Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Luigi Bennardo | -- | 3515 | 2022-04-12 16:32:39 | | | |
| 2 | Lindsay Dong | -1 word(s) | 3514 | 2022-04-13 08:12:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bennardo, L.; Dastoli, S.; Nisticò, S.; Morrone, P.; Patruno, C.; Leo, A.; Citraro, R.; Gallelli, L.; Russo, E.; De Sarro, G. Colchicine in Managing Skin Conditions. Encyclopedia. Available online: https://encyclopedia.pub/entry/21664 (accessed on 12 January 2026).
Bennardo L, Dastoli S, Nisticò S, Morrone P, Patruno C, Leo A, et al. Colchicine in Managing Skin Conditions. Encyclopedia. Available at: https://encyclopedia.pub/entry/21664. Accessed January 12, 2026.
Bennardo, Luigi, Stefano Dastoli, Steven Nisticò, Pietro Morrone, Cataldo Patruno, Antonio Leo, Rita Citraro, Luca Gallelli, Emilio Russo, Giovambattista De Sarro. "Colchicine in Managing Skin Conditions" Encyclopedia, https://encyclopedia.pub/entry/21664 (accessed January 12, 2026).
Bennardo, L., Dastoli, S., Nisticò, S., Morrone, P., Patruno, C., Leo, A., Citraro, R., Gallelli, L., Russo, E., & De Sarro, G. (2022, April 12). Colchicine in Managing Skin Conditions. In Encyclopedia. https://encyclopedia.pub/entry/21664
Bennardo, Luigi, et al. "Colchicine in Managing Skin Conditions." Encyclopedia. Web. 12 April, 2022.
Copy Citation
Colchicine is a natural alkaloid with anti-inflammatory properties used to treat various disorders, including some skin diseases. Colchicine could be, as a single therapy or in combination with other drugs, a possible treatment to manage several skin diseases.
colchicine
dermatology
pustular dermatoses
1. Introduction
Colchicine is a natural alkaloid derived from the plant Colchicum autumnale (Figure 1). Nowadays, it is used in various conditions, including gout, familial Mediterranean fever, and in different rheumatological conditions. It is administered orally, at a dosage varying from 0.5 to 2 mg per day. Its metabolism is primarily and quickly hepatic; after that, the drug is excreted through the bile and the kidneys [1]. Plasmatic concentrations and bioavailability may vary between individuals, so the profile of the response and adverse events (AEs) to colchicine may vary accordingly. The AEs are mainly gastrointestinal symptoms, such as vomiting, diarrhea, and nausea, and affect up to 10% of patients treated with the labelled dosage. Other AEs include muscular damage and anagen or telogen effluvium, according to the drug dosage [2][3][4]. Other AEs, such as cardiac arrhythmias, cardiac failure, kidney disease, and myelosuppression are related to the overdosage.
Colchicine accumulates at a higher dosage in leukocytes than in other blood cells, reducing their functional ability, especially in neutrophils, as well as reducing their migration and their ability to degranulate by inhibiting microtubule polymerization. This accumulation reaches its peak about 48 h after drug assumption. Colchicine also seems involved in inhibiting the cyclooxygenases COX-1 and COX-2. There is no clear limit that establishes the therapeutic, toxic, and lethal doses of colchicine in children or adults.
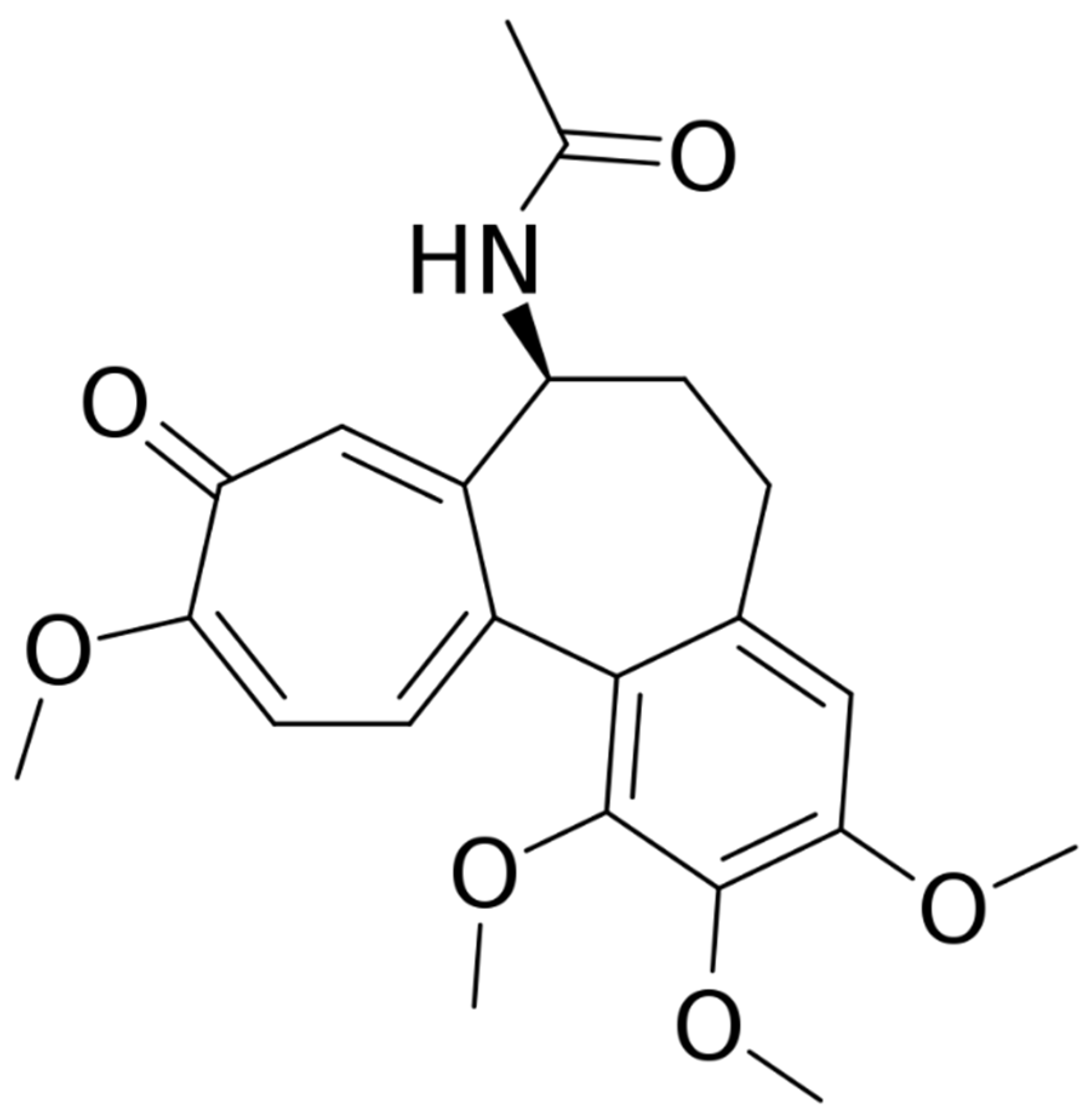
Figure 1. Structure of colchicine.
