
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcin Cichocki | -- | 2201 | 2022-04-12 09:42:52 | | | |
| 2 | Conner Chen | -5 word(s) | 2196 | 2022-04-12 11:13:57 | | | | |
| 3 | Conner Chen | Meta information modification | 2196 | 2022-04-12 11:14:49 | | | | |
| 4 | Conner Chen | Meta information modification | 2196 | 2022-04-13 04:18:22 | | |
Video Upload Options
Urological cancers, namely prostate, bladder, kidney, testicular, and penile cancers, are common conditions that constitute almost one-quarter of all malignant diseases in men. Urological cancers tend to affect older individuals, and their development is influenced by modifiable metabolic, behavioral, and environmental risk factors. Carotenoids may have cancer-fighting properties and protect against cancer development, slow its spread, and reduce the risk of cancer deaths in humans.
1. Prostate Cancer
Prostate cancer (PCa) is a common malignancy among men worldwide. In 2018, PCa was reported as the second most common noncutaneous cancer in men worldwide (an estimated 1.3 million new cases) and the fifth most common cause of cancer death in men. There is wide geographical diversity in its prevalence, with age-standardized rates (ASR) per 100,000 of 97.2 in the United States and less than 20 in Asia and developing countries [1]. The most common pathological type of PCa is acinar adenocarcinoma, which accounts for more than 99% of all prostate tumors [2]. Family history of PCa, older age, and race are the only undisputed risk factors for PCa development. Asian men have low PCa risk compared with men from the Western world. However, when Japanese men moved from Japan to California, their risk of PCa rose, approaching that of American men, which implied a role of dietary or environmental factors, possibly including dietary carotenoid intake as a contributing factor [3]. There have been a few proposed mechanisms through which carotenoids may act to reduce cancer development, as shown in Figure 1.
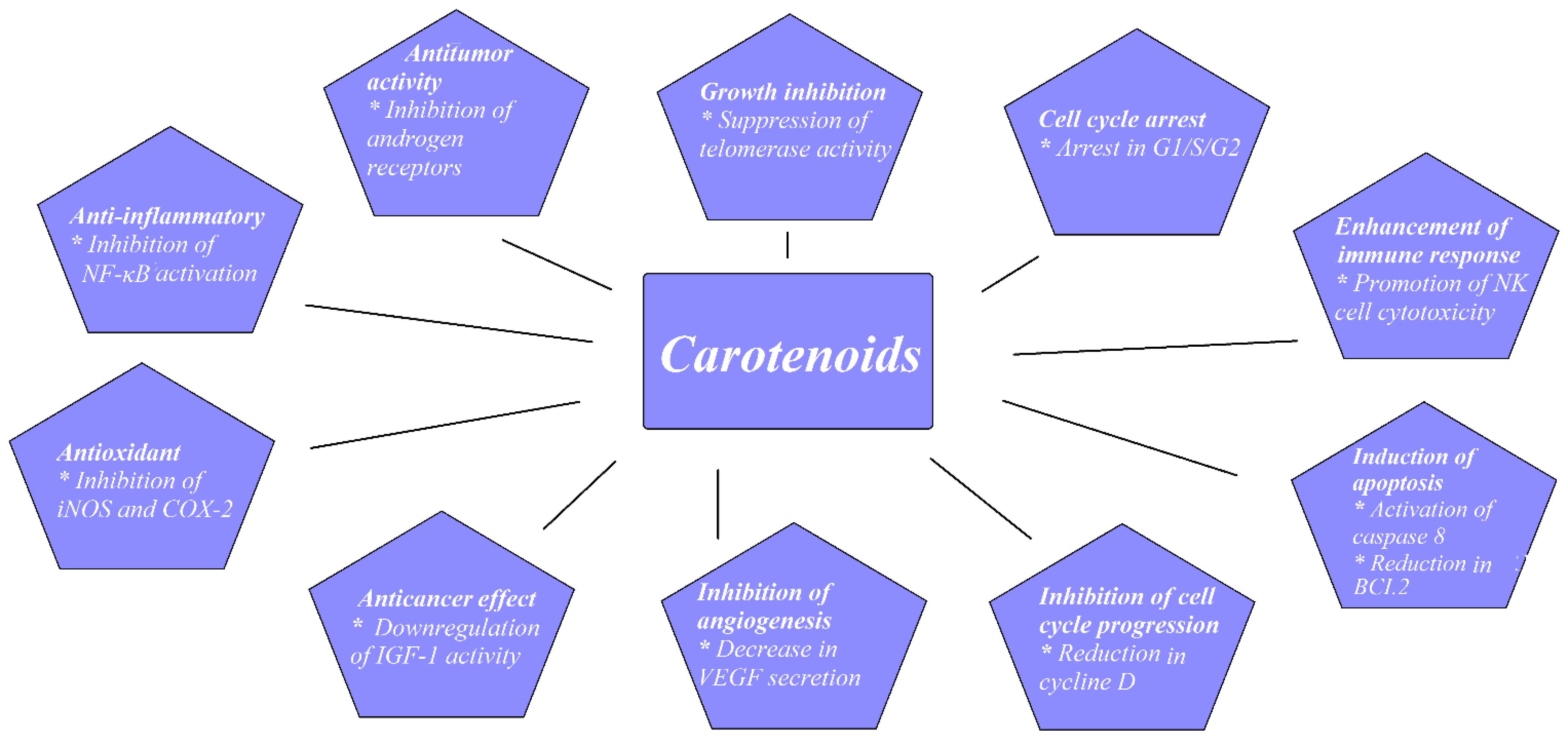
1.1. Experimental Studies
1.2. Epidemiological Studies
| Author | Number of Studies | Measure | Results |
|---|---|---|---|
