified, especially for UL spasticity. In this paper, a total of 28 studies focused on the automatic assessment of UL spasticity using a robotic device was reviewed. From the comprehensive analysis of the above studies, various limitations and challenges in the development of optimal assessment systems for UL spasticity have been identified.Firstly, a significant issue to continue improving is the development and standardization of effective strategies to guarantee the patient's safety during robot-aided limb mobilization. In this way, the use of collaborative robots that are considered intrinsically safe systems could be a feasible alternative to end-point devices. In the case of exoskeleton-based strategies, the integration of recent advances in soft robotic technology can enhance the comfort and safeguarding of patients by default.Secondly, the evaluation of the degree of spasticity throughout the entire range of joint motion is needed. In some cases, the morphology of devices or the administration setup can reduce the complete range of joint motion. Therefore, it is essential that the characteristics of the robotic device would not restrict the whole range of motion of the target joint.It is necessary that the collaboration between researchers and medical practitioners in order to build appropriate biomechanical models of reference (dynamic and kinematic) to better identify the gap between physical movement and theoretical patterns of motion. More comprehensive reference models of spastic muscle behavior would allow understanding of the extent of the functional limitations derived from spasticity.In this sense, it is also important to stress better usage of gathered data from robotic and automatic systems in order to increase the resolution of the spastic descriptors. Currently, five-point ordinal scales for scoring the degree of spasticity are still the golden standard. However, the analysis of the richer performance-based data measured by robotic systems can lead to more descriptive spasticity outcomes, and consequently, to optimally tailored protocols of rehabilitation.For concluding, it is our opinion that the benefits offered by robot-aided assessment systems can complement the holistic rehabilitation cycle and that these kinds of systems will become a complementary tool in daily clinical practice.
References
- Biering-Sørensen, F.; Nielsen, J.B.; Klinge, K. Spasticity-assessment: A review. Spinal Cord 2006, 44, 708–722.
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenbeg Lecture. Neurology 1980, 30, 1303.
- Kuo, C.L.; Hu, G.C. Post-stroke Spasticity: A Review of Epidemiology, Pathophysiology, and Treatments. Int. J. Gerontol. 2018, 12, 280–284.
- Rekand, T. Clinical assessment and management of spasticity: A review: Clinical assessment and management of spasticity. Acta Neurol. Scand. 2010, 122, 62–66.
- Kwakkel, G.; Meskers, C.G.M. Botulinum toxin A for upper limb spasticity. Lancet Neurol. 2015, 14, 969–971.
- Thibaut, A.; Chatelle, C.; Ziegler, E.; Bruno, M.A.; Laureys, S.; Gosseries, O. Spasticity after stroke: Physiology, assessment and treatment. Brain Inj. 2013, 27, 1093–1105.
- Kinnear, B.Z.; Lannin, N.A.; Cusick, A.; Harvey, L.A.; Rawicki, B. Rehabilitation Therapies After Botulinum Toxin-A Injection to Manage Limb Spasticity: A Systematic Review. Phys. Ther. 2014, 94, 1569–1581.
- Hesse, S.; Reiter, F.; Konrad, M.; Jahnke, M.T. Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: A randomized, double-blind, placebo-controlled trial. Clin. Rehabil. 1998, 12, 381–388.
- Hoare, B.J.; Imms, C. Upper-Limb Injections of Botulinum Toxin-A in Children With Cerebral Palsy: A Critical Review of the Literature and Clinical Implications for Occupational Therapists. Am. J. Occup. Ther. 2004, 58, 389–397.
- Petek Balci, B. Spasticty measurement. Arch. Neuropsychiatry 2018, 55, s49–s53.
- Sherwood, A.; McKay, W.B. Spasticity and Upper Motor Neuron Dysfunction. In Wiley Encyclopedia of Biomedical Engineering; Akay, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; p. ebs1109.
- Wang, H.; Huang, P.; Li, X.; Samuel, O.W.; Xiang, Y.; Li, G. Spasticity Assessment Based on the Maximum Isometrics Voluntary Contraction of Upper Limb Muscles in Post-stroke Hemiplegia. Front. Neurol. 2019, 10, 465.
- Johnson, G.R. Outcome measures of spasticity. Eur. J. Neurol. 2002, 9, 10–16.