2. Colchicine in Managing Skin Conditions
2.1. Psoriasis
Psoriasis is characterized by erythematous-squamous plaques affecting mainly extensor surfaces but spreading to all body areas. Different clinical phenotypes of psoriasis have been reported, including palmoplantar, inverse, guttate, pustular, and others. It may be associated with comorbidities, such as arthritis [5][6][7]. The currently approved therapies include topical corticosteroids and vitamin D derivatives [8][9][10], as well as systemic therapies based on traditional immunosuppressive drugs, such as methotrexate and cyclosporine, and novel biologic drugs targeting tumor necrosis factor (TNF) alpha, anti-interleukin (IL) 17, and anti-IL 23 [11][12][13][14][15]. Topical or systemic colchicine was proposed in the late 1970s and early 1980s to manage this condition [16][17].
Kadbey et al. reported the improvement of plaque psoriasis with topical 1% colchicine in 12 subjects [17]. Wahba and colleagues treated 22 patients at a dosage of 0.02 mg per kg per day. Eight out of nine patients that reported mild psoriasis showed a complete or an almost complete clearing, and patients affected by arthritis reported a reduction of joint pain. Patients suffering from more severe forms of psoriasis reported minimal-to-no clearing. They administered colchicine to five patients previously treated with systemic drugs, such as methotrexate, and reached skin clearance. These subjects maintained the results for 8 months in approximately 80% of the cases [18]. A Japanese group described the case of a 32-year-old woman responding to a combination therapy of methotrexate and colchicine [19]. Zachariae and colleagues reported a complete remission of the disease in three-quarters of their patients [20].
2.2. Palmoplantar Pustolosis
Palmoplantar pustulosis is an uncommon, chronic, pustular condition characterized by the presence of sterile pustules on the palms and soles [21]. Acrodermatitis continua of Hallopeau may be considered a subvariant, affecting single fingers [22]. Colchicine has been traditionally used to manage this subvariant, with contrasting results. Takigawa and colleagues treated 32 patients by using a dosage that varied from 1 to 2 mg per day, decreasing the dose to 0.5/1 mg per day in responding patients. Thirteen patients showed the complete disappearance of pustules, while 14 patients showed a reduction in pustules. One patient did not benefit from the treatment, and four stopped the treatment due to its side effects, mainly nausea and diarrhea. Eight cases experienced a reactivation of the condition 3 months after the treatment discontinuation [23]. Twenty-two patients affected by palmoplantar pustulosis entered a double-blind, crossover trial, using oral colchicine at the dosage of 0.5 mg twice per day or a placebo. No significant improvement on the skin condition was recorded [24]. English and colleagues treated 10 patients with no significant difference compared to the placebo [25]. A Danish group performed a double-blind crossover trial on 27 patients treated with 0.5 mg of colchicine three times daily; 10 patients only experienced a reduction in pustule formation, whereas the redness, scaling, and subjective symptoms were unchanged, and a high incidence of gastrointestinal side effects was reported [26]. Wong and colleagues reported a case series stduy of three patients affected by severe palmoplantar pustulosis and their significant improvement of the condition after the treatment with colchicine [27].
2.3. Neutrophilic Eccrine Hidradenitis
Neutrophilic eccrine hidradenitis is a very uncommon neutrophilic condition that is histologically characterized by the necrosis of the eccrine glands, as well as a local neutrophilic infiltration. It is usually observed in patients affected by acute leukemia or other malignancies during chemotherapy. Belot and colleagues described an idiopathic case of a middle-aged female patient treated with colchicine, reporting clinical improvements after just one month [28].
2.4. Eccrine Hidradenitis
Eccrine hidradenitis, or idiopathic hidradenitis, is an uncommon condition affecting children and young adults characterized by tender, red lumps on the soles of the feet, and less often, on the palms. Histologically, a neutrophilic and lymphocytic infiltration is present at the base of the eccrine glands. Scheer and colleagues reported the efficacy of a 5-month cycle of 0.5 mg per day of colchicine in a 7-year-old boy, with no AEs. The condition disappeared after 3 days, but colchicine was continued as a preventive measure [29].
2.5. Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease affecting areas with a high density of apocrine glands and is characterized by subcutaneous nodules that may evolve into fistulas with purulent secretion [30][31][32]. Various treatments have been proposed to manage this condition, such as topical and systemic antibiotics, as well as retinoids [33], with no lasting results [34]. Recently, biologic drugs, such as anti-TNF alpha and anti-IL agents traditionally used to manage other chronic inflammatory diseases [35] have been proposed, with inconsistent results. HS is often associate with a high impairment in the quality of life of patients [36][37][38].
An open, prospective pilot study evaluated the efficacy of colchicine at 0.5 mg twice per day for up to 4 months in the management of HS. Colchicine treatment did not result in a clinically relevant improvement of disease severity. Some patients experienced nausea and diarrhea as AEs [39].
An open prospective study treated 20 patients (10 females and 10 males) with a combination of 100 mg of minocycline administered orally once per day and 0.5 mg of colchicine administered twice per day, for 6 months, followed by a maintenance regimen of 0.5 mg of colchicine twice a day for 3 months. The efficacy of the treatment was evaluated every 3 months, for 9 months, using the Physician’s Global Assessment scale, the Hurley scoring system, and the Dermatology Life Quality Index. All the patients showed an improvement in all the scores after 3 months of treatment and continued to improve over time, suggesting colchicine as a good agent in combination therapy [40]. A Greek study divided 44 patients into three groups, where the first group received 1 mg per day of colchicine as monotherapy, the second group received both colchicine and doxycycline at 100 mg per day, and the third group received both colchicine and doxycycline at 40 mg per day. The three groups reported an improvement in all measured scores, but there was no statistically significant difference among the groups [41].
These data seem to suggest that colchicine may be useful in treating HS, especially when associated with antibacterial drugs.