| Chen et al., 2013 [25] | 17 studies; 6 cohort, 11 NCC | Effect of: Lycopene intake on risk of PCa Serum lycopene on risk of PCa Lycopene intake on risk of advanced PCa Serum lycopene on risk of advanced PCa |
Not significant Not significant Not significant Not significant |
| Wang et al., 2015 [26] | 34 studies; 10 cohort 11 NCC 13 CC |
Effect of: α-carotene, β-carotene, lycopene intake on PCa α-carotene, β-carotene, lycopene blood concentrations on PCa |
Significant inverse association between dietary α-carotene intake and PCa (RR: 0.81; CI: 0.76–0.99). No difference for β-carotene and lycopene intake. Only blood levels of lycopene were significantly associated with reduced PCa risk (RR: 0.81; CI: 0.69–0.96) |
| Rowles et al., 2017 [27] | 42 studies; 19 CC 13 NCC 8 cohort 2 case-cohort |
Effect of: Lycopene intake on PCa Lycopene circulating concentrations on PCa |
Dietary intake (RR = 0.88, CI: 0.78–0.98) and circulating concentrations (RR = 0.88, CI: 0.79–0.98) of lycopene were significantly associated with reduced PCa risk |
2. Kidney Cancer
References
- World Health Organization. Regional Office for Europe. In World Cancer Report: Cancer Research for Cancer Development; IARC: Lyon, France, 2020; ISBN 9789283204473.
- Marcus, D.M.; Goodman, M.; Jani, A.B.; Osunkoya, A.O.; Rossi, P.J. A Comprehensive Review of Incidence and Survival in Patients with Rare Histological Variants of Prostate Cancer in the United States from 1973 to 2008. Prostate Cancer Prostatic Dis. 2012, 15, 283–288.
- Kimura, T.; Sato, S.; Takahashi, H.; Egawa, S. Global Trends of Latent Prostate Cancer in Autopsy Studies. Cancers 2021, 13, 359.
- Tang, Y.; Parmakhtiar, B.; Simoneau, A.R.; Xie, J.; Fruehauf, J.; Lilly, M.; Zi, X. Lycopene Enhances Docetaxel’s Effect in Castration-Resistant Prostate Cancer Associated with Insulin-like Growth Factor I Receptor Levels. Neoplasia 2011, 13, 108–119.
- Gong, X.; Marisiddaiah, R.; Zaripheh, S.; Wiener, D.; Rubin, L.P. Mitochondrial β-Carotene 9, 10 Oxygenase Modulates Prostate Cancer Growth via NF-ΚB Inhibition: A Lycopene-Independent Function Running Title: BCO2 Inhibits NF-ΚB Signaling in Prostate Cancer. Mol. Cancer Res. 2016, 14, 966–975.
