Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mrudula Patel | -- | 1410 | 2022-04-08 10:30:57 | | | |
| 2 | Jessie Wu | Meta information modification | 1410 | 2022-04-08 11:14:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Patel, M. Oral Cavity and Candida albicans. Encyclopedia. Available online: https://encyclopedia.pub/entry/21504 (accessed on 07 February 2026).
Patel M. Oral Cavity and Candida albicans. Encyclopedia. Available at: https://encyclopedia.pub/entry/21504. Accessed February 07, 2026.
Patel, Mrudula. "Oral Cavity and Candida albicans" Encyclopedia, https://encyclopedia.pub/entry/21504 (accessed February 07, 2026).
Patel, M. (2022, April 08). Oral Cavity and Candida albicans. In Encyclopedia. https://encyclopedia.pub/entry/21504
Patel, Mrudula. "Oral Cavity and Candida albicans." Encyclopedia. Web. 08 April, 2022.
Copy Citation
Candida colonisation of the oral cavity increases in immunocompromised individuals which leads to the development of oral candidiasis. In addition, host factors such as xerostomia, smoking, oral prostheses, dental caries, diabetes and cancer treatment accelerate the disease process. Candida albicans is the primary causative agent of this infection, owing to its ability to form biofilm and hyphae and to produce hydrolytic enzymes and candialysin.
candida
Candida albicans
oral cavity
1. Role of Saliva and Mucosa
Saliva plays a major role in the preservation of oral health. It lubricates the oral cavity and through constant flow and swallowing, mechanically removes excess bacteria and food debris. Therefore, as a first defence, the quantity of saliva is important. Individuals with xerostomia generally have altered microbial community. Torres et al. (2002) found high Candidacounts and multiple Candida species in subjects with xerostomia [1]. Patients with head and neck cancers going through radiation therapy often have hyposalivation and imbalance in oral flora increasing the rate of Candida colonisation and infection [2][3][4]. A low salivary flow rate causes intense oral cavity colonisation by C. albicans and other yeasts [5][6].
Mucin present in saliva forms part of the protective coating along the oral mucosal surface, thereby preventing adherence of commensals and pathogens, including Candida. In addition, negatively charged epithelial cells cause repulsion of Candida cells, preventing adherence. Nevertheless, these adverse effects on adherence are normally oppressed by other stronger adherence mechanisms responsible for the colonisation and development of infection [7].
Saliva also contains complex host molecules, which are both harmful and beneficial to the microflora. Proteins and glycoproteins provide primary nutrients to the oral flora. Once established, the consortia of microflora produce enzymes that can break down salivary molecules and free up required nutrients, carbohydrates and amino acids [8]. For example, C. albicans can grow in human saliva without the addition of glucose, and in stationary phase, it can survive for more than 400 h [9]. Saliva contains several ions including sodium, potassium, cadmium, chloride, bicarbonate and phosphate. These ions are usually responsible for the buffering properties of saliva. In addition, it contains mucin, proteins and glycoproteins which provide nutrients to the microorganisms, facilitate their adhesion to the oral surfaces, cause aggregation of microorganisms for clearing from the oral cavity and, to some extent, inhibit the growth of some exogenous microorganisms.
