Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lorenzo Gontrani | -- | 2883 | 2022-04-06 15:44:23 | | | |
| 2 | Lorenzo Gontrani | Meta information modification | 2883 | 2022-04-06 17:35:07 | | | | |
| 3 | Camila Xu | -16 word(s) | 2867 | 2022-04-07 04:17:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gontrani, L.; Tagliatesta, P.; Donia, D.; , .; Bonomo, M.; Carbone, M. Recent Advances of Deep Eutectic Solvents. Encyclopedia. Available online: https://encyclopedia.pub/entry/21421 (accessed on 08 February 2026).
Gontrani L, Tagliatesta P, Donia D, , Bonomo M, Carbone M. Recent Advances of Deep Eutectic Solvents. Encyclopedia. Available at: https://encyclopedia.pub/entry/21421. Accessed February 08, 2026.
Gontrani, Lorenzo, Pietro Tagliatesta, Domenica Donia, , Matteo Bonomo, Marilena Carbone. "Recent Advances of Deep Eutectic Solvents" Encyclopedia, https://encyclopedia.pub/entry/21421 (accessed February 08, 2026).
Gontrani, L., Tagliatesta, P., Donia, D., , ., Bonomo, M., & Carbone, M. (2022, April 06). Recent Advances of Deep Eutectic Solvents. In Encyclopedia. https://encyclopedia.pub/entry/21421
Gontrani, Lorenzo, et al. "Recent Advances of Deep Eutectic Solvents." Encyclopedia. Web. 06 April, 2022.
Copy Citation
Deep Eutectic Solvents (DESs) have gained a lot of attention in the last few years because of their vast applicability in a large number of technological processes, the simplicity of their preparation and their high biocompatibility and harmlessness.
Deep Eutectic Solvents
nanoparticles
inorganic synthesis
environmentally friendly media
1. Introduction
According to the general definition currently used (EU 2011), a “nanoparticle” (NP) is a discrete (nano-)object where at least one of its characteristic dimensions falls in the range 1–100 nm. This category thus includes objects with a fixed number of such dimensions, such as nanowires/nanotubes (mono-dimensional, 1D) and nanodiscs/nanoplates (2D), as well as nanometric spherical/globular three-dimensional aggregates. Nanoparticles can be further classified according to their organic/inorganic nature. Dendrimers, lyposomes, and polymeric NPs belong to the former group, whereas fullerenes, quantum dots, and metal/metal oxide NPs to the latter [1][2]. Most importantly, nanomaterials are classified according to their size, shape, and properties, and it is this last feature that has led to the blossoming of nanomaterial research in the last few years. The remarkable properties nanoparticles possess (e.g., optical, magnetic, and electrical) can be exploited in a large number of technology-related fields, ranging from electronics [3][4] to biology and medicine [5][6]. The correlation between size/shape and properties in nanomaterials was thoroughly assessed in several investigations, and it was shown that their properties were dramatically dependent on the shapes, dimensions, and porosities of the synthesized nanoobjects; thus, a detailed control of the synthetic route is needed. The structural differences observed ultimately result in remarkably different performances in real-world applications. Among the factors that can be controlled, the most easily tunable is probably temperature. For instance, in the synthesis of metal oxides by the co-precipitation pathway, the temperature at which the drying/calcination of the hydroxide is carried out can vary widely. Despite its apparent “simplicity”, the effect of this variation can be quite relevant, as was found, for instance, in the synthesis of nickel (II) oxide NiO, a prototypical p-type, wide-band-gap semiconductor. NiO nanoparticles prepared at 400 °C are smaller and more porous, while larger structures are obtained at 600 °C; the band gaps associated with the latter are larger and the electrochemical performances worse, especially when these nanoparticles are introduced into screen-printed electrodes (SPEs) and employed as electrochemical sensors for selective assays or in Faradic pseudo-capacitors. Other factors that have been found to influence the intercalation of precursors and the sintering of particles, the latter leading to particles of larger sizes, are the reagents used to prepare the precursor hydroxide Ni(OH)2: the use of various combinations of nickel salts and inorganic/organic bases as precipitating agents, and the adoption of surfactant-free hydrothermal synthesis or microwave irradiation (which can lead to different results) [7][8][9][10][11][12].
2. Brief Description of Deep Eutectic Solvents
Since the “official” birthday of this family of liquids, which can be traced back to the pivotal paper by Abbott in 2003 [13], a fairly large amount of research has been dedicated to them, especially in the last decade. The key feature of these solvents is their very easy and economic preparation, which involves the simple mixing of at least one hydrogen bond donor (HBD) with at least one hydrogen bond acceptor (HBA). In most cases, this takes place in solid phase and in definite ratios, providing mixtures with remarkably lower melting points than those of their individual components. The sometimes very large decrease seen in the melting temperature, which in the prototypical choline chloride:urea 1:2 system (“Reline”) [14] can be more than 100 °C with respect to the ideal value obtainable from thermodynamics arguments [15][16], has led to the term “deep” being used to distinguish DESs from other non-ideal physical systems where similar phenomena are observed—e.g., water–DMSO mixtures. Deep Eutectic Solvents were divided into four main classes in the original classification by Abbott, three of which contain a halide anion and a quaternary ammonium cation as HBA (in most cases, choline chloride), and differ in their type of HBD: metal chloride in class I, hydrated metal chloride in class II, organic molecules (such as urea, glycerol, and carboxylic acids) in class III (which is the most populated), and metal halides and urea (or acetamide/ethylene glycol) in class IV. Recently, a new class was introduced (“type V” [17]), containing only hydrogen-bond donors and acceptors and no ions. Other non-negligible valuable traits of Deep Eutectic Solvents are their high sustainability, especially in the subfamily NaDES (Natural DES), where the precursors are benign compounds generally obtained from natural feedstocks, such as choline chloride and carboxylic acids; these therefore have a low toxicity and high biocompatibility and biodegradability [18][19][20]. Additionally, DESs are characterized by their high conductivities, viscosities, and surface tensions; have a low volatility and flammability; and have highly tunable physiochemical properties, considering the large number of possible combinations of precursor salts [21]. These last features are shared with ionic liquids (ILs), which can be considered as the forefathers of DESs. However, ILs are composed (almost entirely) of ions [22][23], whereas DESs, apart from the type V ones, are mixtures that contain polar molecules and ions. A sketch of the desirable technological properties of DESs is provided in Figure 1.
