Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hualu Zhou | -- | 3547 | 2022-04-06 14:30:36 | | | |
| 2 | Rita Xu | Meta information modification | 3547 | 2022-04-07 03:33:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhou, H.; Mcclements, D. Organic and Inorganic Nanoparticles in Foods. Encyclopedia. Available online: https://encyclopedia.pub/entry/21411 (accessed on 08 February 2026).
Zhou H, Mcclements D. Organic and Inorganic Nanoparticles in Foods. Encyclopedia. Available at: https://encyclopedia.pub/entry/21411. Accessed February 08, 2026.
Zhou, Hualu, David Mcclements. "Organic and Inorganic Nanoparticles in Foods" Encyclopedia, https://encyclopedia.pub/entry/21411 (accessed February 08, 2026).
Zhou, H., & Mcclements, D. (2022, April 06). Organic and Inorganic Nanoparticles in Foods. In Encyclopedia. https://encyclopedia.pub/entry/21411
Zhou, Hualu and David Mcclements. "Organic and Inorganic Nanoparticles in Foods." Encyclopedia. Web. 06 April, 2022.
Copy Citation
Inorganic or organic nanoparticles are often incorporated into foods to enhance their quality, stability, nutrition, or safety. When they pass through the gastrointestinal environment, the properties of these nanoparticles are altered, which impacts their biological effects and potential toxicity. Consequently, there is a need to understand how different kinds of nanoparticles behave within the gastrointestinal tract.
nanoparticles
gastrointestinal fate
food nanotechnology
1. Introduction
Nanomaterials are being explored for application in the food industry due to their ability to create new or improved properties in foods and packaging materials [1]. The development of food nanomaterials is an interdisciplinary research effort that includes contributions from the physical, chemical, biological, engineering, and pharmaceutical sciences [1][2].
Nanomaterials have at least one dimension below 100 nm, which includes many kinds of edible fibers, sheets, and particles. However, it should be noted that many food components with larger dimensions (100–1000 nm) are also considered as nanomaterials by some researchers [3][4]. This is because there is typically not a distinct change in the physical, chemical, or biological properties of materials when one of their dimensions falls below 100 nm. Instead, there is typically a more gradual transition in these properties as a material becomes smaller. Some researchers have divided nanomaterials into different categories depending on their attributes. For instance, they have been categorized as either soft or hard nanomaterials [4]. Soft nanomaterials are typically created from organic matter (such as proteins, polysaccharides, and lipids) and tend to be digestible and/or fermentable in the human gastrointestinal tract. In contrast, hard nanomaterials are often constructed from inorganic matter (such as metals or metal salts) and tend to be indigestible and/or non-fermentable. Nanomaterials may also be classified as natural or engineered. Naturally occurring nanomaterials include the casein micelles in milk and the oil bodies in seeds. Engineered nanoparticles are typically designed and synthesized to have compositions and physicochemical properties that lead to specific desirable functional attributes [5]. Both inorganic (such as TiO2, Fe2O3, and Ag) or organic (such as polysaccharides, lipids, and proteins) materials can be used to fabricate engineered nanoparticles suitable for food applications [6].
Nanoparticles are the most common type of nanomaterial currently employed in foods [7]. These nanoparticles come in different compositions, sizes, and shapes, with the most frequently used being spheres, ellipsoids, and fibers. The incorporation of these nanoparticles into foods is often used to improve their optical, flavor, textural, stability, safety, and nutritional properties [8][9]. They have also been also as additives in biodegradable food packaging materials designed to prolong the shelf life of foods [10][11][12]. The extent of the research effort in this area is demonstrated by the continued growth in the number of scientific publications on the applications of nanoparticles in foods (Figure 1).