Certain components present in saliva apply selective pressure on the resident microbiota controlling growth and survival. Mucin, fibronectin, proline-rich-protein and secretary IgA actually cause agglutination, binding microorganisms, whereas statherin, histatins, α and β defensins, lactoperoxidase, lactoferrin and lysozymes have antimicrobial activity. Among these salivary antimicrobials, only histatins and defensins proved to have antifungal activity [10]. Statherin is known to reverse the morphological state of C. albicans from hyphae, a pathogenic state to a blastospore state, which can alter the course of infection [11]. Histatin has well-known in vitro antifungal activity [12][13] and its protective property in keeping the counts of Candida low in the oral cavity has been documented [14][15]. For example, reduced salivary flow and anticandidal activity in HIV-infected individuals contributed to them having an increased incidence of oral Candida infections [16]. Defensins are a group of broad-spectrum antimicrobial peptides, produced at mucosal level as components of the innate immunity. Defensins are able to recognize the fungal cell wall and disrupt it through membrane permeabilization. They are cysteine-rich peptides of two families, the α-defensins and β-defensins, which are produced by neutrophils and epithelial cells, respectively [17]. However, the complete mechanism of action of defensins is unknown [18]. Of four β-defensins, hBD-2 and hBD-3 are strongly induced in response to infection, particularly