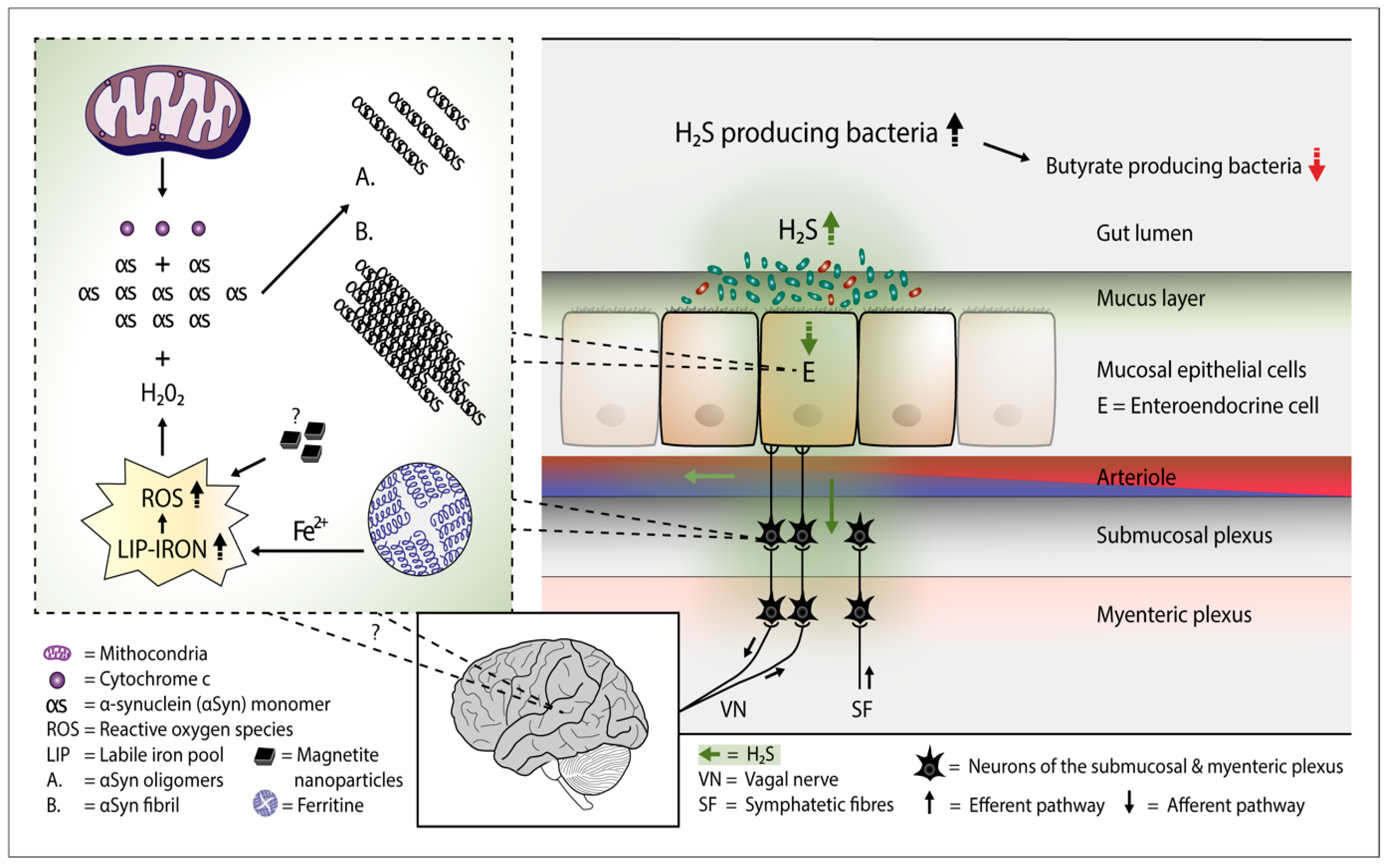
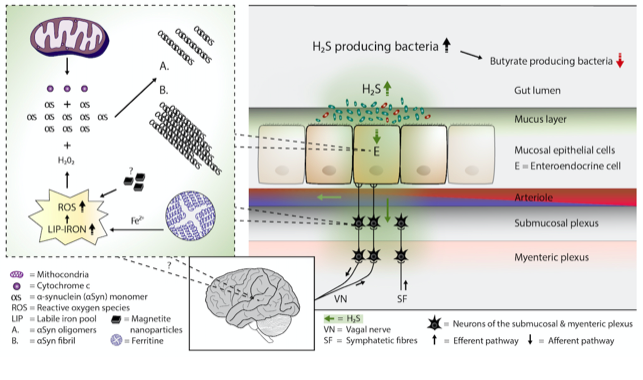
Several bacterial species can generate hydrogen sulfide (H2S). Study evidence favors the view that the microbiome of the gut harbors increased amounts of H2S producing bacteria in Parkinson’s disease. Additionally, H2S can easily penetrate cell membranes and enter the cell interior. In the cells, excessive amounts of H2S can potentially release cytochrome c protein from the mitochondria, increase the iron content of the cytosolic iron pool, and increase the amount of reactive oxygen species. These events can lead to the formation of alpha-synuclein oligomers and fibrils in cells containing alpha-synuclein protein.