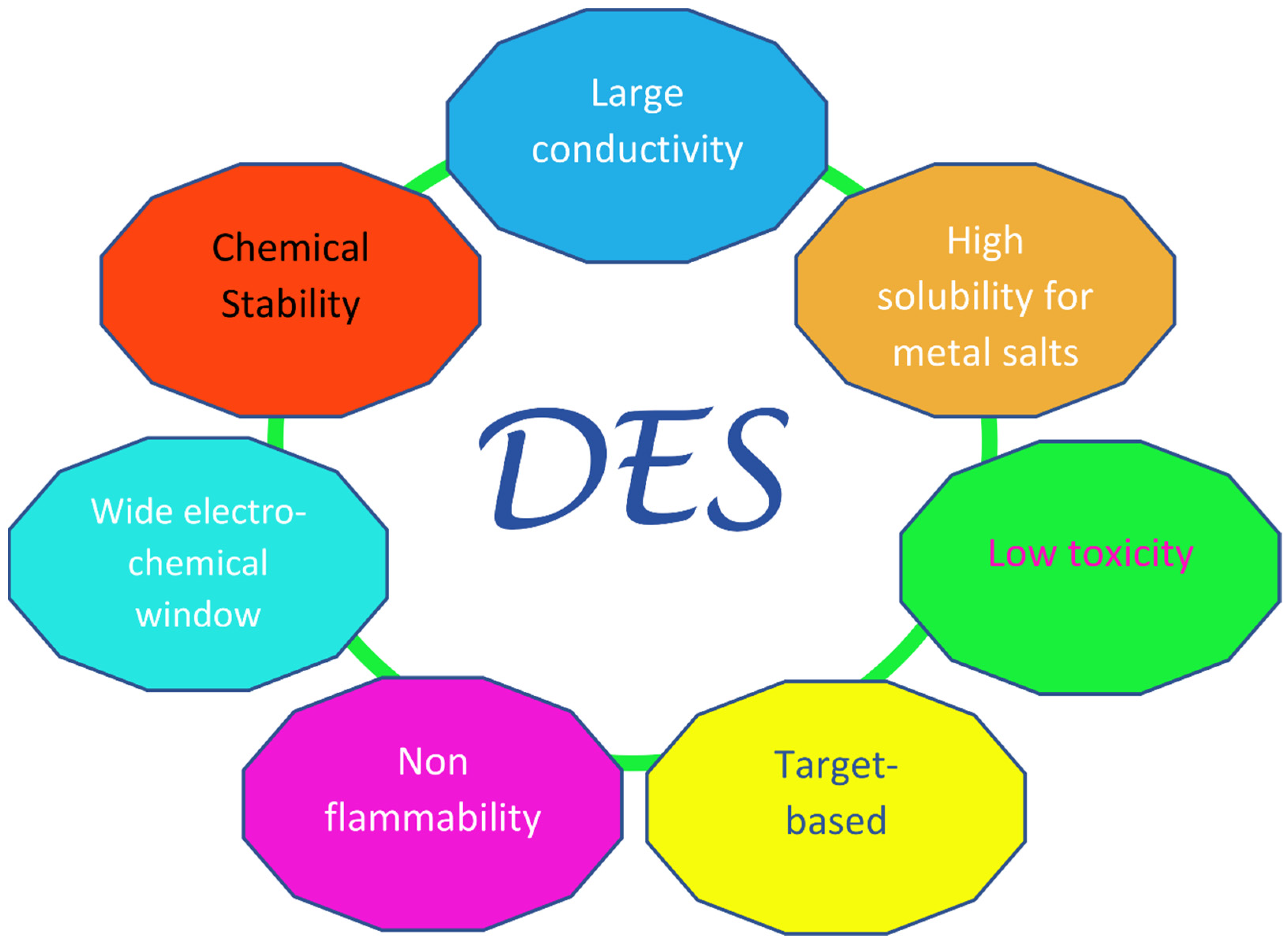 Figure 1. Pictorial diagram of the innovative properties of Deep Eutectic Solvents, inspired by Ref. [21].
Figure 1. Pictorial diagram of the innovative properties of Deep Eutectic Solvents, inspired by Ref. [21].3. DES in Nanoparticle Synthesis from Their Birth to 2022
The investigation of the applicability of ILs and DESs in the field of nanoparticle synthesis started with a focus on the former family, but the amount of research on DES has been growing lately. In addition, some of the features characteristic of ILs can be extended seamlessly to DESs. The most striking property of DESs in the field of nanoparticle synthesis is the high solubility of metal ion salts and complexes in several cases. As already shown by Abbott et al. [24], the solubility of some bulk metal oxides, which are almost insoluble in pure water, is greatly increased in some DESs, reaching values of up to more than 10,000 parts per million in ChCl:malonic acid 1:1 at 50 °C for Cu2O, CuO, and ZnO, and up to 90,000 ppm for ZnO in ChCl:urea 1:2 at 70 °C. In contrast, other oxides, such as iron oxides, are barely soluble in ChCl:urea 1:2. This feature can be used, for instance, to design suitable synthesis protocols based on fractional precipitation. Another key factor is the possibility of these substances acting in a dual capacity as both solvents and templates for nanostructure formation (“target solvent”) [25][26][27][28][29], owing to their structural heterogeneity [30][31][32][33]. In several cases, DESs can behave as reactants as well [34][35][36].