by C. albicans hyphae [19]. Defensins also act as chemoattractants for dendritic cells, neutrophils and T cells [20]. LL-37 cathelicidin is an antimicrobial peptide produced by the epithelial cells of the oral cavity and it is known to interact with the C. albicans cell wall components such as chitin, glucan and mannan, causing inhibition of C. albicans adherence to epithelial cells [21].
Intact epithelial cells, although considered as a physical barrier preventing infections, also play an active role in the initial immune response to pathogens. They are responsible for the release of inflammatory mediators, including inflammatory cells and proinflammatory cytokines, e.g., IL-1α/β, IL-6 and TNF-α as well as antimicrobial factors. In addition to the release of cytokines, they produce four classes of small antimicrobial peptides, such as small anionic peptides, which require zinc as a cofactor, defensins, linear cationic peptides rich in proline or tryptophan and small α-helical cationic peptides, which lack cysteines [22]. This protective effect of IL-1β in invasive C. albicans infection has been demonstrated [23]. Candida albicans pathogenicity and the host response at the mucosal surface are summarised in Figure 1 and Figure 2, respectively [24].
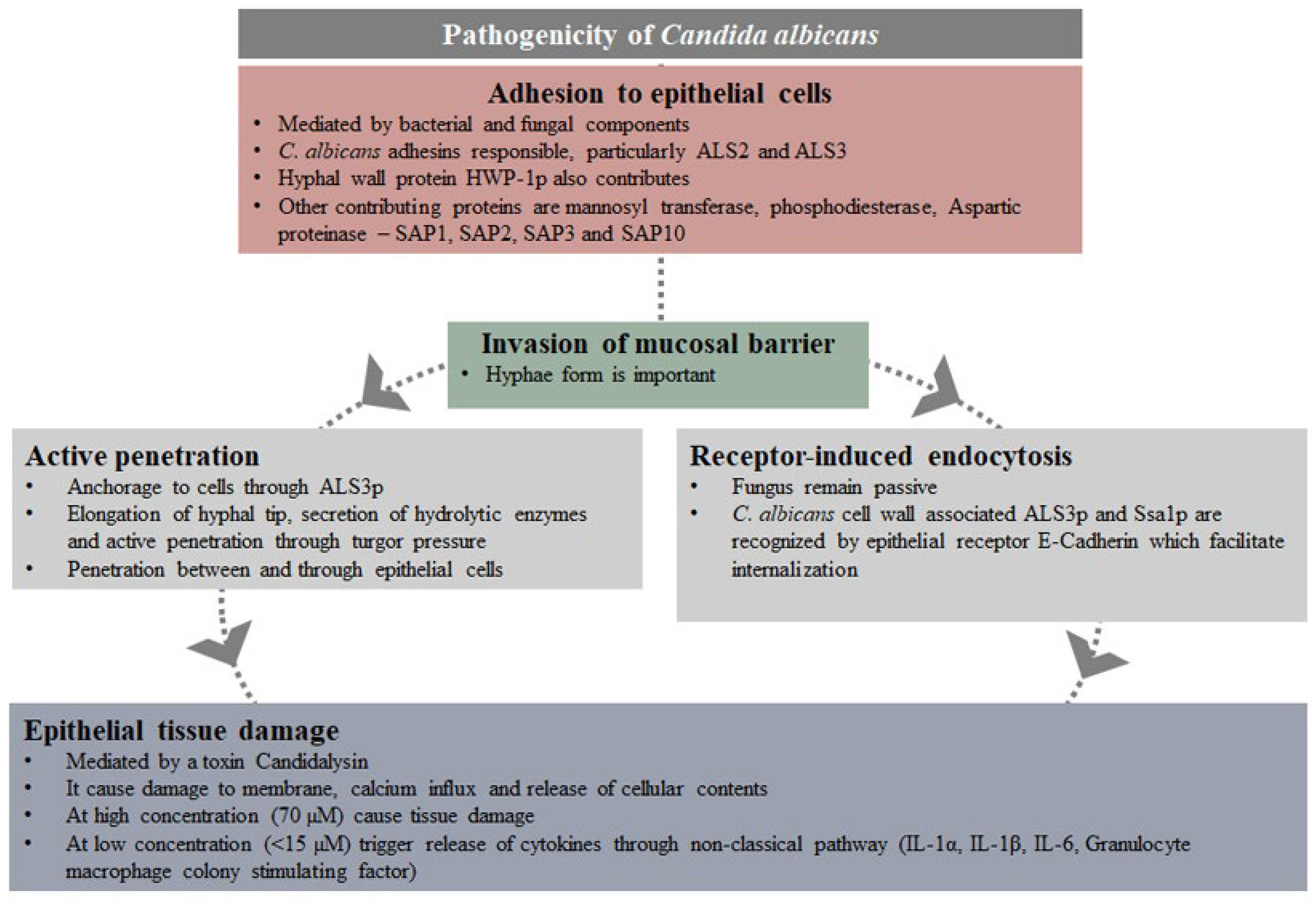
Figure 1. Pathogenicity of Candida albicans.
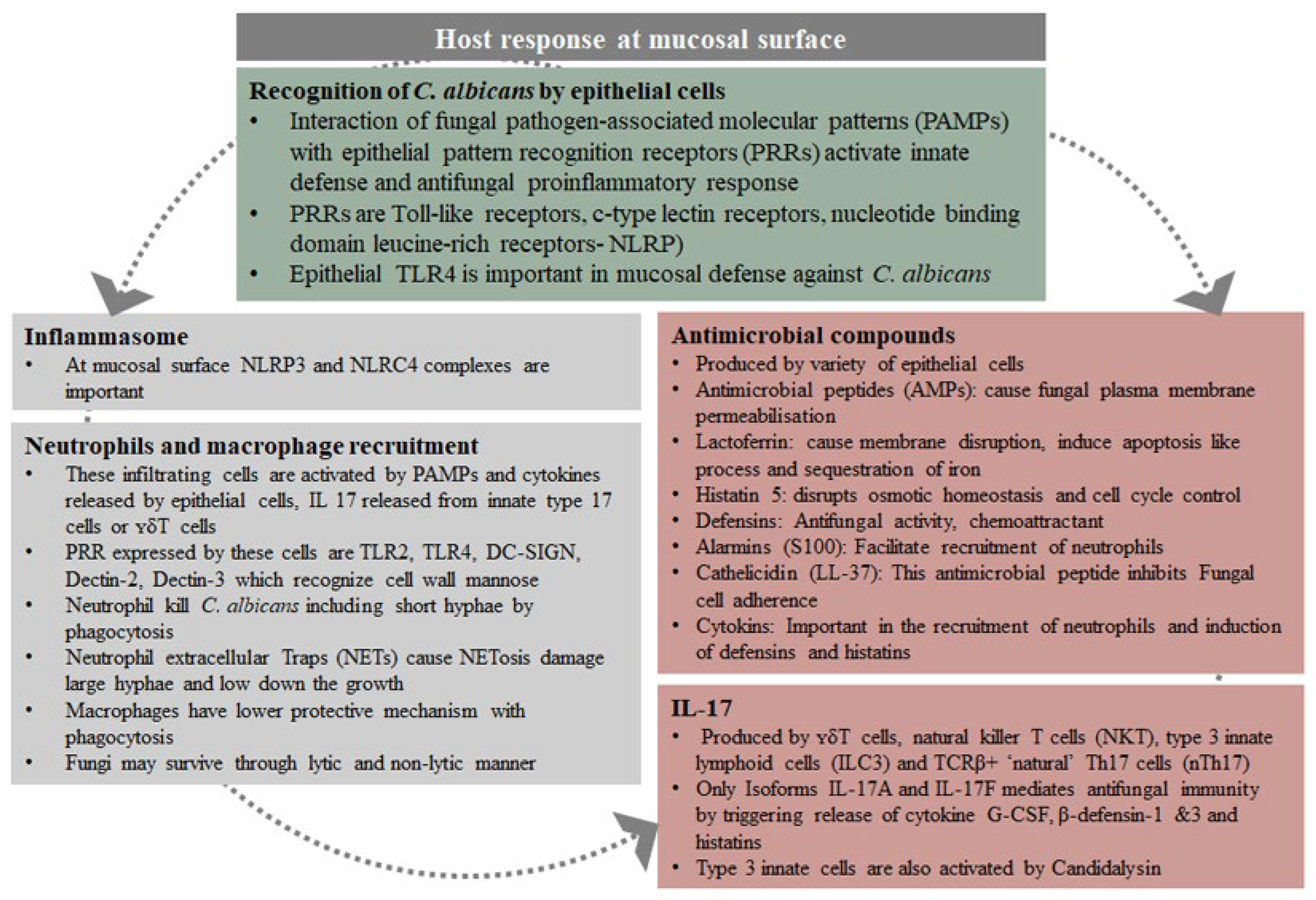
Figure 2. Host response at the mucosal surfaces in Candida albicans infections (Summarised from Richardson et al., 2019) [24].
2. Prevention and Treatment
Many systemic and topical preparations containing antifungal agents such as fluconazole, amphotericin B, miconazole, nystatin and clotrimazole are available for the treatment of oral candidiasis. Treatment is relatively easy, apart from the side effects, threat of over-use and development of resistance. Considering the fact that in the oral cavity C. albicans is carried by a large population of normal healthy individuals and even more by immunocompromised patients, prevention should be the most important approach, rather than treatment. Alternative treatment, such as proven home remedies and phytochemicals, should be considered [25]. In the oral cavity, whatever therapeutic product is used, it is difficult to maintain the therapeutic concentration due to the constant flow of saliva. Therefore, it is important that the subtherapeutic concentrations have some effect on the virulence of the surviving Candida cells, providing additional benefit. The primary objective must be to maintain low Candida counts and reduce their virulence. Moreover, the additional host factors such as high carbohydrate intake, dental caries and hyposalivation should be identified as they influence the adherence, growth and virulence of Candida, and corrective measures should be implemented (Figure 3). This approach is patient-centred and therefore, for compliance, patient education is very important. These lifestyle adjustments can keep the Candida counts in check, and some of the measures may maintain a low level of virulence.
