Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thiyagarajon Ramesh | -- | 1984 | 2022-04-08 09:16:42 | | | |
| 2 | Rita Xu | + 96 word(s) | 2080 | 2022-04-08 10:04:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ramesh, T.; Mayakrishnan, V.; Balakarthikeyan, J.; Kannappan, P.; D.s, P.; , .; Bahakim, D.N. EGFR-Based Targeted Therapy for Colorectal Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/21495 (accessed on 08 February 2026).
Ramesh T, Mayakrishnan V, Balakarthikeyan J, Kannappan P, D.s P, , et al. EGFR-Based Targeted Therapy for Colorectal Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/21495. Accessed February 08, 2026.
Ramesh, Thiyagarajon, Vijayakumar Mayakrishnan, Janani Balakarthikeyan, Priya Kannappan, Prabakaran D.s, , Dr Nasraddin Bahakim. "EGFR-Based Targeted Therapy for Colorectal Cancer" Encyclopedia, https://encyclopedia.pub/entry/21495 (accessed February 08, 2026).
Ramesh, T., Mayakrishnan, V., Balakarthikeyan, J., Kannappan, P., D.s, P., , ., & Bahakim, D.N. (2022, April 08). EGFR-Based Targeted Therapy for Colorectal Cancer. In Encyclopedia. https://encyclopedia.pub/entry/21495
Ramesh, Thiyagarajon, et al. "EGFR-Based Targeted Therapy for Colorectal Cancer." Encyclopedia. Web. 08 April, 2022.
Copy Citation
Colorectal carcinoma (CRC) is the most lethal and common form of cancer in the world. It was responsible for almost 881,000 cancer deaths in 2018. Approximately 25% of cases are diagnosed at advanced stages with metastasis—this poses challenges for effective surgical control and future tumor-related mortality.
colorectal cancer
EGFR
nanocarriers
nanomedicine
1. Introduction
Colorectal cancer (CRC) is the most lethal and common form of cancer in the world. It was responsible for almost 881,000 deaths from cancer [1]. The primary causes of CRC are not known but may involve lifestyle, viruses, smoking, and environmental hazards. Mutation of the adenomatous polyposis coli (APC) gene is likely to occur during the initial stage of CRC development [2]. The advancement of screening technologies, such as the fecal occult blood test, colonoscopy, and colonography, enable the early detection of colorectal cancer. The emergence of less-invasive surgical methods such as endoscopic, laparoscopic, and robotic procedures has contributed to a reduction in the total number of patients requiring operation for resectable colorectal cancer [3]. There are several diagnostic methods available to reduce the incidence of CRC. However, approximately 25% of CRCs are detected at an advanced stage with metastasis. Furthermore, 20% of cases may go on to develop metachronous metastasis. This poses challenges in surgical control and subsequent cancer-related mortality [1]. Controlling the disease is still challenging in patients with advanced-stage CRC, and they require intensive treatments such as chemotherapy with irinotecan or oxaliplatin, signal inhibitors, and antibodies to achieve a satisfactory outcome [3]. Since the primary goal of CRC treatment is to completely eradicate the tumor and metastasis, which is most often accomplished by invasive surgery on account of varying tumor responses to different treatment techniques, it is crucial to choose the optimal treatment strategy for CRC. The treatment is chosen for the patient depending on several criteria, including the type of tumor, stage of the disease, patient age, overall patient health, and patient attitude towards life [2][4]. Despite further current knowledge of the molecular and cellular aspects of cancer, existing treatments still focus on systemic chemo- and radiotherapy. Broad distribution is a common problem with these regimens, which commonly results in inadequate dosage for the treatment of the tumor and/or the production of harmful side effects in normal tissue [4].
It is possible to target specific changes in cancer cell biology that are highly upregulated, when compared to those of the healthy surrounding cells and tissues, by introducing a targeting moiety (ligand, antibody, or peptide) into the nanoparticle system [5]. The addition of a targeting moiety also enhances drug absorption through receptor-mediated endocytosis, which is an active mechanism requiring a much lower concentration gradient across the plasma membrane than basic endocytosis (Figure 1) [6]. With the help of active targeting, both the quantity of drug delivered and therapeutic efficiency can be enhanced while decreasing the side effects of the drug [7].
