
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yanjie Dong | -- | 1238 | 2022-04-08 13:44:12 | | | |
| 2 | Rita Xu | + 3356 word(s) | 4594 | 2022-04-11 05:58:58 | | | | |
| 3 | Rita Xu | -3356 word(s) | 1238 | 2022-04-11 08:51:49 | | | | |
| 4 | Yanjie Dong | + 431 word(s) | 1669 | 2022-04-11 14:23:20 | | | | |
| 5 | Rita Xu | + 11 word(s) | 1680 | 2022-04-12 04:35:05 | | |
Video Upload Options
Muscle contraction by lateral movement of muscle fibers can create acoustic signals with a low frequency, and the signals that occur can be transmitted to the surface of the skin. After collection and filtration, these sound signals will be transferred into electric signals, which can be evaluated quantitatively for perioperative neuromuscular monitoring.
1. Introduction
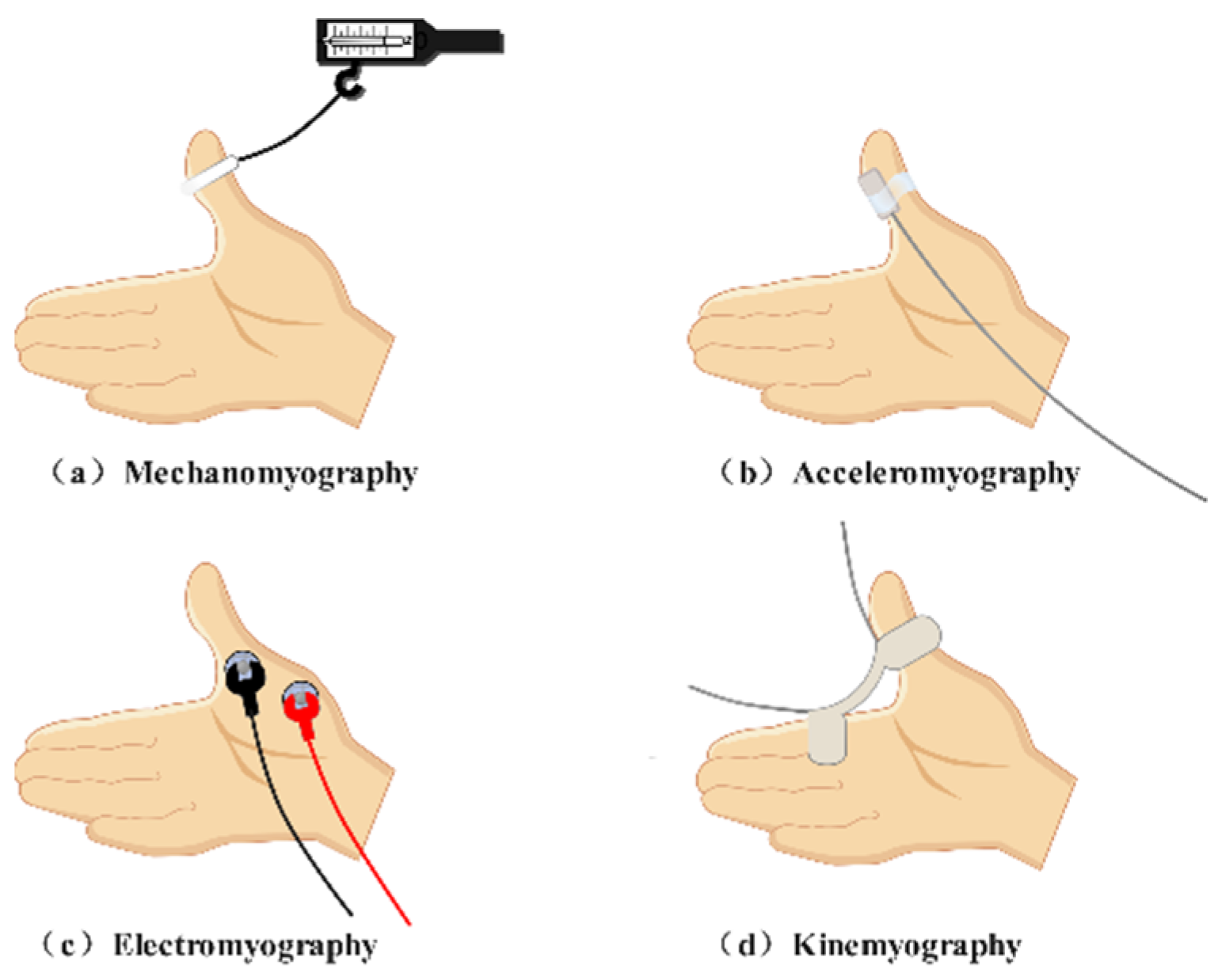
| Neuromuscular Monitoring Methods | Objects Detected | Recording Sites | Drawbacks |
|---|---|---|---|
| Acceleromyography | Acceleration | Adductor pollicis muscle Corrugator supercilii muscle … |
Miscalculation of the block degree Susceptible to outside interference Elaborate setup |
| Mechanomyography | Force | Adductor pollicis muscle | Harsh conditions for the hand posture Adductor pollicis muscle only |
| Electromyography | Compound action potentials | Adductor pollicis muscle Corrugator supercilii muscle Orbicularis oculi muscle Laryngeal muscle Diaphragm… |
Susceptible to electrical interference Not purely reflection of mechanical contraction of muscles |
| Kinemyography | Movement | Adductor pollicis muscle | Harsh conditions for the hand posture Adductor pollicis muscle only Not interchangeable with MMG |
| Author/Year | Sample Size | Sound Detector |
Control | Muscle | Muscle Relaxant |
Main Conclusion |
|---|---|---|---|---|---|---|
| Dascalu 1999 [21] | 25 | Air-coupled microphone | MMG EMG ACC |
Adductor pollicis muscle | Tubocurarine Atracurium Succinylcholine |
PMG could be used for perioperative neuromuscular monitoring |
| Bellemare 2000 [22] | 13 | Condenser microphone | MMG | Adductor pollicis muscle | Rocuronium | PMG was not an alternative method for neuromuscular monitoring at the adductor pollicis muscle when compared with MMG |
| Hemmerling 2002 [23] | 20 | Condenser microphone | ACC | Corrugator supercilii muscle | Mivacurium | PMG was not an alternative method for neuromuscular monitoring at the corrugator supercilii muscle when compared with ACC |
| Hemmerling 2002 [24] | 27 | Condenser microphone | ACC | Corrugator supercilii muscle | Mivacurium | The best recording site at the corrugator supercilii muscle for PMG is located between the anterior midline and the lateral part of the forehead, over the eyebrow |
| Hemmerling 2003 [25] | 28 | Condenser microphone | Cuff pressure method | Laryngeal adductor muscles | Mivacurium | PMG was an alternative method for neuromuscular monitoring at the laryngeal adductor muscles when compared with the cuff pressure method |