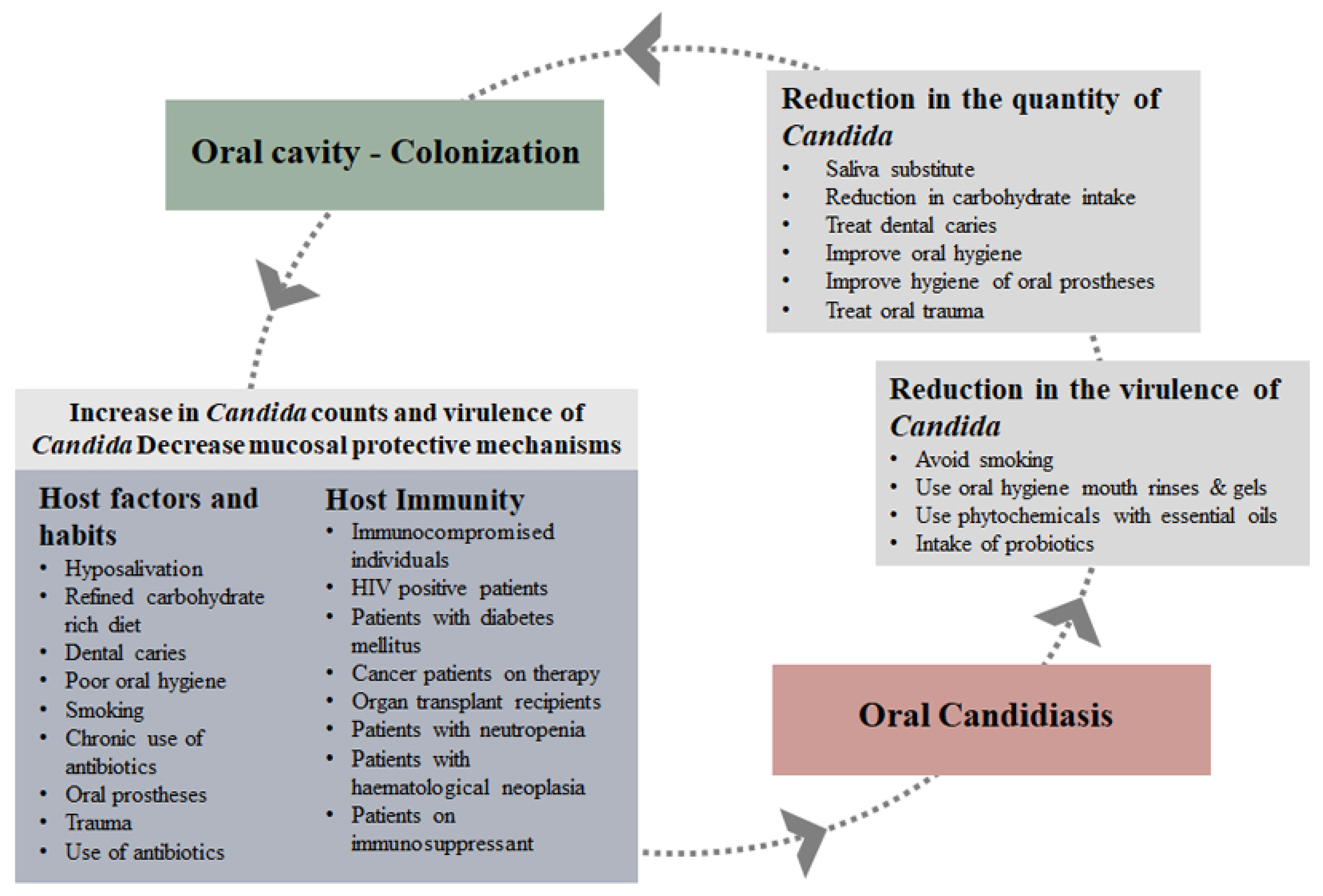
Figure 3. Prevention of development of oral candidiasis.
In the oral cavity C. albicans can be associated with the biofilm formed by oral bacteria; therefore, the removal of biofilm and oral hygiene are important. Mechanical removal of biofilm from hard and soft tissues, using toothpastes, and the use of antimicrobial mouthrinses can keep the numbers low. Although the chlorhexidine gluconate mouthrinses are considered a gold standard for the oral cavity, they cannot be used on a long-term basis and have side effects. A daily-use mouthrinse containing fluoride and triclosan have shown to reduce C. albicans counts by 77% in HIV-positive patients [26]. Mouthrinses containing cetylpyridinium chloride, menthol, eucalyptol and iodine have antifungal activities and can be used to control Candida in the oral cavity [27]. They are not very expensive and have no side effects. Patients with HIV, organ transplants, diabetes mellitus and any immunocompromised individuals should be educated and advised regarding the importance of good oral hygiene. In cancer patients, the few weeks before, after, and during radiation and/or chemotherapy are a crucial time, and it is important to keep the Candida counts low. Similarly oral and denture hygiene in denture wearers is important. They should be educated regarding oral and denture hygiene, and night-time removal of dentures.