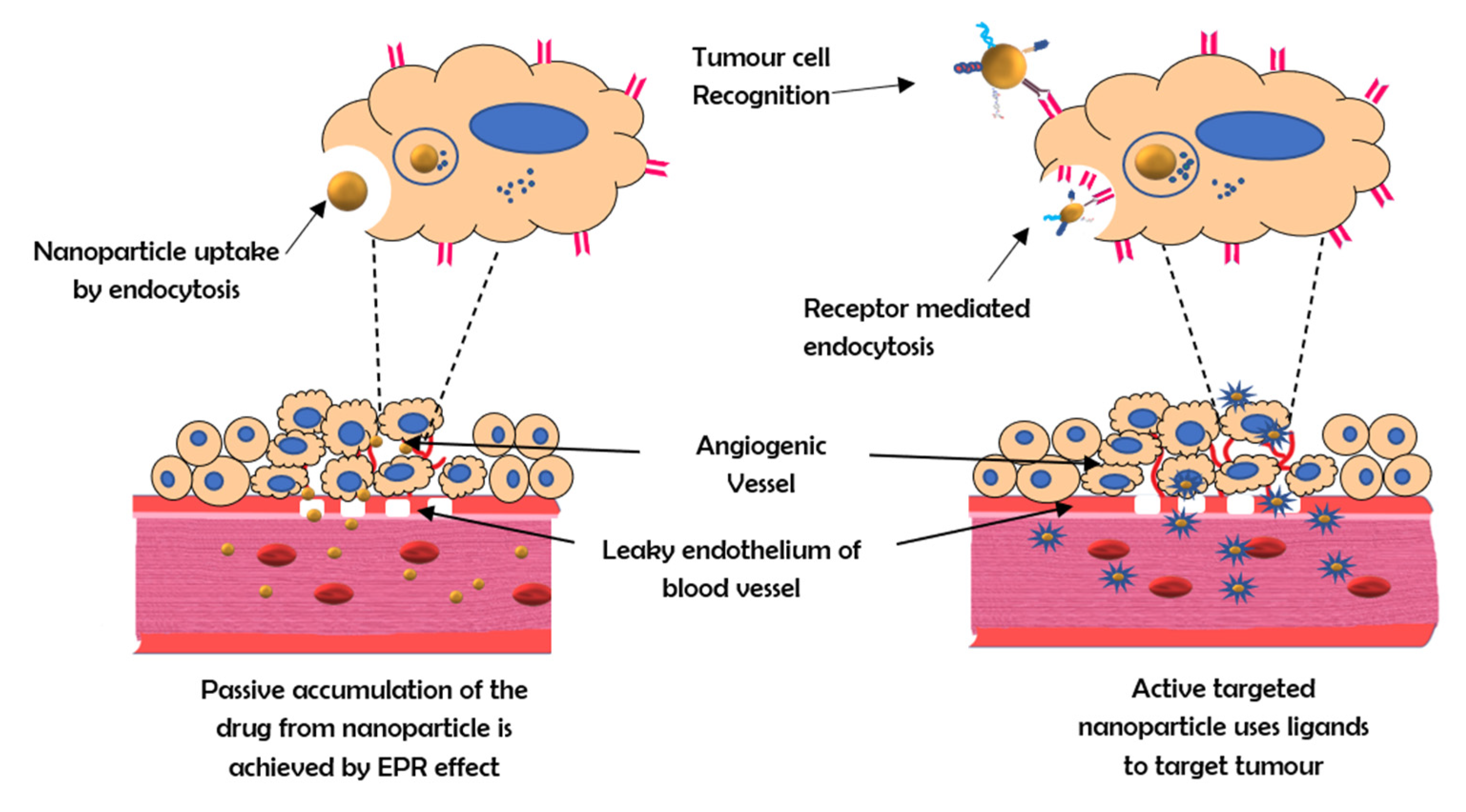
Figure 1. The mechanism of active and passive targeting by nanocarriers.
2. Receptors Used for Targeted Therapy
The strategy of major nanoparticular anti-tumor targeting research is to use antibodies to target disease-associated surface markers on cells. These markers, often receptors, are typically elevated or expressed in particular tumor-associated cells. These receptors can be targeted to deliver chemotherapeutic drugs. They include EGFR, VEGFR, FGFR, HER2, and TGF-b.