1. Introduction
In humans, hydrogen sulfide (H2S) plays various roles in a myriad of physiologic processes relating to inflammatory, immune, endocrine, respiratory, vascular and neuromodulatory actions [1]. Furthermore, H2S is endogenously produced in the human cells by enzymes including cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), which both use L-cysteine as a substrate, and via 3-mercapto-sulfurtransferase (3-MST) pathway that uses 3-mercaptopyruvate as a substrate. Both the brain and colonic tissue generate and modulate H2S production via the activity of CSE and CBS [2]. In healthy humans, the plasma baseline levels of H2S have been reported to lie in the range of 34 μM–274 μM [3,4]. In the gut lumen, the sulfate-reducing bacteria (SRB) and the bacteria of the desulfhydrase enzyme are notable H2S producers. In addition, various bacteria which are homologs of the mammalian CBS, CSE, and 3-MST enzymes are capable of producing H2S. At low concentrations, bacterially produced H2S displays cytoprotective properties by maintaining gut mucus integrity but is toxic to the host at high concentrations [5]. When administered to the human body, H2S diffuses rapidly to blood and, after administration, only a small part of H2S remains in a soluble form [6]. In the blood, H2S combines easily with hemoglobin (Hb) and methemoglobin (metHb) [7,8]. MetHb and H2S form relatively stable metHb-H2S complexes which have a slow reduction rate. Thus, metHb keeps Hb in an oxygen-binding form and acts as a scavenger and regulator of sulfide in the blood [8,9]. In the cells, the main route of H2S elimination is mitochondria, by a chain of several oxidative enzymes [10]. In humans, hydrogen sulfide (H2S) plays various roles in a myriad of physiologic processes relating to inflammatory, immune, endocrine, respiratory, vascular and neuromodulatory actions [1]. Furthermore, H2S is endogenously produced in the human cells by enzymes including cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), which both use L-cysteine as a substrate, and via 3-mercapto-sulfurtransferase (3-MST) pathway that uses 3-mercaptopyruvate as a substrate. Both the brain and colonic tissue generate and modulate H2S production via the activity of CSE and CBS [2]. In healthy humans, the plasma baseline levels of H2S have been reported to lie in the range of 34 μM–274 μM [3][4]. In the gut lumen, the sulfate-reducing bacteria (SRB) and the bacteria of the desulfhydrase enzyme are notable H2S producers. In addition, various bacteria which are homologs of the mammalian CBS, CSE, and 3-MST enzymes are capable of producing H2S. At low concentrations, bacterially produced H2S displays cytoprotective properties by maintaining gut mucus integrity but is toxic to the host at high concentrations [5]. When administered to the human body, H2S diffuses rapidly to blood and, after administration, only a small part of H2S remains in a soluble form [6]. In the blood, H2S combines easily with hemoglobin (Hb) and methemoglobin (metHb) [7][8]. MetHb and H2S form relatively stable metHb-H2S complexes which have a slow reduction rate. Thus, metHb keeps Hb in an oxygen-binding form and acts as a scavenger and regulator of sulfide in the blood [8][9]. In the cells, the main route of H2S elimination is mitochondria, by a chain of several oxidative enzymes [10].