The heterogeneity and microstructure of DESs have attracted great interest. The study by Chen et al. [37] pointed out that in “reline” (ChCl:urea 1:2), “ethaline” (ChCl:ethylene glycol 1:2), and “glyceline” (ChCl:glycerol 1:2 (the classification of this mixture as a DES or as a salt-in-solvent system has recently been debated, and the data are in favor of a 1:3 composition defining a proper DES [38])), the charged and neutral portions of the mixtures tend to separate when the liquid comes into contact with graphite electrodes, with choline and chloride ions being attracted into the Stern layer, while the molecular components are excluded at all the potentials scanned. Similar experiments carried out using Pt(111) electrodes and the same three DESs were conducted at various potentials and at increasing water contents [39]. This entry showed that the addition of water in amounts up to ≈40 wt% did not lead to the decomposition of the interfacial nanostructure, as observed in ILs even at very small water contents. Yet, the cyclic voltammetry analysis reported in the same study indicates that, at such high water concentrations, the nanostructure appears comparable to that of salt solutions. The preservation of the microscopical structure upon hydration was also demonstrated in a neutron diffraction study conducted by Hammond et al. [40], who pointed out that the microscopic features of pure reline were maintained in a water to DES molar ratio of up to 10:1 (around 42 wt% H2O); beyond that limit, the system can be better described as a three-component HBA:HBD:water mixture. Marked heterogeneity was evidenced as well in a molecular dynamics study conducted by Alizadeh et al. [26], who reported the fate of ethaline’s polar and nonpolar molecular groups under electrostatic potential. The calculations suggest that the DES reorganizes in order to maximize the interactions between domains of the same polarity, thus inducing strong heterogeneity in the system.
The oldest syntheses reported mainly concerned the preparation of noble metal nanoparticles with a controlled shape; in most cases, these syntheses employed Abbot’s DES reline [13]: catalysts containing star-like gold NPs were synthesized through the reduction of HAuCl4 by L-ascorbic acid in DES without surfactants or seeds at room temperature ([41], see Figure 2). The shape could be further changed (e.g., to a snowflake or nanothorn) by adjusting the water content. Other noble metal NPS were prepared by electrosynthesis methods: in this regard, DESs can be very valuable, since they possess very wide electrochemical windows just slightly bit smaller than ILs which can be effectively coupled to the large solubility of metal oxides to set up electrochemical cells for the deposition of metal nanoparticles, achieving very efficient control over the nucleation, deposition rate, and size of the crystals obtained [42]. A further very important aspect of noble metal deposition is that DES and ILs can substitute highly toxic cyanide-based electrolytes [43]. Among these examples, the preparation of concave tetrahexahedral Pt nanocrystals by electrodeposition using reline without employing surfactants, seeds, or other chemicals but with a high control of the shape and a high surface energy was reported [44]. Two-dimensional superstructures of aggregated Pd nanoparticles were electrodeposited from choline chloride:urea 1:2 onto glassy carbon foil, with the absorbed species forming an anionic layer that was observed with Ultra Small X-Ray Scattering (USAXS) [45]. Platinum icosahedral nanocrystals with high-index facets and a higher electrocatalytic activity and stability were electro-synthesized in reline [46]. Further details on the role of Deep Eutectic Solvents in the synthesis of plasmonic (Au, Ag, Pt) nanoparticles can be found in the recent review of Des et al. [47], who also describe biocompatible capping strategies that make use of polysaccharides (carrageenan), resulting in highly monodisperse nanoparticles. Other non-electrochemical redox reactions were carried out using environmentally friendly routes based on reline as a solvent: CuCl nanocrystal powder, which is a very useful catalyst for organic synthesis, was obtained either through the synproportion of CuCl2 and Cu [48] or by the reduction of CuCl2 by ascorbic acid [49]. Additionally, spherical, magnetic nanoparticles of ferrous ferrite (Fe3O4) were prepared at 80 °C and successfully tested for the absorption of Cu2+ ions, proving to be superior to NPs prepared in pure water [50]. Further examples of systems prepared in reline with co-precipitation are PbS nano/micro superstructures made from lead (IV) and thioacetamide [51], mesoporous NiO [52], and some examples of iono-thermal reactions at high temperatures/pressures (nanoflower-like α-Ni(OH)2 and NiO [53], Ni2P supported on amorphous/mesoporous Ni3(PO4)2-Ni2P2O7 [54], nanosized SnO crystals [55], Fe2O3 nanospindles as high-capacity anode materials [56], mesoporous Co3O4 sheets or nanoparticles [57], and MnCO3/MnOx mesocrystals [58]). Few studies have employed DESs other than reline; some noteworthy examples utilize ethylene glycol as the HBD partner of ChCl, the latter being used to obtain nanocrystalline SnO2 or SnO2/graphene nanocomposites [59], nanoporous Ag films [60], and Ni-P alloy nanoparticles [61] to deposit Ni deposits [62], Ni-Ti nanocomposite coatings [63], or Ag films [64].