-
EGFR: The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase belonging to the ErbB family of proteins. Ligand binding is required to activate the tyrosine kinase domain. This activates signaling pathways responsible for cell proliferation, angiogenesis migration, continued existence, and adhesion. Since these pathways are essential for the survival of cancer cells, EGFR is a valuable target in the treatment of colorectal carcinoma metastases [8].
-
VEGFR: The vascular endothelial growth factor receptor (VEGFR) is a tyrosine kinase receptor. Binding of the ligand vascular endothelial growth factor (VEGF) to this receptor leads to the activation of the receptor and promotes vasculogenesis and angiogenesis [9]. VEGF overexpression is observed in 40–60% of colorectal cancers and is related to cancer recurrence and decreased survival [10].
-
FGFR: In several essential physiological mechanisms such as homeostasis of tissue metabolism, embryonic development, endocrine function, and wound repair, angiogenesis fibroblast growth factor (FGFR) signaling pathways are crucially significant [11]. Therapy against FGFR2 and its specific isoforms are being considered as novel treatment options for colorectal cancer patients. By administering shRNA to bind FGFR2, CRC development, invasion, and migration can be reduced [12].
-
HER 2: The type I transmembrane glycoprotein human epidermal growth factor receptor 2 (HER2) is involved in signaling pathways that control cell proliferation, survival, and apoptosis in breast cancer. In 20–25% of breast cancer patients, the HER2 gene is amplified, which is connected to an aggressive phenotype and worse prognosis [13]. The efficacy of HER2-targeted therapy is comparable to that of developing therapeutic options for metastatic colorectal cancer, such as immunotherapy with checkpoint inhibitors and BRAF-directed therapy [14].
-
TGF-β: The signaling pathway that is activated by transforming growth factor-beta (TGF-β) is crucial in the regulation of tissue development, proliferation, differentiation, apoptosis, and homeostasis [15]. TGF-β is also a powerful regulator of cell adhesion, motility, and the composition of the extracellular matrix, all of which are implicated in tumor invasion and metastasis. However, TGF-β signaling also stimulates angiogenesis and immunosuppression. TGF-β signaling breakdown in colorectal cancer cells promotes tumor growth in the early stages, whereas the stimulation thereof may enhance cancer invasion and metastasis. Thus, while TGF-β may be used as a target in nanotherapeutic methods, its dual roles in enhancing and suppressing tumorigenesis require it to be treated in a careful and highly selective manner [16].
3. Significance of EGFR as a Target
To design therapeutic approaches, researchers are examining the impact of the epidermal growth factor receptor (EGFR) on the development and prognosis of epithelial malignancies [17][18]. EGFR has emerged as a critical target molecule for enhanced tumor specificity because of its diverse functional roles in cancer [19]. EGFR is a transmembrane tyrosine kinase receptor with a molecular weight of 170 kDa that belongs to the ErbB family of cell membrane receptors. Other receptors in this family, in addition to EGFR (also known as HER1 and ErbB-1), include HER2/c-neu (ErbB-2), HER3 (ErbB-3), and HER4. (ErbB-4). All of these receptors have a cytoplasmic tyrosine kinase-containing domain, a single membrane-spanning region, and an extracellular ligand-binding region [20]. In several malignancies, such as head and neck carcinomas, EGFR expression, which is normally assessed by immunohistochemistry, has been linked to tumor development and poor survival [21]. However, the importance of EGFR protein expression in other cancers, such as lung carcinomas, remains controversial, as the level of EGFR overexpression may vary anywhere from 25 to 82% in colorectal cancers [22].