2.6. Acne Vulgaris
Acne is an inflammatory condition characterized by the blockage and inflammation of the pilosebaceous unit, leading to papulopustular lesions of the affected areas. The regions that are more commonly involved are the face, the back, and the chest. It usually presents during adolescence, but it may appear at any age. It is crucial to treat acne as soon as possible, as the more severe forms may lead to scarring [42][43][44]. Current standard treatments include topical and systemic antibiotics, topical and systemic retinoids, and keratolytic agents [45][46]. Photodynamic therapy has also been proposed [47]. An Italian study reported 12 patients with severe acne vulgaris that were treated for 2 months with colchicine at 1 mg per day, showing no improvement [48]. A Korean group described a 31-year-old female treated for acne conglobata with a combination therapy of isotretinoin, colchicine, and cyclosporin, with reasonable control over the condition [49].
2.7. Urticaria
Urticaria is a characterized by wheals and/or angioedema; it is defined as acute if it occurs for less than 6 weeks, or chronic (chronic urticaria, CU) if it lasts for longer than 6 weeks. Among CU, chronic inducible urticaria (CIU) and chronic spontaneous urticaria (CSU) are identified. The treatment of CU is challenging and currently includes second-generation antihistamines, omalizumab, and cyclosporine [50][51]. Colchicine has also been proposed to manage the disease. Thirteen patients affected by delayed pressure urticaria were enrolled in a double-blind placebo-controlled trial. The researchers were unable to demonstrate any effect of colchicine in managing the disease [52].
2.8. Pyoderma Gangrenosum
Pyoderma gangrenosum (PG) is a neutrophilic dermatosis characterized by a painful, rapidly enlarging ulcer that may mimic various pathologic conditions. It may be associated with some systemic diseases, such as inflammatory bowel diseases and rheumatoid arthritis [53][54]. Different case reports describe the use of colchicine in PG. Lugassy and Ronnen described a case series study of three patients suffering from familial Mediterranean fever, where PG positively responded to colchicine [55]. Two cases of PG associated with inflammatory bowel disease that were refractory to other treatments were managed with colchicine at 1 mg per day with the resolution of all lesions in 3 months, suggesting colchicine as a first-line treatment for refractory PG [56].
2.9. Sweet Syndrome
Acute febrile neutrophilic dermatosis, also known as Sweet syndrome (SS), is a neutrophilic dermatosis characterized by skin, and sometimes mucosal, inflammation with blisters and fever. It may be associated with infections, inflammatory bowel disease, internal malignancies, rheumatological conditions, immunodeficiency, and various drugs. In case of treatment inefficacy, various immunosuppressants have been experimented with [57][58]. A Japanese group described a 29-year-old woman affected by SS responding to 0.5 mg of colchicine three times a day after failing previous treatments with potassium iodide and oral prednisone (10 mg per day). The patient started to respond 2 days after the beginning of therapy, with a reduction of plaques and aphthae. A complete response was obtained in 1 week [59].
2.10. Erythema Nodosum
Erythema nodosum is an inflammatory condition involving the subcutaneous fat (panniculitis). It may be associated with inflammatory intestinal conditions, such as Crohn’s disease [60], bacterial and viral infections, as well as drugs and vaccines [61]. Systemic steroids, non-steroidal anti-inflammatory drugs, and oral potassium iodide may be used to manage this condition. Colchicine has been reported as a possible treatment, although no clinical study is present in medical literature, only old case reports [62].
2.11. Actinic Keratosis
Actinic keratoses (AK) are precancerous lesions found on photodamaged skin [63]. They may be considered a precursor of squamous cell carcinoma, characterized by the abnormal and quick growth of keratinocytes in the epidermis, often secondary to chronic ultraviolet or sunlight exposure [64][65]. Various treatments have been traditionally proposed to manage this condition, such as topical imiquimod, sodium-diclofenac, piroxicam, 5-fluorouracil, cryotherapy, photodynamic therapy, and surgery [66][67]. Topical colchicine was proposed to manage such conditions since the late 1960s [68]. A Swiss group recruited 20 patients with hypertrophic, multiple AK on the forehead. Ten of them applied 1% colchicine in hydrophilic gel twice daily, while the others were treated with the vehicle. Seventy percent of patients in the colchicine arm showed a complete response with no relapse at the 2-month follow-up; no systemic absorption was reported [69].
2.12. Cutaneous Vasculitis
Cutaneous vasculitis (CV) is a heterogeneous group of disorders characterized by inflamed capillaries and veins in the dermis. They can be classified as capillaritis, small vessel vasculitis, medium vessel vasculitis, or large vessel vasculitis [70][71]. CV are generally characterized by petechiae, palpable purpura, and infiltrated erythema, indicating dermal, superficial, small-vessel vasculitis, or, less commonly, by nodular erythema, deep ulcers, livedo racemosa, and digital gangrene, implicating deep dermal, or subcutaneous, muscular-vessel vasculitis [72][73][74]. The treatment is usually anti-inflammatories with steroids and immunosuppressants [75]. Colchicine has been proposed in the management of various disorders in this spectrum of conditions.
2.13. Erythema Induratum
Erythema induratum (EI), also called nodular vasculitis (NV), refers to a chronic lobular panniculitis that may or may not be associated with vasculitis. If it is secondary to tuberculosis, the disease is called Bazin’s EI; otherwise, it is called EI of Withfield [76]. A case series study described three patients with EI, one reporting a previous tubercular infection and two with no infection. Traditional therapies, including multidrug anti-tubercular therapies (rifampicin, isoniazid, ethambutol, and pyrazinamide), corticosteroids, methotrexate, cyclosporine, mycophenolate, and dapsone showed minor-to-no improvements. In contrast, the treatment with colchicine at 0.5 mg twice per day combined with a low dose of corticosteroids led to a complete/almost complete remission in all the patients [77].
2.14. Cutaneous Amyloidosis
Amyloidosis is a condition characterized by the stacking of various insoluble proteins (amyloids) in amounts that cause dysfunction in an organ. This stacking may involve all organs or may be confined to a single one, depending on the insoluble protein involved [78]. The two most common primary cutaneous amyloidoses are lichen and macular amyloidosis, reporting similar histological features [79]. A case series study described 15 patients with primary localized cutaneous amyloidosis, of which eight had macular amyloidosis and seven had lichen amyloidosis. They received oral colchicine at 0.5 mg twice per day for 3 months. Pruritus, and size of the papules, decreased in both subgroups of patients [80].