2. H2S Releases Cytochrome c from the Mitochondria - Start for Alpha-Synuclein Aggregation
Figure 1. Plausible pathophysiological mechanism of Parkinson's disease. Overgrowth of H2S producing gut bacteria raises H2S concentrations in the gut cells and blood. In the gut cells, excessively increased H2S releases cytochrome c (Cyt c) from the mitochondria and increases cytosolic ferrous iron levels. Consequently, the amount of reactive oxygen species (ROS) increases. The presence of magnetite nanoparticles originating from the Desulfovibrio species can further increase the emergence of ROS. The co-occurrence of alpha-synuclein (aSyn), Cyt c, and ROS (especially hydrogen peroxide) leads to aSyn aggregation. Emerged aSyn aggregates (oligomers and fibrils) may spread in a prion-like manner to the lower brain stem via the vagal nerve. In the blood H2S combines with hemoglobin. Part of H2S may remain in a free form and possibly induce aSyn aggregation even in the brain neurons.
3. H2S Producing Small Intestinal Bacteria and PD
Apart from the changes in the colonic microbiota composition, changes in the quantities of small intestinal bacteria likely relate to the pathogenesis of PD as it suggests the high prevalence of small intestinal bacterial overgrowth (SIBO) in the PD population. According to a large meta-analysis, based on lactulose-hydrogen breath test results, a strong association was reported to exist between SIBO and PD [81]. Although increased hydrogen production from the small intestinal microbiome seems to take place in several PD subjects, it is not yet known which bacterial genera or species in the small intestine explain this increase. Hydrogen-utilizing bacteria such as Desulfovibrio species and Bilophila wadsworthia likely benefit from these circumstances. At a general level, the small intestinal microbiome composition has been found to differ markedly from the fecal microbiome composition in humans [82]. A study performed on SIBO and non-SIBO subjects provides interesting information about the duodenal microbiome changes in the SIBO subjects at a general level [82]. In that study, over four-fold higher relative abundance of the class Gammaproteobacteria and three-fold higher relative abundance of the class Deltaproteobacteria was reported to be present in the duodenal aspirates of SIBO subjects when compared to non-SIBO subjects. Of further interest, the family Enterobacteriaceae represented 89 % of the total relative abundance of the Gammaproteobacteria in the duodenum of the SIBO subjects [83]. Notably, hydrogen sulfide-producing genera such as Escherichia, Klebsiella, and Proteus belong to the family Enterobacteriaceae. As to the family members of the class Deltaproteobacteria, especially concerning the family Desulfovibrionaceae, they are best known for their ability to produce H2S. Swallowed bacteria from the oral cavity, emerging, e.g., from the areas of periodontal infections, may possibly play a role in modulating the small intestinal microbiome composition. In a large-scale cohort study, periodontitis was shown to increase the risk of PD [84]. Subgingival deposits occurring with periodontal disease, including cases with aggressive periodontitis, have been reported to contain SRB (which mainly represent the class Deltaproteobacteria) and, among others, bacterial genera such as Porphyromonas and Prevotella [85,86]. Of interest, in a Finnish study on oral mucosal biofilm, significantly increased abundances of the Prevotella and Veillonella genera were observed in PD patients [87].
Apart from the changes in the colonic microbiota composition, changes in the quantities of small intestinal bacteria likely relate to the pathogenesis of PD as it suggests the high prevalence of small intestinal bacterial overgrowth (SIBO) in the PD population. According to a large meta-analysis, based on lactulose-hydrogen breath test results, a strong association was reported to exist between SIBO and PD [11]. Although increased hydrogen production from the small intestinal microbiome seems to take place in several PD subjects, it is not yet known which bacterial genera or species in the small intestine explain this increase. Hydrogen-utilizing bacteria such as Desulfovibrio species and Bilophila wadsworthia likely benefit from these circumstances. At a general level, the small intestinal microbiome composition has been found to differ markedly from the fecal microbiome composition in humans [12]. A study performed on SIBO and non-SIBO subjects provides interesting information about the duodenal microbiome changes in the SIBO subjects at a general level [12]. In that study, over four-fold higher relative abundance of the class Gammaproteobacteria and three-fold higher relative abundance of the class Deltaproteobacteria was reported to be present in the duodenal aspirates of SIBO subjects when compared to non-SIBO subjects. Of further interest, the family Enterobacteriaceae represented 89 % of the total relative abundance of the Gammaproteobacteria in the duodenum of the SIBO subjects [13]. Notably, hydrogen sulfide-producing genera such as Escherichia, Klebsiella, and Proteus belong to the family Enterobacteriaceae. As to the family members of the class Deltaproteobacteria, especially concerning the family Desulfovibrionaceae, they are best known for their ability to produce H2S. Swallowed bacteria from the oral cavity, emerging, e.g., from the areas of periodontal infections, may possibly play a role in modulating the small intestinal microbiome composition. In a large-scale cohort study, periodontitis was shown to increase the risk of PD [14]. Subgingival deposits occurring with periodontal disease, including cases with aggressive periodontitis, have been reported to contain SRB (which mainly represent the class Deltaproteobacteria) and, among others, bacterial genera such as Porphyromonas and Prevotella [15][16]. Of interest, in a Finnish study on oral mucosal biofilm, significantly increased abundances of the Prevotella and Veillonella genera were observed in PD patients [17].