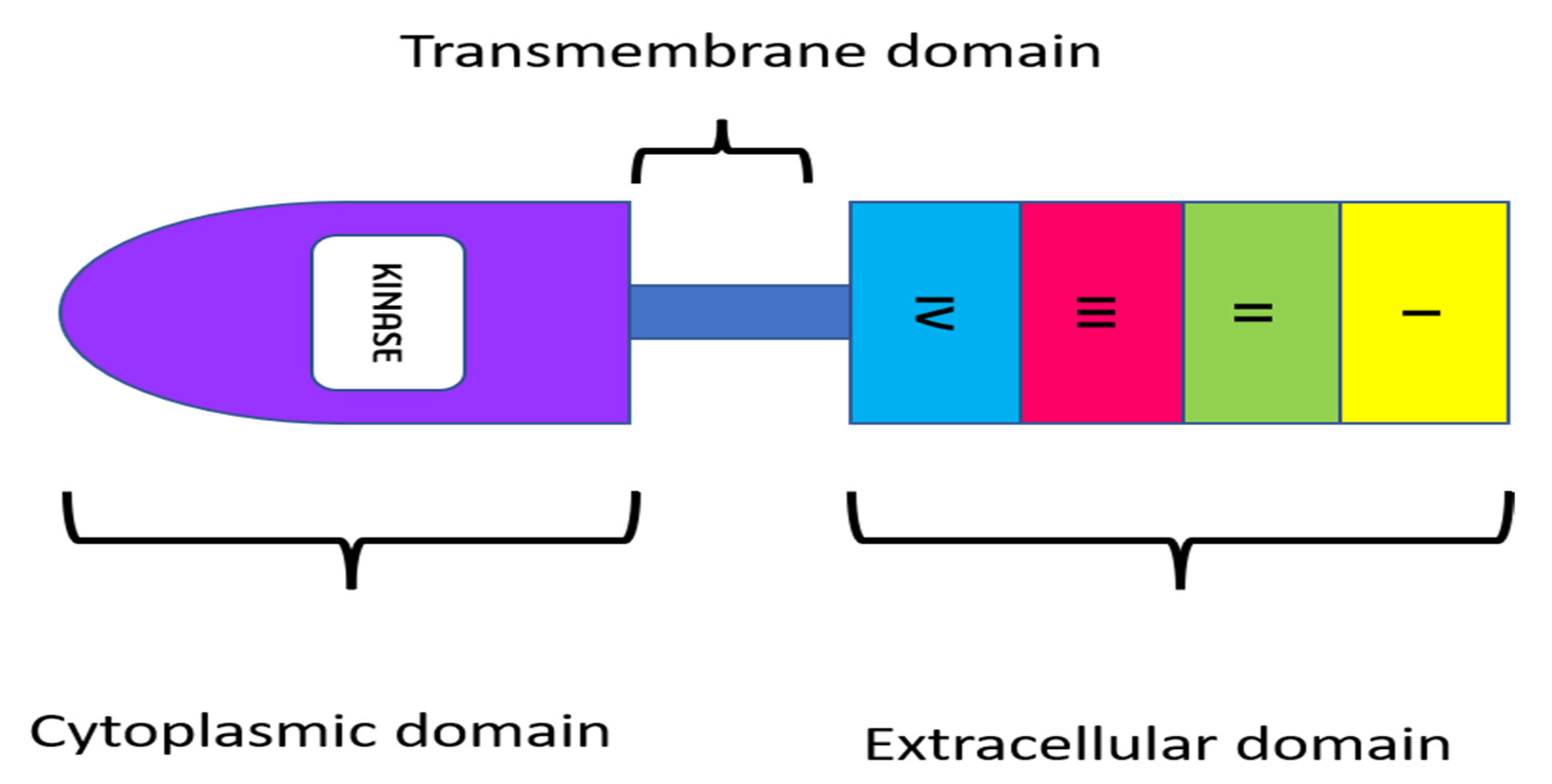
Figure 2. Structure of EGFR receptor.
In addition to EGFR expression levels, ligand binding to EGFR is important in functions that modulate various aspects of colorectal cancers. ADAM17 is a critical enzyme in the regulation of the release of EGFR ligands, which include EGF, amphiregulin, and heparin-binding EGF [23]. Ligand binding to the extracellular domain phosphorylates the tyrosine kinase domain, activating signaling pathways involved in cell proliferation, angiogenesis, migration, continued existence, and adhesion. Since cancer cells rely on these pathways, EGFR is a useful target in the therapy of colorectal cancer metastases [8]. EGF, transforming growth factor (TGF-), β-cellulin, epiregulin (EREG), epigen (EPGN), amphiregulin (AREG), and heparin-binding EGF are the most common EGFR ligands [24]. Conventional EGFR activation begins when a ligand binds to the extracellular domain, inducing a conformational change that causes monomer EGFRs to dimerize [25]. This brings cytoplasmic regions closer together, enabling autophosphorylation of tyrosine residues in the regulatory region. Autophosphorylated tyrosine residues function as anchors for various genes, including GRB2, SHC, SRC, and PI3K, which recruit and activate the RAS, AKT, and STAT signaling pathways, thereby initiating essential cellular processes [26]. EGFR is often mutated in patients with solid tumors. Overexpression of the EGFR protein and kinase-activating mutations are the two forms of pathogenic EGFR changes found in cancer. The kinase-activating mutations that result in enhanced EGFR tyrosine kinase activity might occur as a result of, or in addition to, anti-EGFR therapy. While these markers can be useful tools in the diagnosis of colorectal cancer, current methods of diagnosis primarily use stool samples and colonoscopies [27]. Colorectal cancer is currently diagnosed using stool samples and colonoscopies. The cancer is classified as either colonic or rectal, depending on its location. Both types of tumors exhibit obvious similarities; however, due to their differences in location, their molecular properties likewise differ. Treatment depends on the tumor’s response to chemotherapy or anti-EGFR monoclonal antibodies [28].