2.15. Cutaneous Sarcoidosis
Sarcoidosis is a condition characterized by the formations of granulomas in various parts of the body, including the skin (cutaneous sarcoidosis) [81]. Various treatments have been proposed, such as corticosteroids, immunosuppressants, and biologic drugs [82]. Wise reported three patients affected by facial cutaneous sarcoidosis that rapidly responded, with a long remission, to combined therapy with systemic colchicine and a topical corticosteroid ointment [83].
2.16. Hereditary Angioedema
Hereditary angioedema is a spectrum of genetic-based conditions characterized by the recurrent attacks of self-limiting oedemas of the skin, gastrointestinal tract, and airways. It is classified into three types based on the pathogenesis [84]. A Turkish group [85] reported using oral colchicine with a partial remission of symptoms in a patient suffering from type 1 hereditary angioedema concomitantly affected by familial Mediterranean fever, showing some usefulness of the drug in this condition.
2.17. Acquired Perforating Dermatosis
Perforating dermatoses (PD) are a heterogeneous group of cutaneous diseases defined by the transepidermal elimination of dermal tissue. Four classical forms are described: the acquired reactive perforating collagenosis that eliminates collagen fibers, elastosis perforans serpiginosa that eliminates elastic fibers, Kyrle disease that eliminates keratin, and perforating folliculitis [86]. No first-line treatment is currently available for PD [87]. Grover and colleagues reported the case of a 68-year-old woman affected by diabetes mellitus and chronic kidney disease that successively developed acquired reactive perforating collagenosis. The patient did not respond to oral antihistamines, potent topical corticosteroids, and topical retinoids, so a therapy of oral colchicine, initially 0.5 mg once a day, then twice a day, was administered, with a rapid and complete response in 4 weeks [88].
2.18. Linear IgA Bullous Disease
Linear IgA bullous disease (LIBD), or chronic bullous dermatosis in children, is an autoimmune condition characterized by blisters on the skin and mucous membranes. The diagnostic finding is the presence of the immunoglobulin A deposition at the dermo-epidermal junction using immunofluorescence. It may be associated with inflammatory bowel disease, solid and lymphoid malignancies, and rheumatoid arthritis. No association with gluten-sensitive enteropathy is reported [89][90]. The traditional therapy relies on oral dapsone; alternative treatments have been proposed in case of inefficacy, AEs, or contraindication (i.e., favism), among which colchicine is included. Banodkar and al-Suwaid reported a case series study of eight patients, five boys and three girls, aged between 3 and 9 years, treated with 0.5 mg of oral colchicine twice per day. Five patients responded to the monotherapy, while three patients needed a small dose of steroids to reach remission. No severe side effects were observed [91].
2.19. Pemphigus
Pemphigus is a group of autoimmune diseases characterized by the formation of erosions and/or flaccid bullae of the skin and/or mucosae. All variants of pemphigus are characterized by the development of autoantibodies to the desmosomal proteins of the epidermis [92]. An Israeli group reported treating two cases of IgA pemphigus with 0.5 mg of colchicine three times per day, with the resolution of the condition in two weeks. [93]. A Japanese group used colchicine and a topical steroid in the treatment of pemphigus foliaceous, with good results [94].
2.20. Bullous Pemphigoid
Bullous pemphigoid (BP) is an autoimmune disease characterized by subepidermal blistering. BP is caused by autoantibodies that are directed to the antigens of the hemidesmosome, BP180 and BP230 [95]. A Greek group reported a combination therapy of oral steroids and an immunosuppressant in 15 patients to treat mucosal membrane pemphigoid. Out of the various drugs used, colchicine resulted in the best control rate of the condition (8 out of 12, 66%) [96].
2.21. Epidermolisis Bullosa
Evidence for treating epidermolysis bullosa (EB), both congenital and acquisita, with colchicine is limited to case series studies. However, they suggest that the drug may be an effective treatment modality in EB [97]. A Japanese group described a 65-year-old woman with EB acquisita that did not respond to steroids; the treatment with 1 mg per day of colchicine led to good results [98]. Megahed and Scharffetter-Kochanek reported the cases of a 71-year-old-woman and a 65-year-old-man, who were both resistant to traditional therapies, that responded to a dosage of 2 mg of colchicine per day, lowered down to 1 mg per day after six months [99].
2.22. Dermatitis Herpetiformis
Dermatitis herpetiformis (DH) is a vesico-bullous disease mainly associated with celiachia. Only one case series of DH with four patients is reported; they were treated with 0.6 mg of colchicine three or four times per day, leading to improvement in three out of four patients. All patients reported diarrhea that led to the dose reduction/suspension of the drug, with a recurrence of the condition [100].
2.23. Subcorneal Pustular Dermatosis
Subcorneal pustular dermatosis, or Sneddon–Wilkinson’s disease, is a condition characterized by pustular lesions on the trunk and flexural areas, such as the armpits and groin [101]. Pavithran reported a patient developing AEs with a combination therapy of dapsone and corticosteroids. Oral colchicine at 0.5 mg twice per day was started, with a complete resolution. The dose was dropped to 0.5 mg once per day for maintenance, with no recurrence [102].
2.24. Stomatitis
Colchicine is currently used for different types of mouth inflammatory conditions, with good results. Quintana-Ortega and colleagues performed a multicenter study on 13 young patients affected by periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome. The mean age at the start of the colchicine treatment was 6, and the median dosage was 0.02 mg per kg per day. Treatment was continued for 1 year; as a result, a decreased number of flares and a shortened duration of the disease episodes were recorded [103]. This efficacy was confirmed by an extensive study involving 400 children affected by PFAPA. The patients started on colchicine treatment for 12 consecutive months (0.5 mg per day of colchicine in children < 5years of age, 1 mg per day for children between 5 and 10 years of age, and 1.5 mg per day for children > 10 years of age). Only 358 patients continued the prophylactic therapy due to AEs, reducing the mean time of recurrence from 18.8 ± 7.9 to 49.5 ± 17.6 days. These results were particularly significant in the Mediterranean fever (MEFV) variant [104].