4. Viral Infections, PD, and H2S Producing Gut Bacteria
Influenza infection evidently increases the risk of PD. In a population-based case–control study in Canada, a significant association was reported to exist between a history of severe influenza and PD [88]. Recently, a case–control study based on the data of over 60 000 individuals from the Danish National Patient Registry showed that a history of influenza was significantly associated with a later occurrence of PD [89]. In addition, the Spanish flu (influenza A subtype H1N1) has been considered to be a risk factor for a later PD occurrence. As to animal studies, it has been shown that influenza A virus (H5N1) can induce a disruption of the mucus layer integrity of the small intestine and cause enteric dysbiosis by increasing the relative amounts of Gammaproteobacteria and Bacilli [90]. In a mice study, the influenza A (PR8) virus infection was demonstrated to induce a significant increase in the intestinal Escherichia coli species [91]. As to another study, influenza A (PR8) infection caused a significant increase in the Enterobacteriaceae population in the stool samples of the wild-type mice [92]. In addition, influenza A virus H3N2 infection has been reported to increase the fecal content of the genus Escherichia in mice [93]. As to human studies, an interesting cross-sectional study on alterations in the gut microbiota of 30 patients with COVID-19 infection, 24 patients with influenza (H1N1), and 30 matched healthy controls showed that the relative abundance of the genus Escherichia-Shigella was significantly higher in the fecal samples of H1N1 patients than in controls [94].
Hepatitis C virus (HCV) infection has been reported to be a significant risk factor for Parkinson’s disease [95]. HCV infection can be classified into three types, which consist of the persistently normal serum alanine transferase type, chronic hepatitis, and liver cirrhosis. In all these stages, an enrichment of the family Enterobacteriaceae present in the gut microbiome has been a consistent finding [96]. Evidently, influenza and hepatitis C virus infections may be inductors for the development of PD by inducing gut dysbacteriosis, whereby an overgrowth of the H2S-producing members of the family Enterobacteriaceae, especially the genus Escherichia, play a crucial role. Clinical studies on fecal microbiome content in PD provide support for the view that the Enterobacteriaceae family is an important player in the pathogenesis of PD. Notably, this family has been reported to be significantly more abundant in the fecal samples of PD patients when compared to healthy controls [80,97,98]. Postural instability and gait difficulty have been reported to correlate with the relative abundance of the Enterobacteriaceae in the fecal samples of PD patients [97]. In addition, an increase in Enterobacteriaceae in the fecal samples has been reported to be associated with the non-tremor dominant subtype of PD [99].
Influenza infection evidently increases the risk of PD. In a population-based case–control study in Canada, a significant association was reported to exist between a history of severe influenza and PD [18]. Recently, a case–control study based on the data of over 60 000 individuals from the Danish National Patient Registry showed that a history of influenza was significantly associated with a later occurrence of PD [19]. In addition, the Spanish flu (influenza A subtype H1N1) has been considered to be a risk factor for a later PD occurrence. As to animal studies, it has been shown that influenza A virus (H5N1) can induce a disruption of the mucus layer integrity of the small intestine and cause enteric dysbiosis by increasing the relative amounts of Gammaproteobacteria and Bacilli [20]. In a mice study, the influenza A (PR8) virus infection was demonstrated to induce a significant increase in the intestinal Escherichia coli species [21]. As to another study, influenza A (PR8) infection caused a significant increase in the Enterobacteriaceae population in the stool samples of the wild-type mice [22]. In addition, influenza A virus H3N2 infection has been reported to increase the fecal content of the genus Escherichia in mice [23]. As to human studies, an interesting cross-sectional study on alterations in the gut microbiota of 30 patients with COVID-19 infection, 24 patients with influenza (H1N1), and 30 matched healthy controls showed that the relative abundance of the genus Escherichia-Shigella was significantly higher in the fecal samples of H1N1 patients than in controls [24].