4. Monoclonal Antibodies for EGFR Targeted Therapy
Given EGFR’s functional roles in various cellular activities, numerous strategies have been developed to specifically target and inhibit EGFR-mediated effects. Small-molecule tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (mAbs) are two different therapeutic methods used to target EGFR in diverse human cancers [29]. Clinical trials have demonstrated that monoclonal antibodies and TKIs are effective anti-EGFR therapies [30]. The prevention of receptor dimerization, autophosphorylation of the cytoplasmic domain, and downstream signaling take place due to competitive inhibition of EGFR ligands by monoclonal antibodies, which is particularly developed to be directed towards the extracellular region of EGFR [31]. The EGFR monoclonal antibodies that have been approved for clinical use are cetuximab and panitumumab. Their antitumor effect is mediated by specialized structures with various roles. Cetuximab and panitumumab work similarly: They bind to the extracellular domain of EGFR, inhibiting ligand interaction and causing TK internalization and destruction. As a result, they promote apoptosis by blocking EGFR downstream pathways. Anti-EGFR mAbs, especially those of the IgG1 subclass, may also activate the host immune system (antibody-dependent cellular cytotoxicity and complement-mediated cytotoxicity) to destroy the cancer cell. Regardless of the anti-EGFR medicine employed, numerous clinical studies have indicated that cetuximab and panitumumab produce comparable effects [32]. This is mediated by the mAb variable domain fragment (Fv), which is made up of portions of the light and heavy chains of the antibody. Despite their specificity, the production of EGFR mAbs was not without its hurdles. The mAb variable domain fragment (Fv) is a crucial component. It is made up of portions of the antibody’s light and heavy chains. Immunizing mice against EGFR proteins yielded the first mAbs. However, human anti-mouse antibody reactions such as allergic reactions and lower efficacy resulted from these murine antibodies. The combination of a variable murine region with antigenic activity with a constant human region led to the production of fewer allergenic chimeric antibodies such as cetuximab [33].
Cetuximab
Cetuximab (ERBITUX) is an FDA-approved chimeric (human–murine) IgG1 monoclonal antibody that competitively binds the extracellular domain of EGFR with a high affinity [10][29]. Since 2004, cetuximab has been approved as a single drug or in combination with irinotecan for the treatment of metastatic colorectal cancer with EGFR overexpression in patients with chemotherapy-resistant malignancies [34]. Cetuximab’s capacity to obstruct the EGFR pathway has been demonstrated in preclinical and clinical research. It has been shown in preclinical studies that cetuximab alone possesses cytostatic action; however, the use of cetuximab in combination with other chemotherapeutic drugs (such as platinum-derived compounds and irinotecan) enhances the antitumoral activity of the individual regimens. One explanation for this synergy is that limiting EGFR signaling is insufficient for cytotoxicity in the majority of cell lines, yet inhibiting EGFR makes cells more sensitive to chemotherapy [32]. Cetuximab is metabolized and eliminated by the reticuloendothelial system. Its clearance is unaffected by kidney or liver function; therefore, its pharmacokinetics are the same in people with normal or impaired renal function [35]. Cetuximab’s anticancer actions are mediated by a variety of pathways (Figure 3).