3. Conclusions
Oral colchicine may be considered a safe second- or third-line treatment in several skin diseases, including eccrine hidradenitis, PG, EN, EI, storage diseases, perforating dermatoses, bullous diseases, and pustular dermatoses. Although mainly based on small case series studies, the evidence seems to be promising. For other conditions, such as psoriasis, HS, vasculitis, palmoplantar pustulosis, acne, and urticaria, contrasting results exist. For psoriasis, pustular variants (as reported for other pustular dermatoses) seem to respond better to colchicine, while the efficacy of this drug in the management of arthropathic psoriasis has not been fully proven. Oral colchicine is usually used with dosages between 0.5 mg and 2.5 mg per day, with dosages higher than 2 mg mainly used to manage patients affected by flares of familial Mediterranean fever. It would be wise to not overcome the dose of 2 mg per day due to the risk of side effects. In most dermatological patients, a dosage of 1–1.5 mg per day was usually enough to reach clinical efficacy. If colchicine must be used for longer period of time, it is better to lower the daily dose to 0.5–1 mg per day, in order to reduce the risk of systemic side effects. Topical colchicine might be an interesting, safe, and effective second-line treatment for psoriatic plaques, although it has rarely been investigated. While not responding to monotherapy, HS seems to respond when colchicine is associated with antibiotics. Vasculitis seems to have a different response profile according to the subtype, with conditions such as Behçet’s disease, where colchicine is the main treatment; urticarial vasculitis, in which colchicine is considered an effective treatment; or leukocytoclastic vasculitis, where the efficacy of the drug remains controversial. An upcoming randomized trial of the Vasculitis Clinical Research Consortium should shed light on the usefulness of this molecule. Colchicine may be considered as first-line therapy in other conditions, such as aphthous stomatitis, both in the syndromic variants and in VAS. Topical colchicine may be considered as an effective alternative to treat actinic keratosis. The rate of side effects reported after using oral colchicine may be considered low and, in most cases, it is limited to gastrointestinal manifestations, especially if the dosage does not exceed 1.5 mg per day.
References
- Rao, A.; Konda, C. Colchicine in dermatology. Indian J. Dermatol. Venereol. Leprol. 2010, 76, 201–205.
- Anzengruber, F.; Graf, V.; Hafner, J.; Meienberger, N.; Guenova, E.; Dummer, R. Efficacy and safety of colchicine in inflammatory skin diseases: A retrospective, monocentric study in a large tertiary center. J. Dermatol. Treat. 2021, 32, 104–109.
- Nistico, S.; Tamburi, F.; Bennardo, L.; Dastoli, S.; Schipani, G.; Caro, G.; Fortuna, M.C.; Rossi, A. Treatment of telogen effluvium using a dietary supplement containing Boswellia serrata, Curcuma longa, and Vitis vinifera: Results of an observational study. Dermatol. Ther. 2019, 32, e12842.
- Combalia, A.; Baliu-Piqué, C.; Fortea, A.; Ferrando, J. Anagen effluvium following acute colchicine poisoning. Int. J. Trichol. 2016, 8, 171–172.
- Dattola, A.; Silvestri, M.; Bennardo, L.; Del Duca, E.; Longo, C.; Bianchi, L.; Nisticò, S. Update of calcineurin inhibitors to treat inverse psoriasis: A systematic review. Dermatol. Ther. 2018, 31, e12728.
- Griffiths, C.E.; Barker, J.N. Pathogenesis and clinical features of psoriasis. Lancet 2007, 370, 263–271.
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475.
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Rizzuto, F.; Dastoli, S.; Patruno, C.; Bianchi, L.; Nisticò, S.P. A novel vehicle for the treatment of psoriasis. Dermatol. Ther. 2020, 33, e13185.
- Segaert, S.; Duvold, L.B. Calcipotriol cream: A review of its use in the management of psoriasis. J. Dermatol. Treat. 2006, 17, 327–337.
- Young, M.; Aldredge, L.; Parker, P. Psoriasis for the primary care practitioner. J. Am. Assoc. Nurse Pract. 2017, 29, 157–178.
- Amoruso, G.; Nisticò, S.; Iannone, L.; Russo, E.; Rago, G.; Patruno, C.; Bennardo, L. Ixekizumab May Improve Renal Function in Psoriasis. Healthcare 2021, 9, 543.
- Iannone, L.F.; Bennardo, L.; Palleria, C.; Roberti, R.; De Sarro, C.; Naturale, M.D.; Dastoli, S.; Donato, L.; Manti, A.; Valenti, G.; et al. Safety profile of biologic drugs for psoriasis in clinical practice: An Italian prospective pharmacovigilance study. PLoS ONE 2020, 15, e0241575.
- Kaushik, S.B.; Lebwohl, M.G. Psoriasis: Which therapy for which patient: Psoriasis comorbidities and preferred systemic agents. J. Am. Acad. Dermatol. 2019, 80, 27–40.
- Dastoli, S.; Iannone, L.F.; Bennardo, L.; Silvestri, M.; Palleria, C.; Nisticò, S.P.; De Sarro, G.; Russo, E. A Rare Case of Drug-Induced Erectile Dysfunction with Secukinumab Solved After Switch to Ixekizumab in A Psoriatic Patient: A Case Report. Curr. Drug Saf. 2020, 15, 69–72.
- Dattola, A.; Silvestri, M.; Tamburi, F.; Amoruso, G.F.; Bennardo, L.; Nisticò, S.P. Emerging role of anti-IL23 in the treatment of psoriasis: When humanized is very promising. Dermatol. Ther. 2020, 33, e14504.
- Gaylarde, P.M.; Sarkany, I. Letter: Colchicine therapy for psoriasis. Arch. Dermatol. 1976, 112, 556–557.
- Kaidbey, K.H.; Petrozzi, J.W.; Kligman, A.M. Topical colchicine therapy for recalcitrant psoriasis. Arch. Dermatol. 1975, 111, 33–36.
- Wahba, A.; Cohen, H. Therapeutic trials with oral colchicine in psoriasis. Acta Derm. Venereol. 1980, 60, 515–520.
- Horiguchi, M.; Takigawa, M.; Imamura, S. Treatment of generalized pustular psoriasis with methotrexate and colchicine. Arch. Dermatol. 1981, 117, 760.
- Zachariae, H.; Kragballe, K.; Herlin, T. Colchicine in generalized pustular psoriasis: Clinical response and antibody-dependent cytotoxicity by monocytes and neutrophils. Arch. Dermatol. Res. 1982, 274, 327–333.
- Raposo, I.; Torres, T. Palmoplantar Psoriasis and Palmoplantar Pustulosis: Current Treatment and Future Prospects. Am. J. Clin. Dermatol. 2016, 17, 349–358.
- Passante, M.; Dastoli, S.; Nisticò, S.P.; Bennardo, L.; Patruno, C. Effectiveness of brodalumab in acrodermatitis continua of Hallopeau: A case report. Dermatol. Ther. 2020, 33, e13170.