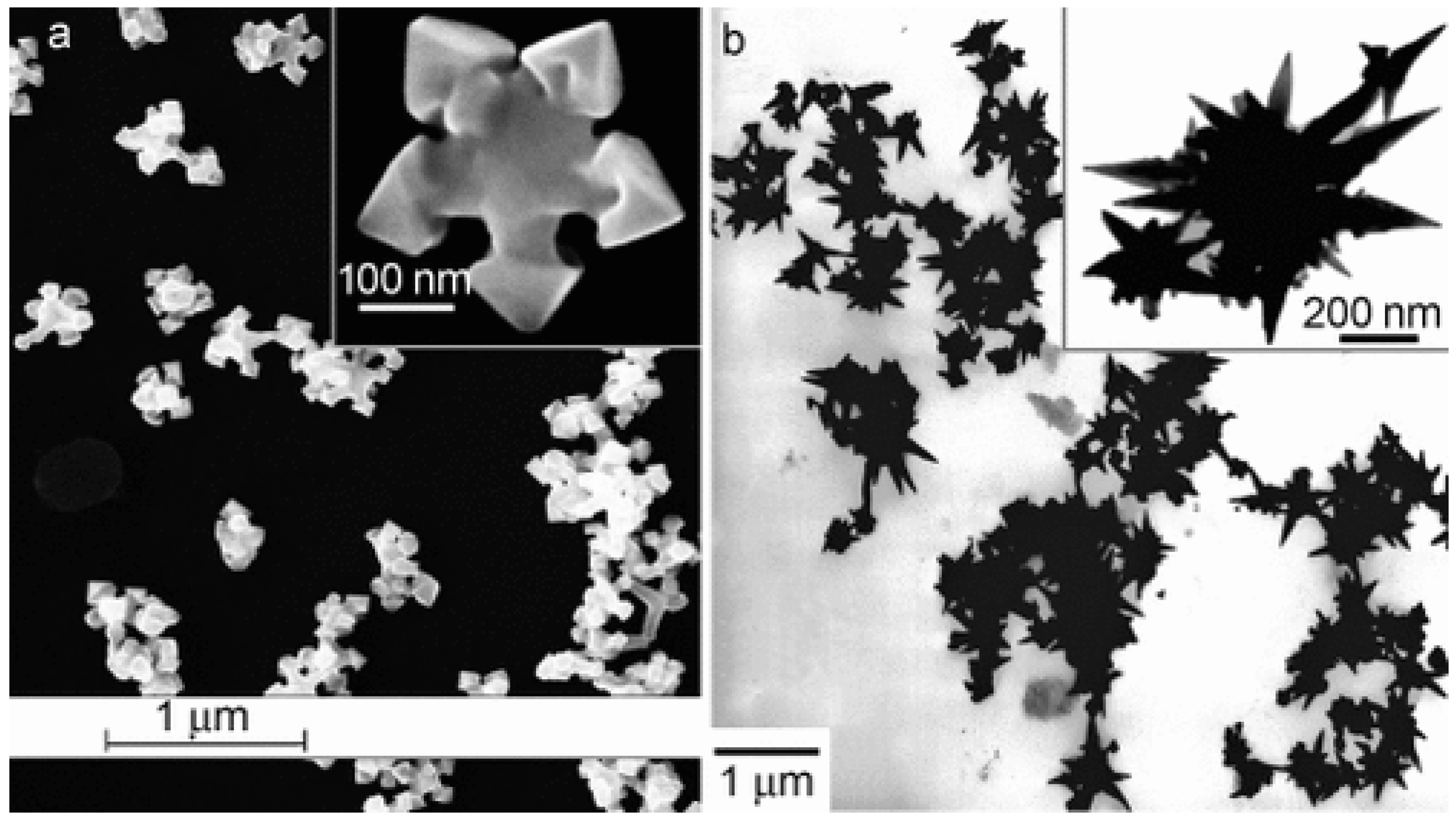 Figure 2. Deep Eutectic Solvents as green media in the synthesis of anisotropic Au nanoparticles (panel (a): flower shape; panel (b): nanothorn). Reproduced with permission from ref. [41]. Copyright 2008 John Wiley and Sons.
Figure 2. Deep Eutectic Solvents as green media in the synthesis of anisotropic Au nanoparticles (panel (a): flower shape; panel (b): nanothorn). Reproduced with permission from ref. [41]. Copyright 2008 John Wiley and Sons.More recently, the increasing need for even greener and more biocompatible alternatives has fostered the usage of new types of environmentally benign and low-cost mixtures, mostly belonging to the NADES family (DESs of natural origin), where the precursors are obtained from renewable feedstocks. For instance, Zainal-Abidin et al. recently reported that graphene is significantly less cytotoxic when it is functionalized with ChCl:glucose/fructose/sucrose) 2:1 or, better yet, ChCl:malonic acid 1:1, with respect to pristine or oxidized graphene owing to surface modifications [65]. NADES mixtures containing glucose, fructose, and sucrose as HBD, choline chloride, and water at different molar ratios, were employed in the synthesis of MoS2 nanosheets by Mohammadpour et al. [66]. The material prepared was stable in aqueous environments, could perform as a catalyst in hydrogen evolution reactions (HERs), and could be obtained in a higher yield compared to other exfoliating agents (average of 44% vs. 20%). New mixtures created by changing the HBA or HBD started to be explored in the last part of the 2010s: an interesting example of those belonging to the second group is the synthesis of calcite nanoparticles by the reaction of CO2 with “calcoline”, a DES composed of choline chloride and calcium chloride [67]. This entry demonstrates that DESs can be successfully exploited in “carbon-reduction” protocols, leading to value-added products. A modification of the acceptor moiety (HBA) was used by Adhikari et al., who reported the use of halide-free DES, where the Cl-anion of reline is replaced by nitrate, for the microwave-assisted reduction of silver salts to organic-soluble oleylamine-capped Ag nanoparticles [68]. Following on from this study, Adhikari et al. later fine-tuned a silver-based DES (1:4 silver triflate:acetamide, “argentous DES”) that allowed them to obtain large amounts of monodispersed colloidal silver nanocrystals of high quality despite the high metal concentration, owing to the “size focusing” effect of the DES that suppressed uncontrolled nanocrystal growth [69]. More recently, these researchers exported their methodology to flow-reactor synthesis employing dimethylammonium nitrate-polyol DES media; they were able to obtain a 1000- to 4000-fold increase in throughput compared to conventional synthesis [70]. The use of ammonium cations as hydrogen bonds, though very common and convenient, is not the only option. In fact, other inorganic salts have recently been used. For example, ionic compounds belonging to the alkali halide family have been proven to form polyol-based DES mixtures (such as CsF/KF:glycerol) that have shown very high selectivities when used as reaction media for copper-catalyzed homocoupling organic reactions [71].
Returning to DESs containing molecules of natural origin, a mixture of caffeic acid with ChCl and ethyleneglycol was used to prepare moleculary imprinted hexagonal boron nitride NPs, which were successfully employed in the solid-phase extraction of flavonoids [72], while tartaric acid was used as a DES component in a recent electrodeposition preparation of a Ti/SnO2–Sb electrode with a high electrochemical activity [73].