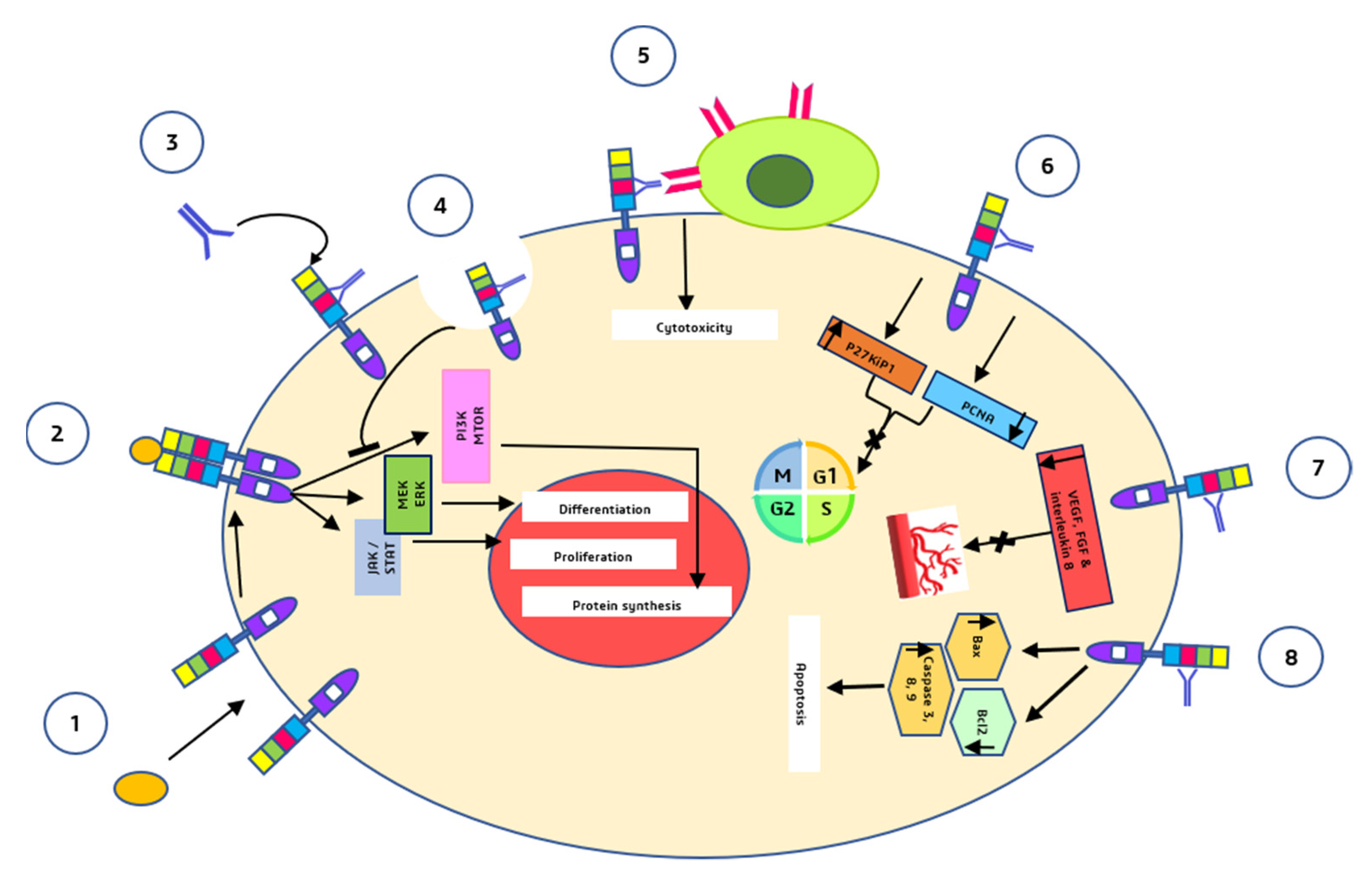

Figure 3. Major anti-tumor mechanisms by which cetuximab acts (1) and (2) are the mechanisms that normally take place in the absence of cetuximab i.e., binding of the ligands to the EGFR monomers, induction of receptor dimerization, and downstream signalling pathways. (3) Cetuximab binds to domain III of EGFR. (4) Receptor internalization mediated by cetuximab and inhibition of downstream signalling pathways. (5) Fc segment of cetuximab binds to natural killer cells and induces ADCC. (6) G1 cell cycle arrest. (7) inhibition of angiogenesis. (8) Induction of apoptosis.
-
Cetuximab binds to the second (L2) EGFR domain and consequently blocks downstream signaling by triggering receptor internalization and blocks the interaction between ligand and receptor [36].