- Takigawa, M.; Miyachi, Y.; Uehara, M.; Tagami, H. Treatment of Pustulosis Palmaris et Plantaris with Oral Doses of Colchicine. Arch. Dermatol. 1982, 118, 458–460.
- Mann, R. Failure of colchicine for palmo-plantar pustulosis. Br. J. Dermatol. 1982, 106, 373.
- English, J.; Fenton, D.; Wilkinson, J. Failure of colchicines for palmo-plantar pustulosis. Clin. Exp. Dermatol. 1983, 8, 207–209.
- Thestrup-Pedersen, K.; Reymann, F. Treatment of pustulosis palmaris et plantaris with colchicine. A double-blind cross-over trial. Acta Derm Venereol. 1984, 64, 76–78.
- Wong, S.S.; Tan, K.C.; Goh, C.L. Long-term colchicine for recalcitrant palmoplantar pustulosis: Treatment outcome in 3 patients. Cutis 2001, 68, 216–218.
- Belot, V.; Perrinaud, A.; Corven, C.; De Muret, A.; Lorette, G.; Machet, L. Hidradénite eccrine neutrophilique idiopathique de l’adulte d’évolution prolongée traitée par colchicine. . Presse Med. 2006, 35, 1475–1478.
- Scheer, H.S.; Kamarashev, J.; Weibel, L. Successful Treatment of Recurrent Idiopathic Plantar Eccrine Hidradenitis with Colchicine. Arch. Dermatol. 2012, 148, 1357–1359.
- Del Duca, E.; Morelli, P.; Bennardo, L.; Di Raimondo, C.; Nisticò, S.P. Cytokine Pathways and Investigational Target Therapies in Hidradenitis Suppurativa. Int. J. Mol. Sci. 2020, 21, 8436.
- Saunte, D.M.L.; Jemec, G.B.E. Hidradenitis Suppurativa: Advances in Diagnosis and Treatment. JAMA 2017, 318, 2019–2032.
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol. 2020, 82, 1045–1058.
- Dattola, A.; Silvestri, M.; Bennardo, L.; Passante, M.; Scali, E.; Patruno, C.; Nisticò, S.P. Role of Vitamins in Skin Health: A Systematic Review. Curr. Nutr. Rep. 2020, 9, 226–235.
- Alikhan, A.; Sayed, C.; Alavi, A.; Alhusayen, R.; Brassard, A.; Burkhart, C.; Crowell, K.; Eisen, D.; Gottlieb, A.B.; Hamzavi, I.; et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J. Am. Acad. Dermatol. 2019, 81, 91–101.
- Roberti, R.; Iannone, L.F.; Palleria, C.; De Sarro, C.; Spagnuolo, R.; Barbieri, M.A.; Vero, A.; Manti, A.; Pisana, V.; Fries, W.; et al. Safety profiles of biologic agents for inflammatory bowel diseases: A prospective pharmacovigilance study in Southern Italy. Curr. Med Res. Opin. 2020, 36, 1457–1463.
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis suppurativa: Current and emerging treatments. J. Am. Acad. Dermatol. 2020, 82, 1061–1082.
- Dattola, A.; Fazia, G.; Tolone, M.; Bennardo, L.; Lanna, C.; Mazzilli, S.; Dastoli, S.; Campione, E.; Bianchi, L.; Segura-Garcia, C.; et al. Psychological symptoms in dermatologic patients during the COVID-19 lockdown period: A double center experience. Ital. J. Dermatol. Venereol. 2021, 156, 392–393.
- Tchero, H.; Herlin, C.; Bekara, F.; Fluieraru, S.; Teot, L. Hidradenitis Suppurativa: A Systematic Review and Meta-analysis of Therapeutic Interventions. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 248, Erratum in Indian J. Dermatol. Venereol. Leprol. 2019, 85, 617.
- Van Der Zee, H.; Prens, E. The Anti-Inflammatory Drug Colchicine Lacks Efficacy in Hidradenitis Suppurativa. Dermatology 2011, 223, 169–173.
- Armyra, K.; Kouris, A.; Markantoni, V.; Katsambas, A.; Kontochristopoulos, G. Hidradenitis suppurativa treated with tetracycline in combination with colchicine: A prospective series of 20 patients. Int. J. Dermatol. 2017, 56, 346–350.
- Liakou, A.I.; Kontochristopoulos, G.; Agiasofitou, E.; Tsantes, A.G.; Papadakis, M.; Marnelakis, I.; Tsante, K.A.; Kapsiocha, A.; Katoulis, A.; Gregoriou, S.; et al. Colchicine Improves Clinical Outcomes and Quality of Life in Hidradenitis Suppurativa Patients: A Retrospective Study. J. Clin. Med. 2021, 10, 4742.
- Knutsen-Larson, S.; Dawson, A.L.; Dunnick, C.A.; Dellavalle, R.P. Acne Vulgaris: Pathogenesis, Treatment, and Needs Assessment. Dermatol. Clin. 2012, 30, 99–106.
- Habeshian, K.A.; Cohen, B.A. Current Issues in the Treatment of Acne Vulgaris. Pediatrics 2020, 145, S225–S230.
- Cannarozzo, G.; Silvestri, M.; Tamburi, F.; Sicilia, C.; Del Duca, E.; Scali, E.; Bennardo, L.; Nisticò, S.P. A new 675-nm laser device in the treatment of acne scars: An observational study. Lasers Med Sci. 2021, 36, 227–231.
- Fox, L.; Csongradi, C.; Aucamp, M.; Du Plessis, J.; Gerber, M. Treatment Modalities for Acne. Molecules 2016, 21, 1063.
- Harper, J.C. Acne vulgaris: What’s new in our 40th year. J. Am. Acad. Dermatol. 2020, 82, 526–527.
- Del Duca, E.; Manfredini, M.; Petrini, N.; Farnetani, F.; Chester, J.; Bennardo, L.; Schipani, G.; Tamburi, F.; Sannino, M.; Cannarozzo, G.; et al. Daylight photodynamic therapy with 5-aminolevulinic acid 5% gel for the treatment of mild-to-moderate inflammatory acne. Ital. J. Dermatol. Venerol. 2021, 156, 46–50.
- Schepis, C.; Siragusa, M.; Palazzo, R.; Guerra, A.P. Failure of Colchicine in the Treatment of Severe Acne Vulgaris. Acta Derm. Venereol. 1999, 79, 491.
- Jeong, S.; Lee, C. Acne conglobata: Treatment with isotretinoin, colchicines, and cyclosporine as compared with surgical intervention. Clin. Exp. Dermatol. 1996, 21, 462–463.