Consumption of refined sugars should be avoided because Candida-associated oral Streptococcus mutans ferment sugars, resulting in the production of acids and extracellular polysaccharides, which facilitate adherence and growth, and enhance hyphae and hydrolytic enzyme production in Candida. In addition, sugars will also support the growth of Candida [28].
Probiotics, for their efficacy towards oral Candida and their virulence, have also been studied [29]. The mode of action of probiotics is inhibition through competition for adhesion, secretary metabolites and stimulation of the immune system of the host.
Many medicinal plant-derived compounds and essential oils have been studied. In vitro and in vivo studies have established the antifungal efficacy of essential oils and their effect on the virulence properties of Candida. Some of these plants are Cinnamomum zeylanicum, Coriandrum sativum and Cymbopogon nardus [30][31][32]. Dodoneae viscosa var. angustifolia (DVA), a medicinal plant that is traditionally used for oral thrush, has also been extensively studied. At high concentrations, DVA has shown antifungal activity against C. albicans isolated from HIV-positive and HIV-negative patients, and at subinhibitory concentrations, it inhibits the virulence properties, such as adherence to epithelial cells and, hyphae and biofilm formation [33]. In addition, DVA-derived flavone also inhibits biofilm formation and acid production by cariogenic bacteria S. mutans. This means that DVA would reduce the Candida counts and improve the oral hygiene, which would further decrease the Candida counts [34]. Nevertheless, beneficial plant-derived compounds can be further explored for their cytotoxic effects and in vivo efficacy and developed into mouthrinses or oral gels [35][36]. In clinical trials, drug formulas prepared from essential oils derived from Pelargonium graveole and Melaleuca alternifolia have shown good efficacy in denture wearers and HIV patients [37][38][39][40].
References
- Torres, S.R.; Peixoto, C.B.; Caldas, D.M.; Silva, E.B.; Akiti, T.; Nucci, M.; de Uzeda, M. Relationship between salivary flow rates and Candida counts in subjects with xerostomia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2002, 93, 149–154.
- Almståhl, A.; Finizia, C.; Carlén, A.; Fagerberg-Mohlin, B.; Alstad, T. Mucosal microflora in head and neck cancer patients. Int. J. Dent. Hyg. 2018, 16, 459–466.
- Karbach, J.; Walter, C.; Al-Nawas, B. Evaluation of saliva flow rates, Candida colonization and susceptibility of Candida strains after head and neck radiation. Clin. Oral Investig. 2012, 16, 1305–1312.
- Tarapan, S.; Matangkasombut, O.; Trachootham, D.; Sattabanasuk, V.; Talungchit, S.; Paemuang, W.; Phonyiam, T.; Chokchaitam, O.; Mungkung, O.O.; Lam-Ubol, A. Oral Candida colonization in xerostomic postradiotherapy head and neck cancer patients. Oral Dis. 2019, 25, 1798–1808.
- Torres, S.R.; Peixoto, C.B.; Caldas, D.M.; Silva, E.B.; Magalhães, F.A.C.; Uzeda, M.; Nucci, M. Clinical aspects of Candida species carriage in saliva of xerotomic subjects. Med. Mycol. 2003, 41, 411–415.
- Buranarom, N.; Komin, O.; Matangkasombut, O. Hyposalivation, Oral health, and Candida colonization in independent dentate elders. PLoS ONE 2020, 15, e0242832.
- Nikou, S.A.; Kichik, N.; Brown, R.; Ponde, N.O.; Ho, J.; Naglik, J.R.; Richardson, J.P. Candidaalbicans interactions with mucosal surfaces during health and disease. Pathogens 2019, 8, 53.
