
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kari Erik Murros | -- | 1554 | 2022-04-12 20:49:10 | | | |
| 2 | Dean Liu | Meta information modification | 1554 | 2022-04-13 05:54:25 | | |
Video Upload Options
Several bacterial species can generate hydrogen sulfide (H2S). Study evidence favors the view that the microbiome of the gut harbors increased amounts of H2S producing bacteria in Parkinson’s disease. Additionally, H2S can easily penetrate cell membranes and enter the cell interior. In the cells, excessive amounts of H2S can potentially release cytochrome c protein from the mitochondria, increase the iron content of the cytosolic iron pool, and increase the amount of reactive oxygen species. These events can lead to the formation of alpha-synuclein oligomers and fibrils in cells containing alpha-synuclein protein.
1. Introduction
2. H2S Releases Cytochrome c from the Mitochondria - Start for Alpha-Synuclein Aggregation
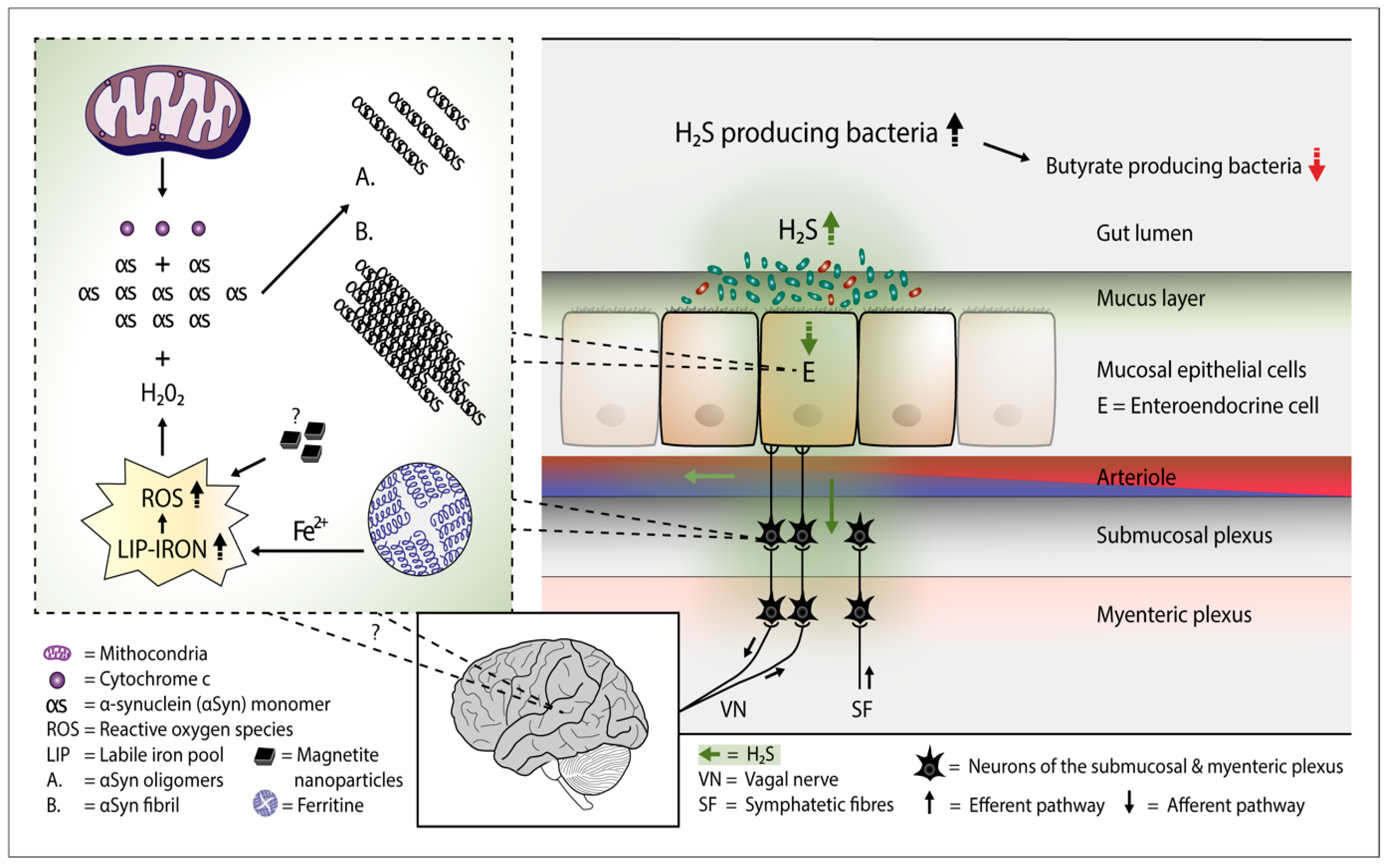
Figure 1. Plausible pathophysiological mechanism of Parkinson's disease. Overgrowth of H2S producing gut bacteria raises H2S concentrations in the gut cells and blood. In the gut cells, excessively increased H2S releases cytochrome c (Cyt c) from the mitochondria and increases cytosolic ferrous iron levels. Consequently, the amount of reactive oxygen species (ROS) increases. The presence of magnetite nanoparticles originating from the Desulfovibrio species can further increase the emergence of ROS. The co-occurrence of alpha-synuclein (aSyn), Cyt c, and ROS (especially hydrogen peroxide) leads to aSyn aggregation. Emerged aSyn aggregates (oligomers and fibrils) may spread in a prion-like manner to the lower brain stem via the vagal nerve. In the blood H2S combines with hemoglobin. Part of H2S may remain in a free form and possibly induce aSyn aggregation even in the brain neurons.