Starting from 2020, the research on DES as media or additives in the synthesis of inorganic materials has blossomed, as it can be easily from the large number of studies published (hundreds). Besides the investigations related to the oldest DES (reline, ethaline and glyceline), the most recent studies are focusing on many new and alternative mixtures, often including biomolecules of natural origin. For instance, the excellent capabilities of glycerol as hydrogen bond donor in DES were further enhanced by the addition of malic acid and D-fructose in a 1:1:1: mole ratio (MaFruGly). MaFruGly was used to disperse Al2O3 into a nanofluid that is capable of extracting polyphenols and other bioactive compounds from olive oil pomaces and leaves [74]. Other examples of such biocompatible mixtures include choline chloride:glucose, which was used to prepare sodium hyaluronate/dopamine/AgNPs hydrogels [75], ChCl:xylitol, which was employed to modify magnetic titania NPs with Fe3O4@TiO2@DES [76]; and choline chloride:gluconic acid DES, which was used to prepare a cobalt-DES precursor that was finally pyrolyzed into Co nanoparticles supported on nitrogen-doped porous carbon (Co@NPC). A relevant portion of the recent studies on this topic has focused on magnetic nanoparticles containing Fe3O4 (or Fe2O3) and organic moieties or enzymes, which could be assembled in a few examples of DESs: acrylic acid-Fe3O4 composites were obtained from acrylic acid:menthol-type V DES used in the detection of pesticides [77], whereas macroporous polyacrylamide γ-maghemite composites were prepared in acetic acid:menthol [78]. A smaller number of studies were dedicated to DES without quaternary ammonium salts, containing metal salts (often hydrated) such as HBA and hydrogen bond donors such as urea or acetamide. This family of DES is known as LADES (Lewis Acid DES)) [79], compared to the more common “BADES” (with Brønsted acids, such as choline chloride + oxalic acid). For instance, some lanthanide-based type IV DES (Ln-DES) containing hydrated nitrates were prepared [80]. These mixtures show unusually low viscosity and surface tensions and the presence of fluxional oligomeric polyanions and polycations, and were employed as reaction media in the combustion synthesis of oxides; more recently, actinide-based type IV DES (An-DES) were obtained by mixing uranyl nitrate hexahydrate UO2(NO3)2·6H2O (UNH) with urea in different ratios, finding 0.2:0.8 to be the optimal UNH:urea mole fraction, with a quite low eutectic temperature of −5.2 °C. This liquid was employed to prepare UO2 nanoparticles through an optimized electrosynthesis path [81].
In summary, the main advantages of DES lie in their friendliness towards ecosystems, their highly tunable physiochemical properties, and their cheap means of preparation and handling. An additional very important and profitable feature is their intrinsic microheterogeneity, with it being possible to confer specific morphologies to the obtained nanoparticles. A possible drawback of the use of DESs is their moderate viscosity, which depends on the nature of their components, with the viscosity being lower for hydrophobic DESs and larger for sugar-based NADESs. Indeed, it has been shown that the decrease in mass diffusivity caused by viscosity affects nanoparticle growth and generally leads to NPs of a larger size [82].
References
- Ealia, S.A.M.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074.
- Wang, Z.; Wang, X.; Zhao, N.; He, J.; Wang, S.; Wu, G.; Cheng, Y. The Desirable Dielectric Properties and High Thermal Conductivity of Epoxy Composites with the Cobweb-Structured SiCnw–SiO2–NH2 Hybrids. J. Mater. Sci. Mater. Electron. 2021, 32, 20973–20984.
- Wang, Z.; Meng, G.; Wang, L.; Tian, L.; Chen, S.; Wu, G.; Kong, B.; Cheng, Y. Simultaneously Enhanced Dielectric Properties and Through-Plane Thermal Conductivity of Epoxy Composites with Alumina and Boron Nitride Nanosheets. Sci. Rep. 2021, 11, 2495.
- Carbone, M.; Briancesco, R.; Bonadonna, L. Antimicrobial Power of Cu/Zn Mixed Oxide Nanoparticles to Escherichia Coli. Environ. Nanotechnol. Monit. Manag. 2017, 7, 97–102.
- Carbone, M. Cu Zn Co Nanosized Mixed Oxides Prepared from Hydroxycarbonate Precursors. J. Alloys Compd. 2016, 688, 202–209.
- Carbone, M. : An Efficient Tool for CH4 Sensing. Appl. Sci. 2020, 10, 6251.
- Carbone, M.; Tagliatesta, P. NiO Grained-Flowers and Nanoparticles for Ethanol Sensing. Materials 2020, 13, 1880.
- Carbone, M.; Aneggi, E.; Figueredo, F.; Susmel, S. NiO-Nanoflowers Decorating a Plastic Electrode for the Non-Enzymatic Amperometric Detection of H2O2 in Milk: Old Issue, New Challenge. Food Control 2022, 132, 108549.
- Carbone, M.; Nesticò, A.; Bellucci, N.; Micheli, L.; Palleschi, G. Enhanced Performances of Sensors Based on Screen Printed Electrodes Modified with Nanosized NiO Particles. Electrochim. Acta 2017, 246, 580–587.
- Carbone, M.; Missori, M.; Micheli, L.; Tagliatesta, P.; Bauer, E.M. NiO Pseudocapacitance and Optical Properties: Does the Shape Win? Materials 2020, 13, 1417.
- Carbone, M.; Maria Bauer, E.; Micheli, L.; Missori, M. NiO Morphology Dependent Optical and Electrochemical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 178–182.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 39, 70–71.
- Meng, X.; Ballerat-Busserolles, K.; Husson, P.; Andanson, J.-M. Impact of Water on the Melting Temperature of Urea + Choline Chloride Deep Eutectic Solvent. New J. Chem. 2016, 40, 4492–4499.
- Arkawaz, A.F.; Abdullah, B.A.; Mustafa Ha, S. Physical, Thermal and Structural Properties of 1 Choline Chloride: 2 Urea Based Ionic Liquids. Singap. J. Sci. Res. 2020, 10, 417–424.
- Lapeña, D.; Bergua, F.; Lomba, L.; Giner, B.; Lafuente, C. A Comprehensive Study of the Thermophysical Properties of Reline and Hydrated Reline. J. Mol. Liq. 2020, 303, 112679.
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-Based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477.
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690.
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071.