- Marsh, P.D.; Do, T.; Beighton, D.; Devine, D.A. Influence of saliva on the oral microbiota. Periodontology 2000 2016, 70, 80–92.
- Valentijn-Benz, M.; Nazmi, K.; Brand, H.S.; Hof, W.V.; Veerman, E.C.I. Growth of Candida albicans in human saliva is supported by low-molecular-mass compounds. FEMS Yeast Res. 2015, 15, fov088.
- Abiko, Y.; Saitoh, M.; Nishimura, M.; Yamazaki, M.; Sawamura, D.; Kaku, T. Role of beta-defensins in oral epithelial health and disease. Med. Mol. Morphol. 2007, 40, 179–184.
- Leito, J.T.D.; Ligtenberg, A.J.M.; Nazmi, K.; Veerman, E.C.I. Identification of salivary components that induce transition of hyphae to yeast in Candida albicans. FEMS Yeast Res. 2009, 9, 1102–1110.
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477.
- Xu, T.; Levitz, S.M.; Diamond, R.D.; Oppenheim, F.G. Anticandidal activity of major human salivary histatins. Infect. Immun. 1991, 59, 2549–2554.
- Jainkittivong, A.; Johnson, D.A.; Yeh, C.K. The relationship between salivary histatin levels and oral yeast carriage. Oral Microbiol. Immunol. 1998, 13, 181–187.
- Sugimoto, J.; Kanehira, T.; Mizugai, H.; Chiba, I.; Morita, M. Relationship between salivary histatin 5 levels and Candida CFU counts in healthy elderly. Gerodontology 2006, 23, 164–169.
- Lin, A.L.; Johnson, D.A.; Patterson, T.F.; Wu, Y.; Lu, D.L.; Shi, Q.; Yeh, C.-K. Salivary anticandidal activity and saliva composition in an HIV-infected cohort. Oral Microbiol. Immunol. 2001, 16, 280–288.
- Diamond, G.; Ryan, L. Beta-defensins: What are they really doing in the oral cavity? Oral Dis. 2011, 17, 628–635.
- Polesello, V.; Segat, L.; Crovella, S.; Zupin, L. Candida infections and human defensins. Protein Pept. Lett. 2017, 24, 747–756.
- Feng, Z.; Jiang, B.; Chandra, J.; Ghannoum, M.; Nelson, S.; Weinberg, A. Human beta-defensins: Differential activity against candidal species and regulation by Candida albicans. J. Dent. Res. 2005, 84, 445–450.
- Vylkova, S.; Li, X.S.; Berner, J.C.; Edgerton, M. Distinct antifungal mechanisms: Beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob. Agents Chemother. 2006, 50, 324–331.
- Tsai, P.W.; Yang, C.Y.; Chang, H.T.; Lan, C.Y. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS ONE 2011, 6, e17755.
- Tomalka, J.; Azodi, E.; Narra, H.P.; Patel, K.; O’Neill, S.; Cardwell, C.; Hall, B.A.; Wilson, J.M.; Hise, A.G. β-Defensin 1 plays a role in acute mucosal defense against Candida albicans. J. Immunol. 2015, 194, 1788–1795.
- Hebecker, B.; Naglik, J.R.; Hube, B.; Jacobsen, I.D. Pathogenicity mechanisms and host response during oral Candida albicans infections. Expert Rev. Anti-Infect. Ther. 2014, 12, 867–879.
- Richardson, J.P.; Moyes, D.L.; Ho, J.; Naglik, J.R. Candida innate immunity at the mucosa. Semin. Cell Dev. Biol. 2019, 89, 58–70.
- Martins, N.; Ferreira, I.C.; Barros, L.; Silva, S.; Henriques, M. Candidiasis: Predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 2014, 177, 223–240.
- Patel, M.; Shackleton, J.A.; Coogan, M.M.; Galpin, J. Antifungal effect of mouth rinses on oral Candida counts and salivary flow in treatment-naïve HIV-infected patients. AIDS Patient Care STDS 2008, 22, 613–618.
