
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Adriano Carotti | -- | 1706 | 2022-04-11 18:14:26 | | | |
| 2 | Catherine Yang | + 4 word(s) | 1710 | 2022-04-12 03:31:45 | | |
Video Upload Options
Pulmonary atresia with ventricular septal defect (PA/VSD) is the extreme form of Fallot's tetralogy in which pulmonary blood flow is ensured from systemic blood flow sources. In the presence of Major Aorto-Pulmonary Collateral Arteries (MAPCAs), the disease assumes the greatest complexity due to the variable pulmonary perfusion patterns, of which MAPCAs are an important, although not the only source. True pulmonary arteries may have varying degrees of hypoplasia, be absent and, more rarely, discontinuous, with unilateral pulmonary perfusion provided by the arterial duct and contralateral by MAPCAs. The variability of the pulmonary perfusion pattern is a determining factor in the complexity of patients with PA/VSD/MAPCAs and the consequent diversity of their surgical management.
1. Preoperative Imaging
1.1. Computed Tomography
1.2. Cardiac Catheterization
Cardiac catheterization is an essential step in the preoperative evaluation of patients with PA/VSD/MAPCAs and is usually performed in the vicinity of surgery. The purpose of cardiac catheterization is to identify the sources of pulmonary blood flow; the presence, size, and confluence of the central pulmonary arteries and their branches in the lungs; areas of stenosis in the lobar or segmental branches; and the origin and course of the MAPCAs and their connections to the true pulmonary arteries. In particular it should be emphasized the key role of the pulmonary vein wedge injection for the demonstration of native pulmonary arteries not detectabled with other methods and its consequent central role in the surgical planning of this peculiar disease.
1.3. Magnetic Resonance Imaging
1.4. 3D Reconstructions of CT or CMR Images
- (1) Acquisition of CT images;
- (2) 3D image segmentation using dedicated 3D medical software;
- (3) Processing and editing of the virtual three-dimensional anatomical model.
2. Surgical Treatment
2.1. Rehabilitation
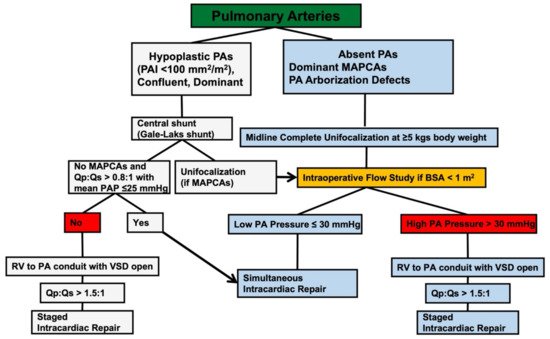
2.2. Unifocalization
2.3. The Role of the Intraoperative Flow-Study in Assessing the Feasibility of Concomitant VSD Closure
2.4. Criteria for Delayed VSD Closure
References
- Ryan, J.R.; Moe, T.G.; Richardson, R.; Frakes, D.H.; Nigro, J.J.; Pophal, S. A Novel Approach to Neonatal Management of Tetralogy of Fallot, With Pulmonary Atresia, and Multiple Aortopulmonary Collaterals. JACC Cardiovasc. Imaging 2015, 8, 103–104.
- Liu, J.; Li, H.; Liu, Z.; Wu, Q.; Xu, Y. Complete Preoperative Evaluation of Pulmonary Atresia with Ventricular Septal Defect with Multi-Detector Computed Tomography. PLoS ONE 2016, 11, e0146380.
- Secinaro, A.; Curione, D. Congenital heart disease in children. In Medical Radiology—Diagnostic Imaging; Springer: Berlin/Heidelberg, Germany, 2019; pp. 987–1009. ISSN1 2197-4187. ISSN2 0942-5373.
- Ciancarella, P.; Ciliberti, P.; Santangelo, T.P.; Secchi, F.; Stagnaro, N.; Secinaro, A. Noninvasive imaging of congenital cardiovascular defects. Radiol. Med. 2020, 125, 1167–1185.
- Secinaro, A.; Curione, D.; Mortensen, K.H.; Santangelo, T.P.; Ciancarella, P.; Napolitano, C.; Del Pasqua, A.; Taylor, A.M.; Ciliberti, P. Dual-source computed tomography coronary artery imaging in children. Pediatr. Radiol. 2019, 49, 1823–1839.