- Yang, C.M.; Lu, Y.L.; Chen, H.Y.; Hu, M.L. Lycopene and the LXRα Agonist T0901317 Synergistically Inhibit the Proliferation of Androgen-Independent Prostate Cancer Cells via the PPARγ-LXRα-ABCA1 Pathway. J. Nutr. Biochem. 2012, 23, 1155–1162.
- Yang, C.M.; Lu, I.H.; Chen, H.Y.; Hu, M.L. Lycopene Inhibits the Proliferation of Androgen-Dependent Human Prostate Tumor Cells through Activation of PPARγ-LXRα-ABCA1 Pathway. J. Nutr. Biochem. 2012, 23, 8–17.
- Renju, G.L.; Muraleedhara Kurup, G.; Bandugula, V.R. Effect of Lycopene Isolated from Chlorella Marina on Proliferation and Apoptosis in Human Prostate Cancer Cell Line PC-3. Tumor Biol. 2014, 35, 10747–10758.
- Soares, N.d.C.P.; Machado, C.L.; Trindade, B.B.; Lima, I.C.d.C.; Gimba, E.R.P.; Teodoro, A.J.; Takiya, C.; Borojevic, R. Lycopene Extracts from Different Tomato-Based Food Products Induce Apoptosis in Cultured Human Primary Prostate Cancer Cells and Regulate TP53, Bax and Bcl-2 Transcript Expression. Asian Pac. J. Cancer Prev. 2017, 18, 339–345.
- Ivanov, N.I.; Cowell, S.P.; Brown, P.; Rennie, P.S.; Guns, E.S.; Cox, M.E. Lycopene Differentially Induces Quiescence and Apoptosis in Androgen-Responsive and -Independent Prostate Cancer Cell Lines. Clin. Nutr. 2007, 26, 252–263.
- Assar, E.A.; Vidalle, M.C.; Chopra, M.; Hafizi, S. Lycopene Acts through Inhibition of IκB Kinase to Suppress NF-ΚB Signaling in Human Prostate and Breast Cancer Cells. Tumor Biol. 2016, 37, 9375–9385.
- Palozza, P.; Sestito, R.; Picci, N.; Lanza, P.; Monego, G.; Ranelletti, F.O. The Sensitivity to β-Carotene Growth-Inhibitory and Proapoptotic Effects Is Regulated by Caveolin-1 Expression in Human Colon and Prostate Cancer Cells. Carcinogenesis 2008, 29, 2153–2161.
- Yang, C.M.; Yen, Y.T.; Huang, C.S.; Hu, M.L. Growth Inhibitory Efficacy of Lycopene and β-Carotene against Androgen-Independent Prostate Tumor Cells Xenografted in Nude Mice. Mol. Nutr. Food Res. 2011, 55, 606–612.
- Elgass, S.; Cooper, A.; Chopra, M. Lycopene Treatment of Prostate Cancer Cell Lines Inhibits Adhesion and Migration Properties of the Cells. Int. J. Med. Sci. 2014, 11, 948–954.
- Kolberg, M.; Pedersen, S.; Bastani, N.E.; Carlsen, H.; Blomhoff, R.; Paur, I. Tomato Paste Alters NF-B and Cancer-Related MRNA Expression in Prostate Cancer Cells, Xenografts, and Xenograft Microenvironment. Nutr. Cancer 2015, 67, 305–315.
- van Blarigan, E.L.; Kenfield, S.A.; Yang, M.; Sesso, H.D.; Ma, J.; Stampfer, M.J.; Chan, J.M.; Chavarro, J.E. Fat Intake after Prostate Cancer Diagnosis and Mortality in the Physicians’ Health Study. Cancer Causes Control 2015, 26, 1117–1126.
- Labbé, D.P.; Zadra, G.; Yang, M.; Reyes, J.M.; Lin, C.Y.; Cacciatore, S.; Ebot, E.M.; Creech, A.L.; Giunchi, F.; Fiorentino, M.; et al. High-Fat Diet Fuels Prostate Cancer Progression by Rewiring the Metabolome and Amplifying the MYC Program. Nat. Commun. 2019, 10, 4358.
- Ioannidou, A.; Watts, E.L.; Perez-Cornago, A.; Platz, E.A.; Mills, I.G.; Key, T.J.; Travis, R.C.; Tsilidis, K.K.; Zuber, V. The Relationship between Lipoprotein A and Other Lipids with Prostate Cancer Risk: A Multivariable Mendelian Randomisation Study. PLoS Med. 2022, 19, e1003859.