-
Through antibody-dependent cell-mediated cytotoxicity (ADCC), cetuximab directs cytotoxic immune effector cells toward EGFR-expressing tumor cells, potentially contributing to its antitumoral impact [37].
-
Cetuximab causes a G1 cell cycle arrest by increasing the cell cycle inhibitor p27kip1 and suppressing proliferating cell nuclear antigen (PCNA) [38].
-
Cetuximab inhibits angiogenesis by restricting the production of pro-angiogenic factors such as interleukin-8, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (FGF) [39].
-
Induction of apoptosis by cetuximab is mediated through two processes: (a) Increased expression of pro-apoptotic proteins such as BAX, caspase-3, caspase-8, and caspase-9 and (b) inactivation of Bcl-2, which is an anti-apoptotic protein [39].
References
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22.
- Gulbake, A.; Jain, A.; Jain, A.; Jain, A.; Jain, S.K. Insight to drug delivery aspects for colorectal cancer. World J. Gastroenterol. 2016, 22, 582–599.
- Noguchi, T.; Ritter, G.; Nishikawa, H. Antibody-based therapy in colorectal cancer. Immunotherapy 2013, 5, 533–545.
- Fay, F.; Scott, C.J. Antibody-targeted nanoparticles for cancer therapy. Immunotherapy 2011, 3, 381–394.
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640.
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193.
- Iqbal, J.; Anwar, F.; Afridi, S. Targeted Drug Delivery Systems and Their Therapeutic Applications in Cancer and Immune Pathological Conditions. Infect. Disord. Drug Targets. 2017, 17, 149–159.
- Piawah, S.; Venook, A.P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancers 2019, 125, 4139–4147.
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676.
- Banerjee, S.; Flores-Rozas, H. Monoclonal antibodies for targeted therapy in colorectal cancer. Cancer Biol. Ther. 2010, 9, 563–571.
- Luo, H.; Zhang, T.; Cheng, P.; Li, D.; Ogorodniitchouk, O.; Lahmamssi, C.; Wang, G.; Lan, M. Therapeutic implications of fibroblast growth factor receptor inhibitors in a combination regimen for solid tumors. Oncol. Lett. 2020, 20, 2525–2536.
- Dariya, B.; Merchant, N.; Aliya, S.; Alam, A.; Nagaraju, G.P. EGFR and FGFR in Growth and Metastasis of Colorectal Cancer; Springer: Singapore, 2018.
- Chen, F.; Ma, K.; Madajewski, B.; Zhuang, L.; Zhang, L.; Rickert, K.; Marelli, M.; Yoo, B.; Turker, M.Z.; Overholtzer, M.; et al. Ultrasmall targeted nanoparticles with engineered antibody fragments for imaging detection of HER2-overexpressing breast cancer. Nat. Commun. 2018, 9, 4141.
- Siena, S.; Sartore-Bianchi, A.; Marsoni, S.; Hurwitz, H.I.; McCall, S.J.; Penault-Llorca, F.; Srock, S.; Bardelli, A.; Trusolino, L. Targeting the human epidermal growth factor receptor 2 (HER2) oncogene in colorectal cancer. Ann. Oncol. 2018, 29, 1108–1119.
- Itatani, Y.; Kawada, K.; Sakai, Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int. J. Mol. Sci. 2019, 20, 5822.
- Xu, Y.; Pasche, B. TGF-β signaling alterations and susceptibility to colorectal cancer. Hum. Mol. Genet. 2007, 16, R14–R20.
- Milane, L.; Duan, Z.; Amiji, M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol. Pharm. 2011, 8, 185–203.
- Vale, C.L.; Tierney, J.F.; Fisher, D.; Adams, R.A.; Kaplan, R.; Maughan, T.S.; Parmar, M.K.; Meade, A.M. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev. 2012, 38, 618–625.
- Koyen Malashevich, A.; Nabeta, G.; Bienfait, S.; Schlafstein, A.; Yu, D.; Daddacha, W. EGFR Role in Cancer: A Potential Therapeutic Target. In Role of Tyrosine Kinases in Gastrointestinal Malignancies; Springer: Singapore, 2018; pp. 225–234.
- Krasinskas, A.M. EGFR Signaling in Colorectal Carcinoma. Patholog. Res. Int. 2011, 2011, 932932.