- Malayeri, A.A.; Spevak, P.J.; Zimmerman, S.L. Utility of a High-Resolution 3D MRI Sequence (3D-SPACE) for Evaluation of Congenital Heart Disease. Pediatr. Cardiol. 2015, 36, 1510–1514.
- Niu, J.; Profirovic, J.; Pan, H.; Vaiskunaite, R.; Voyno-Yasenetskaya, T. G protein βγ subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: Regulation of cell shape and reactive oxygen species production. Circ. Res. 2003, 93, 848–856.
- Schicchi, N.; Secinaro, A.; Muscogiuri, G.; Ciliberti, P.; Leonardi, B.; Santangelo, T.P.; Napolitano, C.; Agliata, G.; Basile, M.C.; Guidi, F.; et al. Multicenter review: Role of cardiovascular magnetic resonance in diagnostic evaluation, pre-procedural planning and follow-up for patients with congenital heart disease. Radiol. Med. 2015, 121, 342–351.
- Grosse-Wortmann, L.; Yoo, S.-J.; Van Arsdell, G.; Chetan, D.; Macdonald, C.; Benson, L.; Honjo, O. Preoperative total pulmonary blood flow predicts right ventricular pressure in patients early after complete repair of tetralogy of Fallot and pulmonary atresia with major aortopulmonary collateral arteries. J. Thorac. Cardiovasc. Surg. 2013, 146, 1185–1190.
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115.
- Byrne, N.; Forte, M.V.; Tandon, A.; Valverde, I.; Hussain, T. A systematic review of image segmentation methodology, used in the additive manufacture of patient-specific 3D printed models of the cardiovascular system. JRSM Cardiovasc. Dis. 2016, 5, 1691.
- Gates, R.N.; Laks, H.; Johnson, K. Side-to-Side Aorto–Gore-Tex Central Shunt. Ann. Thorac. Surg. 1998, 65, 515–516.
- Mumtaz, M.A.; Rosenthal, G.; Qureshi, A.; Prieto, L.; Preminger, T.; Lorber, R.; Latson, L.; Duncan, B.W. Melbourne Shunt Promotes Growth of Diminutive Central Pulmonary Arteries in Patients With Pulmonary Atresia, Ventricular Septal Defect, and Systemic-to-Pulmonary Collateral Arteries. Ann. Thorac. Surg. 2008, 85, 2079–2084.
- D’Udekem, Y.; Alphonso, N.; Nørgaard, M.A.; Cochrane, A.D.; Grigg, L.E.; Wilkinson, J.L.; Brizard, C.P. Pulmonary atresia with ventricular septal defects and major aortopulmonary collateral arteries: Unifocalization brings no long-term benefits. J. Thorac. Cardiovasc. Surg. 2005, 130, 1496–1502.
- Carotti, A.; Albanese, S.; Filippelli, S.; Ravà, L.; Guccione, P.; Pongiglione, G.; Di Donato, R.M. Determinants of outcome after surgical treatment of pulmonary atresia with ventricular septal defect and major aortopulmonary collateral arteries. J. Thorac. Cardiovasc. Surg. 2010, 140, 1092–1103.
- Reddy, V.; Liddicoat, J.R.; Hanley, F.L. Midline one-stage complete unifocalization and repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. J. Thorac. Cardiovasc. Surg. 1995, 109, 832–845.
- Mainwaring, R.D.; Patrick, W.L.; Roth, S.J.; Kamra, K.; Wise-Faberowski, L.; Palmon, M.; Hanley, F.L. Surgical algorithm and results for repair of pulmonary atresia with ventricular septal defect and major aortopulmonary collaterals. J. Thorac. Cardiovasc. Surg. 2018, 156, 1194–1204.
- Itatani, K.; Miyaji, K.; Nakahata, Y.; Ohara, K.; Takamoto, S.; Ishii, M. The lower limit of the pulmonary artery index for the extracardiac Fontan circulation. J. Thorac. Cardiovasc. Surg. 2011, 142, 127–135.
- Reddy, V.; Petrossian, E.; McElhinney, D.B.; Moore, P.; Teitel, D.F.; Hanley, F.L. One-stage complete unifocalization in infants: When should the ventricular septal defect be closed? J. Thorac. Cardiovasc. Surg. 1997, 113, 858–868.