- Antia, C.; Baquerizo, K.; Korman, A.; Bernstein, J.A.; Alikhan, A. Urticaria: A comprehensive review: Epidemiology, diagnosis, and work-up. J. Am. Acad. Dermatol. 2018, 79, 599–614.
- Passante, M.; Napolitano, M.; Dastoli, S.; Bennardo, L.; Fabbrocini, G.; Nisticò, S.P.; Patruno, C. Safety of omalizumab treatment in patients with chronic spontaneous urticaria and COVID-19. Dermatol. Ther. 2021, 34, 6.
- Lawlor, F.; Black, A.K.; Ward, A.M.; Morris, R.; Greaves, M. Delayed pressure urticaria, objective evaluation of a variable disease using a dermographometer and assessment of treatment using colchicine. Br. J. Dermatol. 1989, 120, 403–408.
- Alavi, A.; French, L.; Davis, M.D.; Brassard, A.; Kirsner, R.S. Pyoderma Gangrenosum: An Update on Pathophysiology, Diagnosis and Treatment. Am. J. Clin. Dermatol. 2017, 18, 355–372.
- Braswell, S.F.; Kostopoulos, T.C.; Ortega-Loayza, A.G. Pathophysiology of pyoderma gangrenosum (PG): An updated review. J. Am. Acad. Dermatol. 2015, 73, 691–698.
- Lugassy, G.; Ronnen, M. Case Report: Severe Pyoderma Associated with Familial Mediterranean Fever—Favorable Response to Colchicine in Three Patients. Am. J. Med. Sci. 1992, 304, 29–31.
- Paolini, O.; Hébuterne, X.; Flory, P.; Charles, F.; Rampal, P. Treatment of pyoderma gangrenosum with colchicine. Lancet 1995, 345, 1057–1058.
- Villarreal-Villarreal, C.D.; Ocampo-Candiani, J.; Villarreal-Martínez, A. Sweet Syndrome: A Review and Update. Actas Dermosifiliogr. 2016, 107, 369–378.
- Cohen, P.R. Sweet’s syndrome—A comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J. Rare Dis. 2007, 2, 34.
- Suehisa, S.; Tagami, H. Treatment of acute febrile neutrophilic dermatosis (Sweet’s syndrome) with colchicine. Br. J. Dermatol. 1981, 105, 483.
- Spagnuolo, R.; Dastoli, S.; Silvestri, M.; Cosco, C.; Garieri, P.; Bennardo, L.; Nisticò, S.P. Anti-interleukin 12/23 in the treatment of erythema nodosum and Crohn disease: A case report. Dermatol. Ther. 2019, 32, e12811.
- Cameli, N.; Silvestri, M.; Mariano, M.; Bennardo, L.; Nisticò, S.; Cristaudo, A. Erythema nodosum following the first dose of ChAdOx1-S nCoV-19 vaccine. J. Eur. Acad. Dermatol. Venereol. 2021.
- Wallace, S.L. Erythema nodosum treatment with colchicine. JAMA 1967, 202, 1056.
- Schipani, G.; DEL Duca, E.; Todaro, G.; Scali, E.; Dastoli, S.; Bennardo, L.; Bonacci, S.; DI Raimondo, C.; Pavel, A.B.; Colica, C.; et al. Arsenic and chromium levels in hair correlate with actinic keratosis/non melanoma skin cancer: Results of an observational controlled study. Ital. J. Dermatol. Venereol. 2020.
- Bennardo, L.; Bennardo, F.; Giudice, A.; Passante, M.; Dastoli, S.; Morrone, P.; Provenzano, E.; Patruno, C.; Nisticò, S. Local Chemotherapy as an Adjuvant Treatment in Unresectable Squamous Cell Carcinoma: What Do We Know So Far? Curr. Oncol. 2021, 28, 2317–2325.
- Pentangelo, G.; Nisticò, S.; Provenzano, E.; Cisale, G.; Bennardo, L. Topical 5% Imiquimod Sequential to Surgery for HPV-Related Squamous Cell Carcinoma of the Lip. Medicina 2021, 57, 563.
- Hashim, P.; Chen, T.; Rigel, D.; Bhatia, N.; Kircik, L.H. Actinic Keratosis: Current Therapies and Insights into New Treatments. J. Drugs Dermatol. 2019, 18, s161–s166.
- Dirschka, T.T.; Gupta, G.G.; Micali, G.G.; Stockfleth, E.; Basset-Seguin, N.N.; Del Marmol, V.; Dummer, R.; Jemec, G.B.E.; Malvehy, J.; Peris, K.; et al. Real-world approach to actinic keratosis management: Practical treatment algorithm for office-based dermatology. J. Dermatol. Treat. 2016, 28, 431–442.
- Tutrone, W.D.; Saini, R.; Caglar, S.; Weinberg, J.M.; Crespo, J. Topical therapy for actinic keratoses, II: Diclofenac, colchicine, and retinoids. Cutis 2003, 71, 373–379.
- Grimaître, M.; Etienne, A.; Fathi, M.; Piletta, P.-A.; Saurat, J.-H. Topical colchicine therapy for actinic keratoses. Dermatology 2000, 200, 346–348.
- Sunderkötter, C.H.; Zelger, B.; Chen, K.; Requena, L.; Piette, W.; Carlson, J.A.; Dutz, J.; Lamprecht, P.; Mahr, A.; Aberer, E.; et al. Nomenclature of Cutaneous Vasculitis: Dermatologic Addendum to the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2018, 70, 171–184.
- Micheletti, R.G.; Pagnoux, C. Management of cutaneous vasculitis. Presse Med. 2020, 49, 104033.
- Abenavoli, L.; Bennardo, L.; Nisticò, S.P.; Luzza, F. From gut to skin: The association between celiac disease and cutaneous abnormalities. Minerva Gastroenterol. 2021, 67, 301–303.
- Chen, K.-R.; Carlson, J.A. Clinical Approach to Cutaneous Vasculitis. Am. J. Clin. Dermatol. 2008, 9, 71–92.
- Abenavoli, L.; Dastoli, S.; Bennardo, L.; Boccuto, L.; Passante, M.; Silvestri, M.; Proietti, I.; Potenza, C.; Luzza, F.; Nisticò, S.P. The Skin in Celiac Disease Patients: The Other Side of the Coin. Medicina 2019, 55, 578.
- Carlson, J.A.; Cavaliere, L.F.; Grant-Kels, J.M. Cutaneous vasculitis: Diagnosis and management. Clin. Dermatol. 2006, 24, 414–429.