| Hemmerling 2004 [26] | 15 | Condenser microphone | Balloon pressure MMG | Corrugator supercilii muscle | Mivacurium | PMG was an alternative method for neuromuscular monitoring at the corrugator supercilii muscle when compared with Balloon pressure MMG |
| Hemmerling 2004 [27] | 12 | Condenser microphone | MMG | Hand muscles | Rocuronium | PMG was an alternative method for neuromuscular monitoring at hand muscles muscle when compared with MMG |
| Hemmerling 2004 [28] | 28 | Condenser microphone | MMG | Adductor pollicis muscle | Mivacurium | PMG was an alternative method for neuromuscular monitoring at the adductor pollicis muscle when compared with MMG |
| Hemmerling 2004 [29] | 12 | Condenser microphone | - | Posterior cricoarytenoid muscle /Lateral cricoarytenoid muscle |
Mivacurium | The acoustic signals created by the posterior cricoarytenoid muscle and the lateral cricoarytenoid muscle after the administration of muscle relaxants are different. |
| Deschamps 2005 [30] | 10 | Piezo-electric microphone | MMG | Corrugator supercilii muscle/The first dorsal interosseus muscle | - | An apparent staircase phenomenon was found at the first dorsal interosseus muscle and the adductor pollicis muscle while no obvious staircase phenomenon occured at the corrugator supercilii muscle. |
| Hemmerling 2005 [31] | 12 | Piezo-electric microphone | - | Lateral cricoarytenoid muscle /Strap muscles of the neck |
Mivacurium | PMG signals recorded were different outside and inside of the trachea for recovery time. |
| Michaud 2005 [32] |
15 | Piezo-electric microphone | - | Vastus medialis muscle Adductor pollicis |
Mivacurium | The vastus medialis muscle is an alternative recording site for PMG |
| Michaud 2005 [33] |
14 | Piezo-electric microphone | - | Adductor pollicis muscle | Mivacurium | Whether it is the dominant hand would not influence the rustles of PMG recording at the adductor pollicis muscle |
| Trager 2006 [18] |
14 | Piezo-electric microphone | MMG KMG |
Adductor pollicis muscle | Mivacurium | PMG was an alternative method for neuromuscular monitoring at the adductor pollicis muscle when compared with MMG or KMG |
| Hemmerling 2008 [34] | 28 | Piezo-electric microphone | - | Adductor pollicis muscle | Mivacurium | The potency of mivacurium is greater after a 20 min infusion of propofol compared with a 5 min infusion of propofol |
| Wehbe 2012 [35] |
1 | Piezo-electric microphone | - | Adductor pollicis /Corrugator supercilii muscle |
Not mentioned | “Relaxofon” may be a feasible neuromuscular monitoring device |
2. The development of PMG on perioperative neuromuscular monitoring
The history of PMG-based equipment can be roughly divided into two categories (Figure 2): (1). Theoretical derivation and early exploration; (2). Applied research and technique renovation.
Figure 2. Timeline for the progression of PMG on perioperative neuromuscular monitoring.
3. The merits, demerits and challenges of PMG-based equipment on perioperative neuromuscular monitoring