- Chang, S.S.; Califano, J. Current status of biomarkers in head and neck cancer. J. Surg. Oncol. 2008, 97, 640–643.
- Spano, J.P.; Fagard, R.; Soria, J.C.; Rixe, O.; Khayat, D.; Milano, G. Epidermal growth factor receptor signaling in colorectal cancer: Preclinical data and therapeutic perspectives. Ann. Oncol. 2005, 16, 189–194.
- Pabla, B.; Bissonnette, M.; Konda, V.J. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J. Clin. Oncol. 2015, 6, 133–141.
- Li, Q.H.; Wang, Y.Z.; Tu, J.; Liu, C.W.; Yuan, Y.J.; Lin, R.; He, W.L.; Cai, S.R.; He, Y.L.; Ye, J.N. Anti-EGFR therapy in metastatic colorectal cancer: Mechanisms and potential regimens of drug resistance. Gastroenterol. Rep. 2020, 8, 179–191.
- Dawson, J.P.; Berger, M.B.; Lin, C.C.; Schlessinger, J.; Lemmon, M.A.; Ferguson, K.M. Epidermal growth factor receptor dimerization and activation require ligand-induced conformational changes in the dimer interface. Mol. Cell Biol. 2005, 25, 7734–7742.
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52.
- Thomas, R.; Weihua, Z. Rethink of EGFR in Cancer With Its Kinase Independent Function on Board. Front. Oncol. 2019, 9, 800.
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers. 2015, 1, 15065.
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31.
- El Guerrab, A.; Bamdad, M.; Kwiatkowski, F.; Bignon, Y.-J.; Penault-Llorca, F.; Aubel, C. Anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors as combination therapy for triple-negative breast cancer. Oncotarget 2016, 7, 73618–73637.
- Burgess, A.W.; Cho, H.S.; Eigenbrot, C.; Ferguson, K.M.; Garrett, T.P.; Leahy, D.J.; Lemmon, M.A.; Sliwkowski, M.X.; Ward, C.W.; Yokoyama, S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol. Cell 2003, 12, 541–552.
- Frattini, M.; Saletti, P.; Molinari, F.; De Dosso, S. EGFR signaling in colorectal cancer: A clinical perspective. Gastrointest. Cancer Targets Ther. 2015, 2015, 21.
- You, B.; Chen, E.X. Anti-EGFR monoclonal antibodies for treatment of colorectal cancers: Development of cetuximab and panitumumab. J. Clin. Pharmacol. 2012, 52, 128–155.
- Bou-Assaly, W.; Mukherji, S. Cetuximab (Erbitux). Am. J. Neuroradiol. 2010, 31, 626–627.
- Ebisumoto, K.; Okami, K.; Hamada, M.; Maki, D.; Sakai, A.; Saito, K.; Shimizu, F.; Kaneda, S.; Iida, M. Cetuximab with radiotherapy as an alternative treatment for advanced squamous cell carcinoma of the temporal bone. Auris Nasus Larynx 2018, 45, 637–639.
- Van Krieken, J.H.; Jung, A.; Kirchner, T.; Carneiro, F.; Seruca, R.; Bosman, F.T.; Quirke, P.; Fléjou, J.F.; Plato Hansen, T.; de Hertogh, G.; et al. KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: Proposal for an European quality assurance program. Virchows Arch. 2008, 453, 417–431.
- Hanck-Silva, G.; Fatori Trevizan, L.N.; Petrilli, R.; de Lima, F.T.; Eloy, J.O.; Chorilli, M. A Critical Review of Properties and Analytical/Bioanalytical Methods for Characterization of Cetuximab. Crit. Rev. Anal. Chem. 2020, 50, 125–135.
- Okuyama, K.; Suzuki, K.; Naruse, T.; Tsuchihashi, H.; Yanamoto, S.; Kaida, A.; Miura, M.; Umeda, M.; Yamashita, S. Prolonged cetuximab treatment promotes p27(Kip1)-mediated G1 arrest and autophagy in head and neck squamous cell carcinoma. Sci. Rep. 2021, 11, 5259.
- Martinelli, E.; De Palma, R.; Orditura, M.; De Vita, F.; Ciardiello, F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin. Exp. Immunol. 2009, 158, 1–9.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
723
Revisions:
2 times
(View History)
Update Date:
08 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