- Gontrani, L.; Plechkova, N.V.; Bonomo, M. In-Depth Physico-Chemical and Structural Investigation of a Dicarboxylic Acid/Choline Chloride Natural Deep Eutectic Solvent (NADES): A Spotlight on the Importance of a Rigorous Preparation Procedure. ACS Sustain. Chem. Eng. 2019, 7, 12536–12543.
- Jafari, K.; Fatemi, M.H.; Estellé, P. Deep Eutectic Solvents (DESs): A Short Overview of the Thermophysical Properties and Current Use as Base Fluid for Heat Transfer Nanofluids. J. Mol. Liq. 2021, 321, 114752.
- Kanzaki, R.; Uchida, K.; Song, X.; Umebayashi, Y.; Ishiguro, S. Acidity and Basicity of Aqueous Mixtures of a Protic Ionic Liquid, Ethylammonium Nitrate. Anal. Sci. 2008, 24, 1347–1349.
- Mariani, A.; Bonomo, M.; Gao, X.; Centrella, B.; Nucara, A.; Buscaino, R.; Barge, A.; Barbero, N.; Gontrani, L.; Passerini, S. The Unseen Evidence of Reduced Ionicity: The Elephant in (the) Room Temperature Ionic Liquids. J. Mol. Liq. 2021, 324, 115069.
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. J. Chem. Eng. Data 2006, 51, 1280–1282.
- Carriazo, D.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Resorcinol-Based Deep Eutectic Solvents as Both Carbonaceous Precursors and Templating Agents in the Synthesis of Hierarchical Porous Carbon Monoliths. Chem. Mater. 2010, 22, 6146–6152.
- Raghuwanshi, V.S.; Ochmann, M.; Hoell, A.; Polzer, F.; Rademann, K. Deep Eutectic Solvents for the Self-Assembly of Gold Nanoparticles: A SAXS, UV–Vis, and TEM Investigation. Langmuir 2014, 30, 6038–6046.
- Alizadeh, V.; Geller, D.; Malberg, F.; Sánchez, P.B.; Padua, A.; Kirchner, B. Strong Microheterogeneity in Novel Deep Eutectic Solvents. ChemPhysChem 2019, 20, 1786–1792.
- Parnham, E.R.; Drylie, E.A.; Wheatley, P.S.; Slawin, A.M.Z.; Morris, R.E. Ionothermal Materials Synthesis Using Unstable Deep-Eutectic Solvents as Template-Delivery Agents. Angew. Chem. 2006, 118, 5084–5088.
- Chen, J.; Ali, M.C.; Liu, R.; Munyemana, J.C.; Li, Z.; Zhai, H.; Qiu, H. Basic Deep Eutectic Solvents as Reactant, Template and Solvents for Ultra-Fast Preparation of Transition Metal Oxide Nanomaterials. Chin. Chem. Lett. 2020, 31, 1584–1587.
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. Liquid Structure of the Choline Chloride-Urea Deep Eutectic Solvent (Reline) from Neutron Diffraction and Atomistic Modelling. Green Chem. 2016, 18, 2736–2744.
- McDonald, S.; Murphy, T.; Imberti, S.; Warr, G.G.; Atkin, R. Amphiphilically Nanostructured Deep Eutectic Solvents. J. Phys. Chem. Lett. 2018, 9, 3922–3927.
- Cui, Y.; Rushing, J.C.; Seifert, S.; Bedford, N.M.; Kuroda, D.G. Molecularly Heterogeneous Structure of a Nonionic Deep Eutectic Solvent Composed of N-Methylacetamide and Lauric Acid. J. Phys. Chem. B 2019, 123, 3984–3993.
- Sahu, S.; Banu, S.; Sahu, A.K.; Phani Kumar, B.V.N.; Mishra, A.K. Molecular-Level Insights into Inherent Heterogeneity of Maline Deep Eutectic System. J. Mol. Liq. 2022, 350, 118478.
- Karimi, M.; Rastegar Ramsheh, M.; Mohammad Ahmadi, S.; Reza Madani, M. One-Step and Low-Temperature Synthesis of Monetite Nanoparticles in an All-in-One System (Reactant, Solvent, and Template) Based on Calcium Chloride-Choline Chloride Deep Eutectic Medium. Ceram. Int. 2017, 43, 2046–2050.
- Jhang, P.-C.; Chuang, N.-T.; Wang, S.-L. Layered Zinc Phosphates with Photoluminescence and Photochromism: Chemistry in Deep Eutectic Solvents. Angew. Chem. 2010, 122, 4296–4300.
- Di Carmine, G.; Abbott, A.P.; D’Agostino, C. Deep eutectic solvents: Alternative reaction media for organic oxidation reactions. React. Chem. Eng. 2021, 6, 582–598.
- Wei, X.; Chen, J.; Ali, M.C.; Munyemana, J.C.; Qiu, H. Cadmium Cobaltite Nanosheets Synthesized in Basic Deep Eutectic Solvents with Oxidase-like, Peroxidase-like, and Catalase-like Activities and Application in the Colorimetric Assay of Glucose. Microchim. Acta 2020, 187, 314.
- Bonomo, M.; Gontrani, L.; Capocefalo, A.; Sarra, A.; Nucara, A.; Carbone, M.; Postorino, P.; Dini, D. A Combined Electrochemical, Infrared and EDXD Tool to Disclose Deep Eutectic Solvents Formation When One Precursor Is Liquid: Glyceline as Case Study. J. Mol. Liq. 2020, 319, 114292.
- Hammond, O.S.; Li, H.; Westermann, C.; Al-Murshedi, A.Y.M.; Endres, F.; Abbott, A.P.; Warr, G.G.; Edler, K.J.; Atkin, R. Nanostructure of the Deep Eutectic Solvent/Platinum Electrode Interface as a Function of Potential and Water Content. Nanoscale Horiz. 2019, 4, 158–168.
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. 2017, 129, 9914–9917.