The properties of PMG in perioperative neuromuscular monitoring are as follows: (1) PMG-based equipment is easy to install and apply; (2) PMG-based equipment may present a stable signal that is barely affected by outside factors owing to its low frequency quality; and (3) PMG-based equipment is able to assess neuromuscular monitoring at the adductor pollicis muscle, corrugator supercilii muscle, vastus medialis muscle, first dorsal interosseus muscle, hypothenar muscles, and laryngeal muscles.
The short board of the PMG is equally obvious. On the one hand, the same target muscle of different individuals may create dissimilar muscle sounds. This can be explained by different muscle qualities, internal environments of muscle cells, and firing rates of motor units in different patients. On the other hand, although high-frequency noises have been filtered, some coexisting low-frequency noises, such as heart sounds, breath sounds, and vascular sounds, may still be disturbances.
As the form of PMG-based equipment is still presently on the phase of the assembled prototype, problems related to signal gathering, analysis and display remain to be solved. First, the improvement of sound detectors is the main focus. Recently, microphones with cylindrical and conical acoustic chambers have been developed. This implies that the air-coupled microphones will soon return to the stage center. In addition, the most advanced microphones in previous studies are piezoelectric microphones and condenser microphones with a diameter of 1.6 cm. There is no question that current technology would give birth to more sophisticated sound detectors that are more suitable for gathering sound signals from other small muscles. It is worth noting that some delicate microphones with air chambers have been utilized in the study of prosthesis control [36]. Second, the data presented in previous studies are just filtered raw data. The method of data analysis is an unavoidable topic during the process of productization of PMG-based equipment. Information mining beyond amplitude from both the time and frequency domains of PMG is still a tricky problem. Researchers believe artificial intelligence will be a potential choice. Third, as calibration endows ACC-based equipment with individualized monitoring experience, PMG-based equipment may also need a calibration technique because of different muscle qualities and muscle masses in different individuals. Finally, only hand muscles, the corrugator supercilii muscle, the vastus medialis muscle, and the laryngeal muscles are not sufficient for the increasing number of surgical approaches. It is urgent to determine more alternative recording sites, such as the trapezius muscle, when the prone position is performed during nephrectomy or spinal surgery.
References
- Bowman, W.C. Neuromuscular Block. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S277–S286.
- Larijani, G.E.; Gratz, I.; Silverberg, M.; Jacobi, A.G. Clinical Pharmacology of the Neuromuscular Blocking Agents. DICP 1991, 25, 54–64.