- Ardizzoni, A.; Pericolini, E.; Paulone, S.; Orsi, C.F.; Castagnoli, A.; Oliva, I.; Strozzi, E.; Blasi, E. In vitro effects of commercial mouthwashes on several virulence traits of Candida albicans, viridans streptococci and Enterococcus faecalis colonizing the oral cavity. PLoS ONE 2018, 13, e0207262.
- Ellepola, K.; Truong, T.; Liu, Y.; Lin, Q.; Lim, T.K.; Lee, Y.M.; Cao, T.; Koo, H.; Seneviratne, C.J. Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans–Candida albicans mixed-species biofilms. Infect. Immun. 2019, 87, e00339-19.
- Ribeiro, F.C.; Rossoni, R.D.; de Barros, P.P.; Santos, J.D.; Fugisaki, L.R.O.; Leão, M.P.V.; Junqueira, J.C. Action mechanisms of probiotics on Candida spp. and candidiasis prevention: An update. J. Appl. Microbiol. 2020, 129, 175–185.
- Trindade, L.A.; de Araújo Oliveira, J.; Dias de Castro, R.; de Oliveira Lima, E. Inhibition of adherence of C. albicans to dental implants and cover screws by Cymbopogon nardus essential oil and citronellal. Clin. Oral Investig. 2015, 19, 2223–2231.
- Veilleux, M.-P.; Grenier, D. Determination of the effects of cinnamon bark fractions on Candida albicans and oral epithelial cells. BMC Complement. Altern. Med. 2019, 19, 303.
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.; Rehder, V.L.; Duarte, M.C.; Höfling, J.F. Action of Coriandrum sativum L. Essential oil upon oral Candida albicans biofilm formation. Evid. Based Complement. Altern. Med. 2011, 2011, 985832.
- Naicker, S.D.; Patel, M. Dodonaea viscosa var. angustifolia inhibits germ tube and biofilm formation by C. albicans. Evid. Based Complement. Altern. Med. 2013, 2013, 261978.
- Ngabaza, T.; Moeno, S.; Patel, M. Anti-acidogenic and anti-biofilm activity of 5,6,8-trihydroxy-7-methoxy-2-(4-methoxyphenyl)-4H-chromen-4-one. Microb. Pathog. 2018, 123, 149–152.
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.S.; El-Ganiny, A.M. Back to Nature: Combating Candida albicans biofilm, phospholipase and hemolysin using plant essential oils. Antibiotics 2021, 10, 81.
- Serra, E.; Hidalgo-Bastida, L.A.; Verran, J.; Williams, D.; Malic, S. Antifungal activity of commercial essential oils and biocides against Candida albicans. Pathogens 2018, 7, 15.
- Sabzghabaee, A.M.; Shirdare, Z.; Ebadian, B.; Aslani, A.; Ghannadi, A. Clinical evaluation of the essential oil of Pelargonium graveolens for the treatment of denture stomatitis. Dent. Res. J. 2011, 8 (Suppl. 1), S105–S108.
- Vazquez, J.A.; Zawawi, A.A. Efficacy of alcohol-based and alcohol-free melaleuca oral solution for the treatment of fluconazole-refractory oropharyngeal candidiasis in patients with AIDS. HIV Clin. Trials 2002, 3, 379–385.
- Catalán, A.; Pacheco, J.G.; Martínez, A.; Mondaca, M.A. In vitro and in vivo activity of Melaleuca alternifolia mixed with tissue conditioner on Candida albicans. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2008, 105, 327–332.
- Jandourek, A.; Vaishampayan, J.K.; Vazquez, J.A. Efficacy of melaleuca oral solution for the treatment of fluconazole refractory oral candidiasis in AIDS patients. AIDS 1998, 12, 1033–1037.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
856
Revisions:
2 times
(View History)
Update Date:
08 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