- Liao, H.-G.; Jiang, Y.-X.; Zhou, Z.-Y.; Chen, S.-P.; Sun, S.-G. Shape-Controlled Synthesis of Gold Nanoparticles in Deep Eutectic Solvents for Studies of Structure-Functionality Relationships in Electrocatalysis. Angew. Chem. Int. Ed. 2008, 47, 9100–9103.
- Sales, V.; Paternoster, C.; Mantovani, D.; Kolliopoulos, G. Fe–Mn Alloys Electroforming Process Using Choline Chloride Based Deep Eutectic Solvents. Mater. Proc. 2021, 5, 40.
- Maciej, A.; Łatanik, N.; Sowa, M.; Matuła, I.; Simka, W. Electrodeposition of Copper and Brass Coatings with Olive-Like Structure. Materials 2021, 14, 1762.
- Wei, L.; Fan, Y.-J.; Tian, N.; Zhou, Z.-Y.; Zhao, X.-Q.; Mao, B.-W.; Sun, S.-G. Electrochemically Shape-Controlled Synthesis in Deep Eutectic Solvents—A New Route to Prepare Pt Nanocrystals Enclosed by High-Index Facets with High Catalytic Activity. J. Phys. Chem. C 2012, 116, 2040–2044.
- Hammons, J.A.; Muselle, T.; Ustarroz, J.; Tzedaki, M.; Raes, M.; Hubin, A.; Terryn, H. Stability, Assembly, and Particle/Solvent Interactions of Pd Nanoparticles Electrodeposited from a Deep Eutectic Solvent. J. Phys. Chem. C 2013, 117, 14381–14389.
- Wei, L.; Zhou, Z.-Y.; Chen, S.-P.; Xu, C.-D.; Su, D.; Schuster, M.E.; Sun, S.-G. Electrochemically Shape-Controlled Synthesis in Deep Eutectic Solvents: Triambic Icosahedral Platinum Nanocrystals with High-Index Facets and Their Enhanced Catalytic Activity. Chem. Commun. 2013, 49, 11152.
- Das, N.; Kumar, A.; Rayavarapu, R.G. The Role of Deep Eutectic Solvents and Carrageenan in Synthesizing Biocompatible Anisotropic Metal Nanoparticles. Beilstein J. Nanotechnol. 2021, 12, 924–938.
- Zhang, F.; Lai, J.; Huang, Y.; Li, F.; Luo, G.; Chu, G. A Green Method for Preparing CuCl Nanocrystal in Deep Eutectic Solvent. Aust. J. Chem. 2013, 66, 237.
- Huang, Y.; Shen, F.; La, J.; Luo, G.; Lai, J.; Liu, C.; Chu, G. Synthesis and Characterization of CuCl Nanoparticles in Deep Eutectic Solvents. Part. Sci. Technol. 2013, 31, 81–84.
- Chen, F.; Xie, S.; Zhang, J.; Liu, R. Synthesis of Spherical Fe3O4 Magnetic Nanoparticles by Co-Precipitation in Choline Chloride/Urea Deep Eutectic Solvent. Mater. Lett. 2013, 112, 177–179.
- Querejeta-Fernández, A.; Hernández-Garrido, J.C.; Yang, H.; Zhou, Y.; Varela, A.; Parras, M.; Calvino-Gámez, J.J.; González-Calbet, J.M.; Green, P.F.; Kotov, N.A. Unknown Aspects of Self-Assembly of PbS Microscale Superstructures. ACS Nano 2012, 6, 3800–3812.
- Gu, C.D.; Huang, M.L.; Ge, X.; Zheng, H.; Wang, X.L.; Tu, J.P. NiO Electrode for Methanol Electro-Oxidation: Mesoporous vs. Nanoparticulate. Int. J. Hydrogen Energy 2014, 39, 10892–10901.
- Ge, X.; Gu, C.D.; Lu, Y.; Wang, X.L.; Tu, J.P. A Versatile Protocol for the Ionothermal Synthesis of Nanostructured Nickel Compounds as Energy Storage Materials from a Choline Chloride-Based Ionic Liquid. J. Mater. Chem. A 2013, 1, 13454.
- Zhao, Y.; Zhao, Y.; Feng, H.; Shen, J. Synthesis of Nickel Phosphide Nano-Particles in a Eutectic Mixture for Hydrotreating Reactions. J. Mater. Chem. 2011, 21, 8137.
- Zheng, H.; Gu, C.-D.; Wang, X.-L.; Tu, J.-P. Fast Synthesis and Optical Property of SnO Nanoparticles from Choline Chloride-Based Ionic Liquid. J. Nanopart. Res. 2014, 16, 2288.
- Xiong, Q.Q.; Tu, J.P.; Ge, X.; Wang, X.L.; Gu, C.D. One-Step Synthesis of Hematite Nanospindles from Choline Chloride/Urea Deep Eutectic Solvent with Highly Powerful Storage versus Lithium. J. Power Sources 2015, 274, 1–7.
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Correlation between Microstructure and Electrochemical Behavior of the Mesoporous Co3O4 Sheet and Its Ionothermal Synthesized Hydrotalcite-like α-Co(OH)2 Precursor. J. Phys. Chem. C 2014, 118, 911–923.
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Endowing Manganese Oxide with Fast Adsorption Ability through Controlling the Manganese Carbonate Precursor Assembled in Ionic Liquid. J. Colloid Interface Sci. 2015, 438, 149–158.
- Gu, C.; Zhang, H.; Wang, X.; Tu, J. One-Pot Synthesis of SnO2/Reduced Graphene Oxide Nanocomposite in Ionic Liquid-Based Solution and Its Application for Lithium Ion Batteries. Mater. Res. Bull. 2013, 48, 4112–4117.
- Gu, C.D.; Xu, X.J.; Tu, J.P. Fabrication and Wettability of Nanoporous Silver Film on Copper from Choline Chloride-Based Deep Eutectic Solvents. J. Phys. Chem. C 2010, 114, 13614–13619.
- Zhang, H.; Lu, Y.; Gu, C.-D.; Wang, X.-L.; Tu, J.-P. Ionothermal Synthesis and Lithium Storage Performance of Core/Shell Structured Ni–P Nanoparticles. CrystEngComm 2012, 14, 7942.