- Ashworth, N.; Satkunam, L.; Deforge, D. Treatment for spasticity in amyotrophic lateral sclerosis/motor neuron disease. In The Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2004; p. CD004156.pub2.
- Walton, K. Management of Patients With Spasticity-A Practical Approach. Pract. Neurol. 2003, 3, 342–353.
- Li, S.; Francisco, G.E. New insights into the pathophysiology of post-stroke spasticity. Front. Hum. Neurosci. 2015, 9, 192.
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Currà, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of spasticity: Implications for neurorehabilitation. Biomed Res. Int. 2014, 2014.
- Priori, A.; Cogiamanian, F.; Mrakic-Sposta, S. Pathophysiology of spasticity. Neurol. Sci. 2006, 27, s307–s309.
- Ward, A.B. A literature review of the pathophysiology and onset of post-stroke spasticity. Eur. J. Neurol. 2012, 19, 21–27.
- Mukherjee, A.; Chakravarty, A. Spasticity mechanisms–for the clinician. Front. Neurol. 2010, 1, 149.
- Malhotra, S.; Pandyan, A.; Day, C.; Jones, P.; Hermens, H. Spasticity, an impairment that is poorly defined and poorly measured. Clin. Rehabil. 2009, 23, 651–658.
- Denny-Brown, D. The Cerebral Control of Movement; Liverpool University Press: Liverpool, UK, 1966.
- Burridge, J.; Wood, D.; Hermens, H.J.; Voerman, G.; Johnson, G.; Wijck, F.V.; Platz, T.; Gregoric, M.; Hitchcock, R.; Pandyan, A. Theoretical and methodological considerations in the measurement of spasticity. Disabil. Rehabil. 2005, 27, 69–80.
- Htoon, Z.L.; Sidek, S.N.; Fatai, S.; Rashid, M.M. Estimation of Upper Limb Impedance Parameters Using Recursive Least Square Estimator. In Proceedings of the 2016 International Conference on Computer and Communication Engineering (ICCCE), Kuala Lumpur, Malaysia, 26–27 July 2016; pp. 144–148.
- Sidiropoulos, A.; Karayiannidis, Y.; Doulgeri, Z. Human-robot collaborative object transfer using human motion prediction based on dynamic movement primitives. In Proceedings of the 2019 18th European Control Conference (ECC), Naples, Italy, 25–28 June 2019; pp. 2583–2588.
- Zhou, Z.; Sun, Y.; Wang, N.; Gao, F.; Wei, K.; Wang, Q. Robot-Assisted Rehabilitation of Ankle Plantar Flexors Spasticity: A 3-Month Study with Proprioceptive Neuromuscular Facilitation. Front. Neurorobot. 2016, 10.
- Oña, E.D.; Jardón, A.; Cuesta-Gómez, A.; Sánchez-Herrera-Baeza, P.; Cano-de-la Cuerda, R.; Balaguer, C. Validity of a Fully-Immersive VR-Based Version of the Box and Blocks Test for Upper Limb Function Assessment in Parkinson’s Disease. Sensors 2020, 20, 2773.
- Scano, A.; Molteni, F.; Molinari Tosatti, L. Low-Cost Tracking Systems Allow Fine Biomechanical Evaluation of Upper-Limb Daily-Life Gestures in Healthy People and Post-Stroke Patients. Sensors 2019, 19, 1224.
- Zhang, L.Q.; Chung, S.; Lin, A.; van Rey, E.; Bai, Z.; Grant, T.; Roth, E. A portable intelligent stretching device for treating spasticity and contracture with outcome evaluation. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Engineering in Medicine and Biology, Houston, TX, USA, 23–26 October 2002; Volume 3, pp. 2453–2454.
- Ang, W.S.; Geyer, H.; Chen, I.M.; Ang, W.T. Objective Assessment of Spasticity With a Method Based on a Human Upper Limb Model. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1414–1423.
- Jayaraman, P.P.; Forkan, A.R.M.; Morshed, A.; Haghighi, P.D.; Kang, Y.B. Healthcare 4.0: A review of frontiers in digital health. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2020, 10, e1350.
- Oña, E.D.; Cano-de-la Cuerda, R.; Sánchez-Herrera, P.; Balaguer, C.; Jardón, A. A review of robotics in neurorehabilitation: Towards an automated process for upper limb. J. Healthc. Eng. 2018, 2018, 9758939.