- Cacciatore, S.; Wium, M.; Licari, C.; Ajayi-Smith, A.; Masieri, L.; Anderson, C.; Salukazana, A.S.; Kaestner, L.; Carini, M.; Carbone, G.M.; et al. Inflammatory Metabolic Profile of South African Patients with Prostate Cancer. Cancer Metab. 2021, 9, 29.
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-Inflammatory Activity of β-Carotene, Lycopene and Tri-n-Butylborane, a Scavenger of Reactive Oxygen Species. Vivo 2018, 32, 255–264.
- Su, Q.; Rowley, K.G.; Itsiopoulos, C.; O’dea, K. ORIGINAL COMMUNICATION Identification and Quantitation of Major Carotenoids in Selected Components of the Mediterranean Diet: Green Leafy Vegetables, Figs and Olive Oil. Eur. J. Clin. Nutr. 2002, 56, 1149–1154.
- Kenfield, S.A.; Dupre, N.; Richman, E.L.; Stampfer, M.J.; Chan, J.M.; Giovannucci, E.L. Mediterranean Diet and Prostate Cancer Risk and Mortality in the Health Professionals Follow-up Study. Eur. Urol. 2014, 65, 887–894.
- van Hoang, D.; Pham, N.M.; Lee, A.H.; Tran, D.N.; Binns, C.W. Dietary Carotenoid Intakes and Prostate Cancer Risk: A Case-Control Study from Vietnam. Nutrients 2018, 10, 70.
- Antwi, S.O.; Steck, S.E.; Su, L.J.; Hebert, J.R.; Zhang, H.; Craft, N.E.; Fontham, E.T.H.; Smith, G.J.; Bensen, J.T.; Mohler, J.L.; et al. Carotenoid Intake and Adipose Tissue Carotenoid Levels in Relation to Prostate Cancer Aggressiveness among African-American and European-American Men in the North Carolina–Louisiana Prostate Cancer Project (PCaP). Prostate 2016, 76, 1053–1066.
- Zu, K.; Mucci, L.; Rosner, B.A.; Clinton, S.K.; Loda, M.; Stampfer, M.J.; Giovannucci, E. Dietary Lycopene, Angiogenesis, and Prostate Cancer: A Prospective Study in the Prostate-Specific Antigen Era. J. Natl. Cancer Inst. 2014, 106, djt430.
- Key, T.J.; Appleby, P.N.; Allen, N.E.; Travis, R.C.; Roddam, A.W.; Jenab, M.; Egevad, L.; Tjønneland, A.; Johnsen, N.F.; Overvad, K.; et al. Plasma Carotenoids, Retinol, and Tocopherols and the Risk of Prostate Cancer in the European Prospective Investigation into Cancer and Nutrition study. Am. J. Clin. Nutr. 2007, 86, 672–681.
- Giovannucci, E. Commentary: Serum Lycopene and Prostate Cancer Progression: A Re-Consideration of Findings from the Prostate Cancer Prevention Trial. Cancer Causes Control 2011, 22, 1055–1059.
- Chen, J.; Song, Y.; Zhang, L. Lycopene/Tomato Consumption and the Risk of Prostate Cancer: A Systemic Review and Meta-Analysis of Prospective Studies. J. Nutr. Sci. Vitaminol. (Tokyo) 2013, 59, 2013–2223.
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of Carotene and Lycopene on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0137427.
- Rowles, J.L.; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W. Increased Dietary and Circulating Lycopene Are Associated with Reduced Prostate Cancer Risk: A Systematic Review and Meta-Analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377.
- Cairns, P. Renal Cell Carcinoma. Cancer Biomark. 2011, 9, 461–473.
- Al-Bayati, O.; Hasan, A.; Pruthi, D.; Kaushik, D.; Liss, M.A. Systematic Review of Modifiable Risk Factors for Kidney Cancer. Urol. Oncol. Semin. Orig. Investig. 2019, 37, 359–371.