- Gilchrist, H.; Patterson, J.W. Erythema nodosum and erythema induratum (nodular vasculitis): Diagnosis and management. Dermatol. Ther. 2010, 23, 320–327.
- Wee, E.; Kelly, R.I. Treatment of nodular vasculitis with colchicine. Australas. J. Dermatol. 2017, 58, e79–e82.
- Weidner, T.; Illing, T.; Elsner, P. Primary Localized Cutaneous Amyloidosis: A Systematic Treatment Review. Am. J. Clin. Dermatol. 2017, 18, 629–642.
- Kaltoft, B.; Schmidt, G.; Lauritzen, A.F.; Gimsing, P. Primary localised cutaneous amyloidosis—A systematic review. Dan. Med J. 2013, 60, A4727.
- Chakravarty, K.; Chanda, M. Role of colchicine in primary localised cutaneous amyloidosis. Indian J. Dermatol. Venereol. Leprol. 1995, 61, 268–269.
- Caplan, A.; Rosenbach, M.; Imadojemu, S. Cutaneous Sarcoidosis. Semin. Respir. Crit. Care Med. 2020, 41, 689–699.
- Dai, C.; Shih, S.; Ansari, A.; Kwak, Y.; Sami, N. Biologic Therapy in the Treatment of Cutaneous Sarcoidosis: A Literature Review. Am. J. Clin. Dermatol. 2019, 20, 409–422.
- Wise, R.D. Clinical resolution of facial cutaneous sarcoidosis with systemic colchicine and a topical corticosteroid ointment. Compr. Ther. 2008, 34, 105–110.
- Napolitano, M.; Vastarella, M.; Fabbrocini, G.; Cinelli, E.; Camela, E.; Tranchini, P.; Bennardo, L.; Patruno, C. Hereditary angioedema type III, recurrent pregnancy loss and heterozygous MTHFR mutation. Dermatol. Ther. 2020, 33, e14541.
- Bahceci, S.E.; Genel, F.; Gulez, N.; Nacaroglu, H.T. Coexistence of hereditary angioedema in a case of familial Mediterranean fever with partial response to colchicine. Cent. Eur. J. Immunol. 2015, 40, 115–116.
- Kawakami, T.; Akiyama, M.; Ishida-Yamamoto, A.; Nakano, H.; Mitoma, C.; Yoneda, K.; Suga, Y. Clinical practice guide for the treatment of perforating dermatosis. J. Dermatol. 2020, 47, 1374–1382.
- Lukács, J.; Schliemann, S.; Elsner, P. Treatment of acquired reactive perforating dermatosis—A systematic review. J. Dtsch. Dermatol. Ges. 2018, 16, 825–842.
- Grover, C.; Kharghoria, G.; Sharma, S. Acquired perforating dermatosis: Response to colchicine. Dermatol. Ther. 2020, 33, e14492.
- Vico-Alonso, C.; Palencia-Pérez, S.I. Linear IgA Bullous Dermatosis. N. Engl. J. Med. 2020, 382, 2248.
- Juratli, H.A.; Sárdy, M. Lineare IgA-Dermatose. . Hautarzt 2019, 70, 254–259.
- Banodkar, D.; Al-Suwaid, A.R. Colchicine as a novel therapeutic agent in chronic bullous dermatosis of childhood. Int. J. Dermatol. 1997, 36, 213–216.
- Palleria, C.; Bennardo, L.; Dastoli, S.; Iannone, L.F.; Silvestri, M.; Manti, A.; Nisticò, S.P.; Russo, E.; De Sarro, G. Angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers induced pemphigus: A case series and literature review. Dermatol. Ther. 2019, 32, e12748.
- Hodak, E.; Lapidoth, M.; David, M. Effect of colchicine in the subcorneal pustular dermatosis type of IgA pemphigus. J. Am. Acad. Dermatol. 1999, 40, 91–94.
- Takahashi, A.; Nishijima, C.; Umehara, K.; Kawashima, A.; Inaoki, M. Pemphigus foliaceus successfully treated with colchicine and topical corticosteroid. Eur. J. Dermatol. 2010, 20, 825–826.
- Bennardo, L.; Passante, M.; Cameli, N.; Cristaudo, A.; Patruno, C.; Nisticò, S.P.; Silvestri, M. Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering 2021, 8, 153.
- Chaidemenos, G.; Sidiropoulos, T.; Katsioula, P.; Koussidou-Eremondi, T. Colchicine in the management of mucous membrane pemphigoid. Dermatol. Ther. 2011, 24, 443–445.
- Robinson, K.P.; Chan, J.J. Colchicine in dermatology: A review. Australas. J. Dermatol. 2018, 59, 278–285.
- Tanaka, N.; Dainichi, T.; Ohyama, B.; Yasumoto, S.; Oono, T.; Iwatsuki, K.; Elfert, S.; Fritsch, A.; Bruckner-Tuderman, L.; Hashimoto, T. A case of epidermolysis bullosa acquisita with clinical features of Brunsting-Perry pemphigoid showing an excellent response to colchicine. J. Am. Acad. Dermatol. 2009, 61, 715–719.
- Megahed, M.; Scharffetter-Kochanek, K. Epidermolysis bullosa acquisita–successful treatment with colchicine. Arch. Dermatol. Res. 1994, 286, 35–40.
- Silvers, D.N.; Juhlin, E.A.; Berczeller, P.H.; McSorley, J. Treatment of Dermatitis Herpetiformis with Colchicine. Arch. Dermatol. 1980, 116, 1373–1374.
- Watts, P.J.; Khachemoune, A. Subcorneal Pustular Dermatosis: A Review of 30 Years of Progress. Am. J. Clin. Dermatol. 2016, 17, 653–671.
- Pavithran, K. Colchicine in the treatment of subcorneal pustular dermatosis. Indian J. Dermatol. Venereol. Leprol. 2010, 61, 56–57.
- Quintana-Ortega, C.; Seoane-Reula, E.; Fernández, L.; Camacho, M.; Olbrich, P.; Neth, O.; Murias, S.; Udaondo, C.; Remesal, A.; Calvo, C.; et al. Colchicine treatment in children with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome: A multicenter study in Spain. Eur. J. Rheumatol. 2020, 8, 73–78.
- Gunes, M.; Cekic, S.; Kilic, S.S. Is colchicine more effective to prevent periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis episodes in Mediterranean fever gene variants? Pediatr. Int. 2017, 59, 655–660.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
13 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