Hepatitis C virus (HCV) infection has been reported to be a significant risk factor for Parkinson’s disease [25]. HCV infection can be classified into three types, which consist of the persistently normal serum alanine transferase type, chronic hepatitis, and liver cirrhosis. In all these stages, an enrichment of the family Enterobacteriaceae present in the gut microbiome has been a consistent finding [26]. Evidently, influenza and hepatitis C virus infections may be inductors for the development of PD by inducing gut dysbacteriosis, whereby an overgrowth of the H2S-producing members of the family Enterobacteriaceae, especially the genus Escherichia, play a crucial role. Clinical studies on fecal microbiome content in PD provide support for the view that the Enterobacteriaceae family is an important player in the pathogenesis of PD. Notably, this family has been reported to be significantly more abundant in the fecal samples of PD patients when compared to healthy controls [27][28][29]. Postural instability and gait difficulty have been reported to correlate with the relative abundance of the Enterobacteriaceae in the fecal samples of PD patients [28]. In addition, an increase in Enterobacteriaceae in the fecal samples has been reported to be associated with the non-tremor dominant subtype of PD [30].
5. Bacterially Produced H2S and Risk Factors for PD
Advancing age has been established as the largest risk factor for developing PD. Globally, the prevalence of PD begins rise steeply in the age range of 60–70 years and peaks in the age range of 80–90 years [100]. As to the colonic microbiome in advancing age, a community study on changes in the microbiome composition during the aging process showed that in older people, a sharp and continuous increase takes place in the relative abundance of the H2S-producing Desulfovibrio, Bilophila and Corynebacterium gut bacteria [101]. In case additional and substantial overgrowth of the H2S producing bacterial genera such as Escherichia or gDSV takes place, the risk for PD development will likely increase as a function of age.
In addition to aging, male gender is an established risk factor for PD. Furthemore, with regard to the incidence rates, male to female ratios have been reported to vary from 1.37 to 3.7 [102]. Estrogen, especially 17beta-estradiol which has been reported to display neuroprotective properties may provide an explanation for this difference [103,104]. It has been stated that long-term exposure to estrogen may be important in the PD risk reduction [104]. Experimental studies on ischemia models have shown that 17beta-estradiol prevents the release of Cyt c from mitochondria and by this action, estradiol displays cytoprotective properties [105,106]. Ultimately, given that H2S levels increase in the gut cells and perhaps even at the brain level, estadiol can probably counteract H2S actions by preventing the release of Cyt c from the mitochondrial membrane.
Advancing age has been established as the largest risk factor for developing PD. Globally, the prevalence of PD begins rise steeply in the age range of 60–70 years and peaks in the age range of 80–90 years [31]. As to the colonic microbiome in advancing age, a community study on changes in the microbiome composition during the aging process showed that in older people, a sharp and continuous increase takes place in the relative abundance of the H2S-producing Desulfovibrio, Bilophila and Corynebacterium gut bacteria [32]. In case additional and substantial overgrowth of the H2S producing bacterial genera such as Escherichia or gDSV takes place, the risk for PD development will likely increase as a function of age.
In addition to aging, male gender is an established risk factor for PD. Furthemore, with regard to the incidence rates, male to female ratios have been reported to vary from 1.37 to 3.7 [33]. Estrogen, especially 17beta-estradiol which has been reported to display neuroprotective properties may provide an explanation for this difference [34][35]. It has been stated that long-term exposure to estrogen may be important in the PD risk reduction [35]. Experimental studies on ischemia models have shown that 17beta-estradiol prevents the release of Cyt c from mitochondria and by this action, estradiol displays cytoprotective properties [36][37]. Ultimately, given that H2S levels increase in the gut cells and perhaps even at the brain level, estadiol can probably counteract H2S actions by preventing the release of Cyt c from the mitochondrial membrane.