- Sahin, K.; Cross, B.; Sahin, N.; Ciccone, K.; Suleiman, S.; Osunkoya, A.O.; Master, V.; Harris, W.; Carthon, B.; Mohammad, R.; et al. Lycopene in the Prevention of Renal Cell Cancer in the TSC2 Mutant Eker Rat Model. Arch. Biochem. Biophys. 2015, 572, 36–39.
- Bock, C.H.; Ruterbusch, J.J.; Holowatyj, A.N.; Steck, S.E.; van Dyke, A.L.; Ho, W.J.; Cote, M.L.; Hofmann, J.N.; Davis, F.; Graubard, B.I.; et al. Renal Cell Carcinoma Risk Associated with Lower Intake of Micronutrients. Cancer Med. 2018, 7, 4087–4097.
- Hu, J.; la Vecchia, C.; Negri, E.; DesMeules, M.; Mery, L. Canadian Cancer Registries Epidemiology Research Group Dietary Vitamin C, E, and Carotenoid Intake and Risk of Renal Cell Carcinoma. Cancer Causes Control 2009, 20, 1451–1458.
- Yuan, J.-M.; Gago-Dominguez, M.; Castelao, J.E.; Hankin, J.H.; Ross, R.K.; Yu, M.C. Cruciferous Vegetables in Relation to Renal Cell Carcinoma.; Wiley-Liss, Inc.: New York, NY, USA, 1998; Volume 77.
- Brock, K.E.; Ke, L.; Gridley, G.; Chiu, B.C.H.; Ershow, A.G.; Lynch, C.F.; Graubard, B.I.; Cantor, K.P. Fruit, Vegetables, Fibre and Micronutrients and Risk of US Renal Cell Carcinoma. Br. J. Nutr. 2012, 108, 1077–1085.
- Bosetti, C.; Scotti, L.; Dal Maso, L.; Talamini, R.; Montella, M.; Negri, E.; Ramazzotti, V.; Franceschi, S.; la Vecchia, C. Micronutrients and the Risk of Renal Cell Cancer: A Case-Control Study from Italy. Int. J. Cancer 2007, 120, 892–896.
- Ho, W.J.; Simon, M.S.; Yildiz, V.O.; Shikany, J.M.; Kato, I.; Beebe-Dimmer, J.L.; Cetnar, J.P.; Bock, C.H. Antioxidant Micronutrients and the Risk of Renal Cell Carcinoma in the Women’s Health Initiative Cohort. Cancer 2015, 121, 580–588.
- Lee, J.E.; Giovannucci, E.; Smith-Warner, S.A.; Spiegelman, D.; Willett, W.C.; Curhan, G.C. Intakes of Fruits, Vegetables, Vitamins A, C, and E, and Carotenoids and Risk of Renal Cell Cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2445–2452.
- Bertoia, M.; Albanes, D.; Mayne, S.T.; Männistö, S.; Virtamo, J.; Wright, M.E. No Association between Fruit, Vegetables, Antioxidant Nutrients and Risk of Renal Cell Carcinoma. Int. J. Cancer 2010, 126, 1504–1512.
- Nicodemus, K.K.; Sweeney, C.; Folsom, A.R. Evaluation of Dietary, Medical and Lifestyle Risk Factors for Incident Kidney Cancer in Postmenopausal Women. Int. J. Cancer 2004, 108, 115–121.
- van Dijk, B.A.C.; Schouten, L.J.; Oosterwijk, E.; Hulsbergen-Van De Kaa, C.A.; Kiemeney, L.A.L.M.; Goldbohm, R.A.; Schalken, J.A.; van den Brandt, P.A. Carotenoid and Vitamin Intake, von Hippel-Lindau Gene Mutations and Sporadic Renal Cell Carcinoma. Cancer Causes Control 2008, 19, 125–134.
- Lee, J.E.; Männistö, S.; Spiegelman, D.; Hunter, D.J.; Bernstein, L.; van den Brandt, P.A.; Buring, J.E.; Cho, E.; English, D.R.; Flood, A.; et al. Intakes of Fruit, Vegetables, and Carotenoids and Renal Cell Cancer Risk: A Pooled Analysis of 13 Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1730–1739.
- Zhang, S.; Jia, Z.; Yan, Z.; Yang, J. Consumption of Fruits and Vegetables and Risk of Renal Cell Carcinoma: A Meta-Analysis of Observational Studies. Oncotarget 2017, 8, 27892.