- Shakhnovich, V.; Smith, P.B.; Guptill, J.T.; James, L.P.; Collier, D.N.; Wu, H.; Livingston, C.E.; Zhao, J.; Kearns, G.L.; Benjamin, D.K.; et al. Obese Children Require Lower Doses of Pantoprazole Than Nonobese Peers to Achieve Equal Systemic Drug Exposures. J. Pediatr. 2018, 193, 102–108.e1.
- Chau, I.; Horn, K.; Dullenkopf, A. Neuromuscular monitoring during modified rapid sequence induction: A comparison of TOF-Cuff® and TOF-Scan®. Australas. Emerg. Care 2020, 23, 217–220.
- Zhang, X.-F.; Li, D.-Y.; Wu, J.-X.; Jiang, Q.-L.; Zhu, H.-W.; Xu, M.-Y. Comparison of deep or moderate neuromuscular blockade for thoracoscopic lobectomy: A randomized controlled trial. BMC Anesthesiol. 2018, 18, 195.
- Hunter, J. Reversal of residual neuromuscular block: Complications associated with perioperative management of muscle relaxation. Br. J. Anaesth. 2017, 119, i53–i62.
- Batistaki, C.; Vagdatli, K.; Tsiotou, A.; Papaioannou, A.; Pandazi, A.; Matsota, P. A multicenter survey on the use of neuromuscular blockade in Greece. Does the real-world clinical practice indicate the necessity of guidelines? J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 202–214.
- Klein, A.A.; Meek, T.; Allcock, E.; Cook, T.M.; Mincher, N.; Morris, C.; Nimmo, A.F.; Pandit, J.J.; Pawa, A.; Rodney, G.; et al. Recommendations for Standards of Monitoring During Anaesthesia and Recovery 2021: Guideline from the Association of Anaesthetists. Anaesthesia 2021, 76, 1212–1223.
- Checketts, M.R.; Alladi, R.; Ferguson, K.; Gemmell, L.; Handy, J.M.; Klein, A.; Love, N.J.; Misra, U.K.; Morris, C.; Nathanson, M.H.; et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2015, 71, 85–93.
- Söderström, C.M.; Eskildsen, K.Z.; Gätke, M.R.; Staehr-Rye, A.K. Objective Neuromuscular Monitoring of Neuromuscular Blockade in Denmark: An Online-Based Survey of Current Practice. Acta Anaesthesiol. Scand. 2017, 61, 619–626.
- Hemmerling, T.M.; Le, N. Brief review: Neuromuscular monitoring: An update for the clinician. Can. J. Anaesth. 2007, 54, 58–72.
- Murphy, G.S. Neuromuscular Monitoring in the Perioperative Period. Anesth. Analg. 2018, 126, 464–468.
- Naguib, M.; Brull, S.; Johnson, K.B. Conceptual and technical insights into the basis of neuromuscular monitoring. Anesthesia 2017, 72, 16–37.
- Duţu, M.; Ivaşcu, R.; Tudorache, O.; Morlova, D.; Stanca, A.; Negoiţă, S.; Corneci, D. Neuromuscular monitoring: An update. Rom. J. Anaesth. Intensiv. Care 2018, 25, 55–60.
- Dahaba, A.A.; Suljevic, I.; Xiao, Z.Y.; Wang, K. Mindray 3-directional NMT Module (a new generation “Tri-axial” neuromuscular monitor) versus the Relaxometer mechanomyograph and versus the TOF-Watch SX acceleromyograph. Int. J. Clin. Monit. Comput. 2018, 33, 853–862.
- Colegrave, N.; Billard, V.; Motamed, C.; Bourgain, J.L. Comparison of the Tof-Scan™ Acceleromyograph to Tof-Watch Sx™: Influence of Calibration. Anaesth. Crit. Care Pain Med. 2016, 35, 223–227.
- Bowdle, A.; Jelacic, S. Progress towards a standard of quantitative twitch monitoring. Anesthesia 2020, 75, 1133–1135.
- Trager, G.; Michaud, G.; Deschamps, S.; Hemmerling, T.M. Comparison of phonomyography, kinemyography and mechanomyography for neuromuscular monitoring. Can. J. Anaesth. 2006, 53, 130–135.
- Lee, W. The latest trend in neuromuscular monitoring: Return of the electromyography. Anesth. Pain Med. 2021, 16, 133–137.