3. H2S Producing Small Intestinal Bacteria and PD
4. Viral Infections, PD, and H2S Producing Gut Bacteria
5. Bacterially Produced H2S and Risk Factors for PD
References
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896.
- Linden, D.R.; Sha, L.; Mazzone, A.; Stoltz, G.J.; Bernard, C.E.; Furne, J.K.; Levitt, M.D.; Farrugia, G.; Szurszewski, J.H. Production of the gaseous signal molecule hydrogen sulfide in mouse tissue. J. Neurochem. 2008, 106, 1577–1585.
- Furne, J.; Saeed, A.; Levitt, M.D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R479–R1485.
- Karunya, R.; Jayaprakash, K.S.; Gaikwad, R.; Sajeesh, P.; Ramshad, K.; Muraleedharan, K.M.; Dixit, M.; Thangaraj, P.R.; Sen, A.K. Rapid measurement of hydrogen sulfide in human blood plasma using a microfluid method. Sci. Rep. 2019, 9, 3258.
- Buret, A.G.; Allain, T.; Motta, J.-P.; Wallace, J.L. Effects of hydrogen sulfide on the microbiome: From toxicity to therapy. Antioxid. Redox Sign. 2022, 36, 211–219.
- Haouzi, P.; Sonobe, T.; Judenherc-Haouzi, A. Hydrogen sulfide intoxication induced brain injury and methylene blue. Neurobiol. Dis. 2020, 133, 104474.
- Klingerman, C.M.; Trushin, N.; Prokopczyk, B.; Haouzi, P. H2S concentration in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R630–R638.
- Bianco, C.L.; Savitsky, A.; Feelisch, M.; Cortese-Krott, M.M. Investigations on the role of hemoglobin in sulfide metabolism by intact human red blood cells. Biochem. Pharmacol. 2018, 149, 163–174.
- Jensen, B.; Fago, A. Reactions of ferric hemoglobin and myoglobin with hydrogen sulfide under physiologic conditions. J. Inorg. Biochem. 2018, 182, 133–140.
- Zaorska, E.; Tomasova, L.; Loszelewski, D.; Ostaszewski, R.; Ufnal, M. Hydrogen sulfide in pharmacotherapy, beyond the hydrogen sulfide-donors. Biomolecules 2020, 10, 323.
- Li, X.; Feng, X.; Jiang, Z.; Jiang, Z. Association of small intestinal bacterial overgrowth with Parkinson’s disease: A systematic review and meta-analysis. Gut Pathog. 2021, 13, 25.
- Leite, G.G.S.; Weitsman, S.; Parodi, G.; Celly, S.; Sedighi, R.; Sanchez, M.; Morales, W.; Villanueva-Millan, M.J.; Barlow, G.M.; Mathur, R.; et al. Mapping the segmental microbiomes in the human small bowel in comparison with stool: A REIMAGINE study. Digest. Dis. Sci. 2020, 65, 2595–2604.
- Leite, G.; Morales, W.; Weitsman, S.; Celly, S.; Parodi, G.; Mathur, R.; Barlow, G.M.; Sedighi, R.; Millan, M.J.V.; Rezaie, A.; et al. The duodenal microbiome is altered in small intestinal bacterial overgrowth. PloS ONE 2020, 15, e02344906.
- Chen, C.-K.; Wu, Y.-T.; Chang, Y.-C. Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: A population-based retrospective matched-cohort study. PeerJ 2017, 10, e3647.
- Shi, M.; Wei, Y.; Hu, W.; Nie, Y.; Wu, X.; Lu, R. The subgingival micrbiome of periodontal pockets with different probing depths in chronic and aggressive periodontitis: A pilot study. Front. Cell. Infect. Microbiol. 2018, 8, 124.
- Kushkevych, I.; Coufalová, M.; Vitezová, M.; Ritmann, S.K.-M.R. Sulfate-reducing bacteria of the oral cavity and their relation with periodontitis-recent advances. J. Clin. Med. 2020, 9, 2347.
- Pereira, P.A.B.; Aho, V.T.E.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Oral and nasal microbiota in Parkinson’s disease. Park. Rel. Dis. 2017, 38, 61–67.