- Abbott, A.P.; El Ttaib, K.; Ryder, K.S.; Smith, E.L. Electrodeposition of Nickel Using Eutectic Based Ionic Liquids. Trans. IMF 2008, 86, 234–240.
- Hou, Y.; Peng, Z.; Liang, J.; Fu, S. Ni–Ti Nanocomposite Coatings Electro-Codeposited from Deep Eutectic Solvent Containing Ti Nanoparticles. J. Electrochem. Soc. 2020, 167, 042502.
- Abbott, A.P.; Ttaib, K.E.; Frisch, G.; Ryder, K.S.; Weston, D. The Electrodeposition of Silver Composites Using Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2012, 14, 2443.
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F. From Nanoengineering to Nanomedicine: A Facile Route to Enhance Biocompatibility of Graphene as a Potential Nano-Carrier for Targeted Drug Delivery Using Natural Deep Eutectic Solvents. Chem. Eng. Sci. 2019, 195, 95–106.
- Mohammadpour, Z.; Abdollahi, S.H.; Safavi, A. Sugar-Based Natural Deep Eutectic Mixtures as Green Intercalating Solvents for High-Yield Preparation of Stable MoS2 Nanosheets: Application to Electrocatalysis of Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 5896–5906.
- Karimi, M.; Jodaei, A.; Khajvandi, A.; Sadeghinik, A.; Jahandideh, R. In-Situ Capture and Conversion of Atmospheric CO2 into Nano-CaCO3 Using a Novel Pathway Based on Deep Eutectic Choline Chloride-Calcium Chloride. J. Environ. Manag. 2018, 206, 516–522.
- Adhikari, L.; Larm, N.E.; Bhawawet, N.; Baker, G.A. Rapid Microwave-Assisted Synthesis of Silver Nanoparticles in a Halide-Free Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2018, 6, 5725–5731.
- Adhikari, L.; Larm, N.E.; Baker, G.A. Argentous Deep Eutectic Solvent Approach for Scaling Up the Production of Colloidal Silver Nanocrystals. ACS Sustain. Chem. Eng. 2019, 7, 11036–11043.
- Adhikari, L.; Larm, N.E.; Baker, G.A. Batch and Flow Nanomanufacturing of Large Quantities of Colloidal Silver and Gold Nanocrystals Using Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2020, 8, 14679–14689.
- Antenucci, A.; Bonomo, M.; Ghigo, G.; Gontrani, L.; Barolo, C.; Dughera, S. How Do Arenediazonium Salts Behave in Deep Eutectic Solvents? A Combined Experimental and Computational Approach. J. Mol. Liq. 2021, 339, 116743.
- Li, X.; Row, K.H. Preparation of Deep Eutectic Solvent-Based Hexagonal Boron Nitride-Molecularly Imprinted Polymer Nanoparticles for Solid Phase Extraction of Flavonoids. Microchim. Acta 2019, 186, 753.
- Sun, Y.; Cheng, S.; Mao, Z.; Lin, Z.; Ren, X.; Yu, Z. High Electrochemical Activity of a Ti/SnO2–Sb Electrode Electrodeposited Using Deep Eutectic Solvent. Chemosphere 2020, 239, 124715.
- Mitar, A.; Prlić Kardum, J. Intensification of Mass Transfer in the Extraction Process with a Nanofluid Prepared in a Natural Deep Eutectic Solvent. Chem. Eng. Technol. 2020, 43, 2286–2294.
- Li, W.; Zhao, X.; Huang, T.; Ren, Y.; Gong, W.; Guo, Y.; Wang, J.; Tu, Q. Preparation of Sodium Hyaluronate/Dopamine/AgNPs Hydrogel Based on the Natural Eutetic Solvent as an Antibaterial Wound Dressing. Int. J. Biol. Macromol. 2021, 191, 60–70.
- Li, H.; Wang, Y.; He, X.; Chen, J.; Xu, F.; Liu, Z.; Zhou, Y. A Green Deep Eutectic Solvent Modified Magnetic Titanium Dioxide Nanoparticles for the Solid-Phase Extraction of Chymotrypsin. Talanta 2021, 230, 122341.
- Jamshidi, F.; Nouri, N.; Sereshti, H.; Shojaee Aliabadi, M.H. Synthesis of Magnetic Poly (Acrylic Acid-Menthol Deep Eutectic Solvent) Hydrogel: Application for Extraction of Pesticides. J. Mol. Liq. 2020, 318, 114073.
- Vallejo-Macías, M.T.; Recio-Colmenares, C.L.; Pelayo-Vázquez, J.B.; Gómez-Salazar, S.; Carvajal-Ramos, F.; Soltero-Martínez, J.F.; Vázquez-Lepe, M.; Mota-Morales, J.D.; Pérez-García, M.G. Macroporous Polyacrylamide γ-Fe2O3 Nanoparticle Composites as Methylene Blue Dye Adsorbents. ACS Appl. Nano Mater. 2020, 3, 5794–5806.
- Qin, H.; Hu, X.; Wang, J.; Cheng, H.; Chen, L.; Qi, Z. Overview of Acidic Deep Eutectic Solvents on Synthesis, Properties and Applications. Green Energy Environ. 2020, 5, 8–21.
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. Structure and Properties of “Type IV” Lanthanide Nitrate Hydrate:Urea Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 4932–4940.
- Gupta, R.; Gamare, J.; Sahu, M.; Pandey, K.; Gupta, S.K. Electrochemical and Thermodynamic Insights on Actinide Type (IV) Deep Eutectic Solvent. J. Mol. Liq. 2021, 329, 115550.
- Douglas, F.J.; MacLaren, D.A.; Murrie, M. A Study of the Role of the Solvent during Magnetite Nanoparticle Synthesis: Tuning Size, Shape and Self-Assembly. RSC Adv. 2012, 2, 8027.
More
Information
Subjects:
Chemistry, Inorganic & Nuclear
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.0K
Entry Collection:
Chemical Bond
Revisions:
3 times
(View History)
Update Date:
07 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