- Fuchs-Buder, T.; Claudius, C.; Skovgaard, L.T.; Eriksson, L.I.; Mirakhur, R.K.; Viby-Mogensen, J. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: The Stockholm revision. Acta Anaesthesiol. Scand. 2007, 51, 789–808.
- Dascalu, A.; Geller, E.; Moalem, Y.; Manoah, M.; Enav, S.; Rudick, Z. Acoustic monitoring of intraoperative neuromuscular block. Br. J. Anaesth. 1999, 83, 405–409.
- Bellemare, F.; Couture, J.; Donati, F.; Plaud, B. Temporal Relation between Acoustic and Force Responses at the Adductor Pollicis during Nondepolarizing Neuromuscular Block. Anesthesiology 2000, 93, 646–652.
- Hemmerling, T.M.; Donati, F.; Babin, D.; Beaulieu, P. Duration of control stimulation does not affect onset and offset of neuromuscular blockade at the corrugator supercilii muscle measured with phonomyography or acceleromyography. Can. J. Anaesth. 2002, 49, 913–917.
- Hemmerling, T.; Donati, F.; Beaulieu, P.; Babin, D. Phonomyography of the corrugator supercilii muscle: Signal characteristics, best recording site and comparison with acceleromyography. Br. J. Anaesth. 2002, 88, 389–393.
- Hemmerling, T.M.; Babin, D.; Donati, F. Phonomyography as a Novel Method to Determine Neuromuscular Blockade at the Laryngeal Adductor Muscles: Comparison with the Cuff Pressure Method. Anesthesiology 2003, 98, 359–363.
- Hemmerling, T.M.; Michaud, G.; Babin, D.; Trager, G.; Donati, F. Comparison of phonomyography with balloon pressure mechanomyography to measure contractile force at the corrugator supercilii muscle. Can. J. Anaesth. 2004, 51, 116–121.
- Hemmerling, T.M.; Michaud, G.; Trager, G.; Deschamps, S. Phonomyographic measurements of neuromuscular blockade are similar to mechanomyography for hand muscles. Can. J. Anaesth. 2004, 51, 795–800.
- Hemmerling, T.M.; Michaud, G.; Trager, G.; Deschamps, S.; Babin, D.; Donati, F. Phonomyography and Mechanomyography Can Be Used Interchangeably to Measure Neuromuscular Block at the Adductor Pollicis Muscle. Anesth. Analg. 2004, 99, 377–381.
- Hemmerling, T.M.; Michaud, G.; Trager, G.; Donati, F. Simultaneous Determination of Neuromuscular Blockade at the Adducting and Abducting Laryngeal Muscles Using Phonomyography. Anesth. Analg. 2004, 98, 1729–1733.
- Deschamps, S.; Trager, G.; Mathieu, P.A.; Hemmerling, T.M. The staircase phenomenon at the corrugator supercilii muscle in comparison with the hand muscles. Br. J. Anaesth. 2005, 95, 372–376.
- Hemmerling, T.M.; Michaud, G.; Deschamps, S.; Trager, G. An External Monitoring Site at the Neck Cannot Be Used to Measure Neuromuscular Blockade of the Larynx. Anesth. Analg. 2005, 100, 1718–1722.
- Michaud, G.; Trager, G.; Deschamps, S.; Hemmerling, T.M. Monitoring neuromuscular blockade at the vastus medialis muscle using phonomyography. Can. J. Anaesth. 2005, 52, 795–800.
- Michaud, G.; Trager, G.; Deschamps, S.; Hemmerling, T.M. Dominance of the Hand Does Not Change the Phonomyographic Measurement of Neuromuscular Block at the Adductor Pollicis Muscle. Anesth. Analg. 2005, 100, 718–721.
- Hemmerling, T.M.; Le, N.; Decarie, P.; Cousineau, J.; Bracco, D. Total intravenous anesthesia with propofol augments the potency of mivacurium. Can. J. Anaesth. 2008, 55, 351–357.
- Wehbe, M.; Mathieu, P.A.; Hemmerling, T.M. Relaxofon: A neuromuscular blockade monitor for patients under general anesthesia. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 150–153.
- Posatskiy, A.; Chau, T. The effects of motion artifact on mechanomyography: A comparative study of microphones and accelerometers. J. Electromyogr. Kinesiol. 2012, 22, 320–324.