- Harris, M.A.; Tsui, J.K.; Marion, S.A.; Shen, H.; Teschke, K. Asoociation of Parkinson’s disease with infections and occupational exposure to possible vectors. Mov. Disord 2012, 27, 1111–1117.
- Cocoros, N.M.; Svensson, E.; Szépligeti, S.K.; Vestergaard, S.V.; Szentkúti, P.; Thomsen, R.W.; Borghammer, P.; Sorensen, H.T.; Henderson, V.W. Long-term risk of Parkinson’s disease following influenza and other infections. JAMA Neurol. 2021, 78, 1461–1470.
- Yildiz, S.; Mazel-Sanchez, B.; Kandasamy, M.; Manicassamy, B.; Schmolke, M. Influenza A virus infection impacts systemic microbiota dynamics and causes quantitative enteric dysbiosis. Microbiome 2018, 6, 9.
- Wang, J.; Li, F.; Wei, H.; Lian, Z.-X.; Sun, R.; Tian, Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 2014, 211, 2397–2410.
- Deriu, E.; Boxx, G.M.; He, X.; Pan, C.; Benavidez, S.D.; Cen, L.; Rozengurt, N.; Shi, W.; Cheng, G. Influenza virus affects interstinal microbiota and secondary Salmonella infection in the gut through type 1 interferons. PloS Pathog. 2016, 15, e1005572.
- Sencio, V.; Barthelemy, A.; Tavares, L.P.; Machado, M.G.; Soulard, D.; Cuinat, C.; Queiroz-Junior, C.M.; Noordine, M.-L.; Salomé-Desnoulez, S.; Deryuter, L.; et al. Gut dysbiosis during influenza contributes to pulmonary pneumococcal superinfection through altered short-chain fatty acid production. Cell Rep. 2020, 30, 2934–2947.
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the gut microbiota in patients with Coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 2020, 71, 2669–2678.
- Wu, W.Y.-Y.; Kang, K.-H.; Chen, S.L.-S.; Chiu, S.Y.-H.; Yen, A.M.-F.; Fann, J.C.-T.; Su, C.-W.; Liu, H.-C.; Lee, C.-Z.; Fu, W.-M.; et al. Hepatitis C virus infection: A risk for Parkinson’s disease. J. Viral Hepat. 2015, 22, 784–791.
- El-Mowafy, M.; Elgaml, A.; El-Mesery, M.; Sultan, S.; Ahmed, T.A.E.; Gomaa, A.I.; Aly, M.; Mottawea, W. Changes of gut-microbiota-liver axis in hepatitis C virus infections. Biology 2021, 10, 55.
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F.; Qin, B. Structural changes of gut microbiota in Parkinson’s disease and its correlations with clinical features. Sci. China Life Sci. 2017, 60, 1223–1233.
- Scheperjans, F.; Aho, V.; Pereira, P.A.B.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinicla phenotype. Mov. Disord. 2015, 30, 350–358.
- Unger, M.M.; Spiegel, J.; Dillmann, K.-U.; Grundmann, D.; Philippelt, H.; Bürmann, J.; Fassbender, K.; Scwiertz, A.; Schäfer, K.-H. Short chain fatty acids and gut microbiota differ between patients witn Parkinson’n disease and age-matched controls. Park. Rel. Dis. 2016, 32, 66–72.
- Vascellari, S.; Melis, M.; Palmas, V.; Serra, A.; Perra, D.; Santoru, M.L.; Oppo, V.; Uva, P.; Atzori, L.; Morelli, M.; et al. Clinical phenotypes of Parkinson’s disease associate with distinct gut microbiota and metabolome enterotypes. Biomolecules 2021, 11, 144.
- GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2018, 17, 939–953.
- Xu, C.; Zhu, H.; Qiu, P. Aging progression of human microbiota. BMC Microbiol. 2019, 19, 236.
- Gillies, G.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex differences in Parkinson’s disease. Front. Neuroendocrin. 2014, 35, 370–384.
- Bourque, M.; Dluzen, D.E.; Di Paolo, T. Neuroprotective actions of sex steroids in Parkinson’s disease. Front Neuroendocrinol. 2009, 30, 142–157.
- Gatto, N.M.; Deapen, D.; Stoyanoff, S.; Pinder, R.; Bordelon, T.; Ritz, B. Lifetime exposure to estrogens and Parkinson’s disease in California teachers. Park. Rel. Dis. 2014, 20, 1149–1156.
- Bagetta, C.; Chiappetta, O.; Amantea, D.; Iannone, M.; Rotiroti, D.; Costa, A.; Nappi, G.; Corasaniti, M.T. Estradiol reduces cytochrome c translocation and minimizes hippocampal damage caused by transient global ischemia in rat. Neurosci. Lett. 2004, 368, 87–91.
- Morkuniene, R.; Arandarcikaite, O.; Borutaite, V. Estradiol prevents release of cytochrome c form mitochondria and inhibits ischemia-induced apoptosis in perfused heart. Exp. Gerontol. 2006, 41, 704–708.