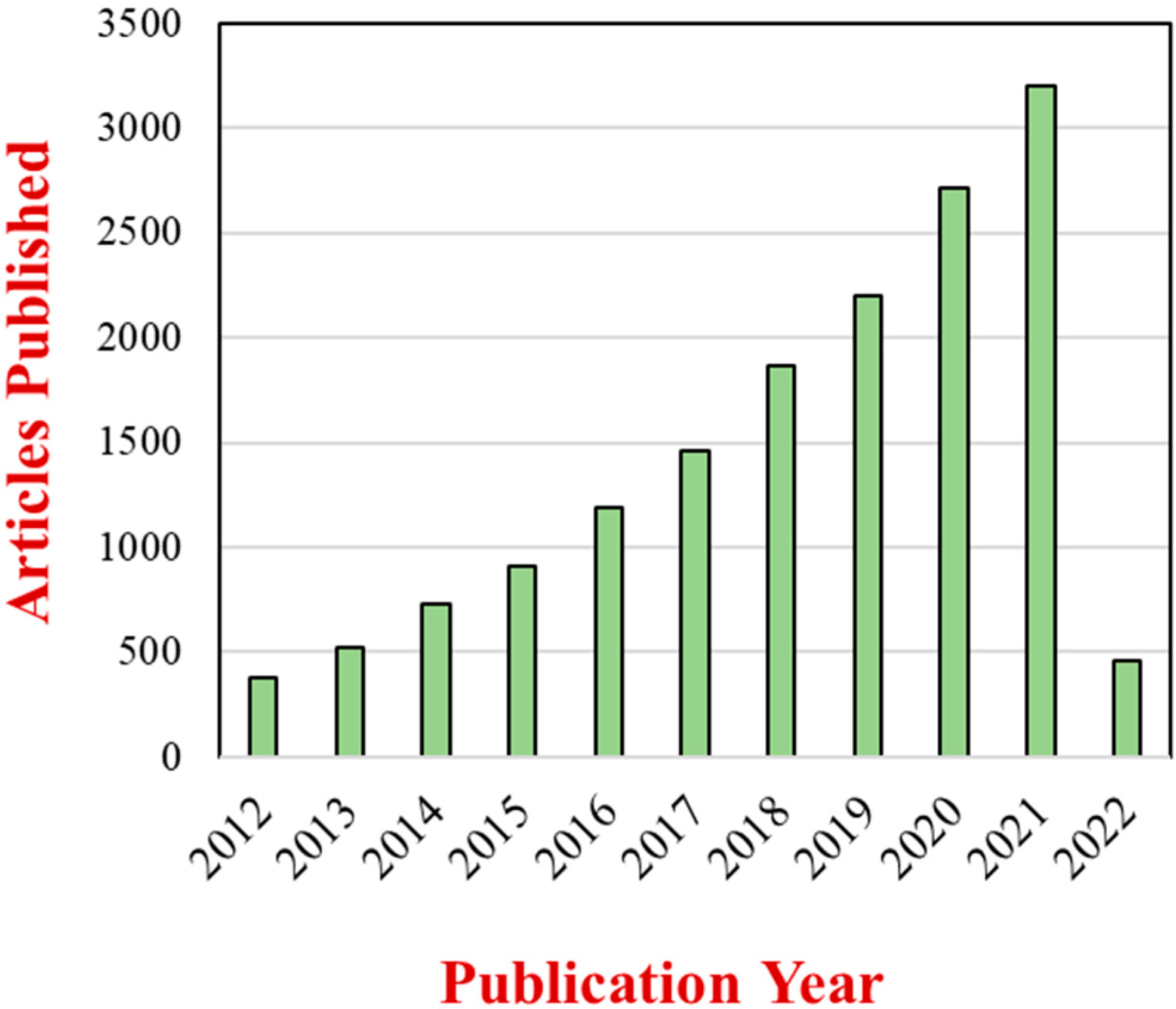
Figure 1. Number of articles with two topic keywords, “nanoparticles” and “foods”, published from 2012 to March 2022 based on a Web of Science Core Collection source.
After ingestion, any nanoparticles present within a food enter the human gastrointestinal tract (GIT) and are exposed to the various conditions that exist in the mouth, esophagus, stomach, small intestine, and colon [8][9]. Exposure to these conditions changes the properties of the nanoparticles, which alters their GIT fate and potential toxicity. Moreover, the nanoparticles themselves may interfere with the different physicochemical and physiological processes occurring within the GIT, such as digestion, transport, metabolism, and absorption. As a result, their presence may alter the digestion and absorption of other components within foods, thereby affecting their pharmacokinetics and bioavailability.
2. Intrinsic Properties of Nanoparticles
The intrinsic properties of nanoparticles, such as their size, shape, composition, and interfacial properties, potentially have a major effect on their applications within foods (Figure 2). These properties influence the optical, rheological, flavor, and nutritional attributes of foods and beverages. Moreover, they also affect their behavior within the GIT and therefore impact their digestibility and bioavailability. Therefore, it is important to be able to control and characterize the intrinsic properties of nanoparticles added to foods.
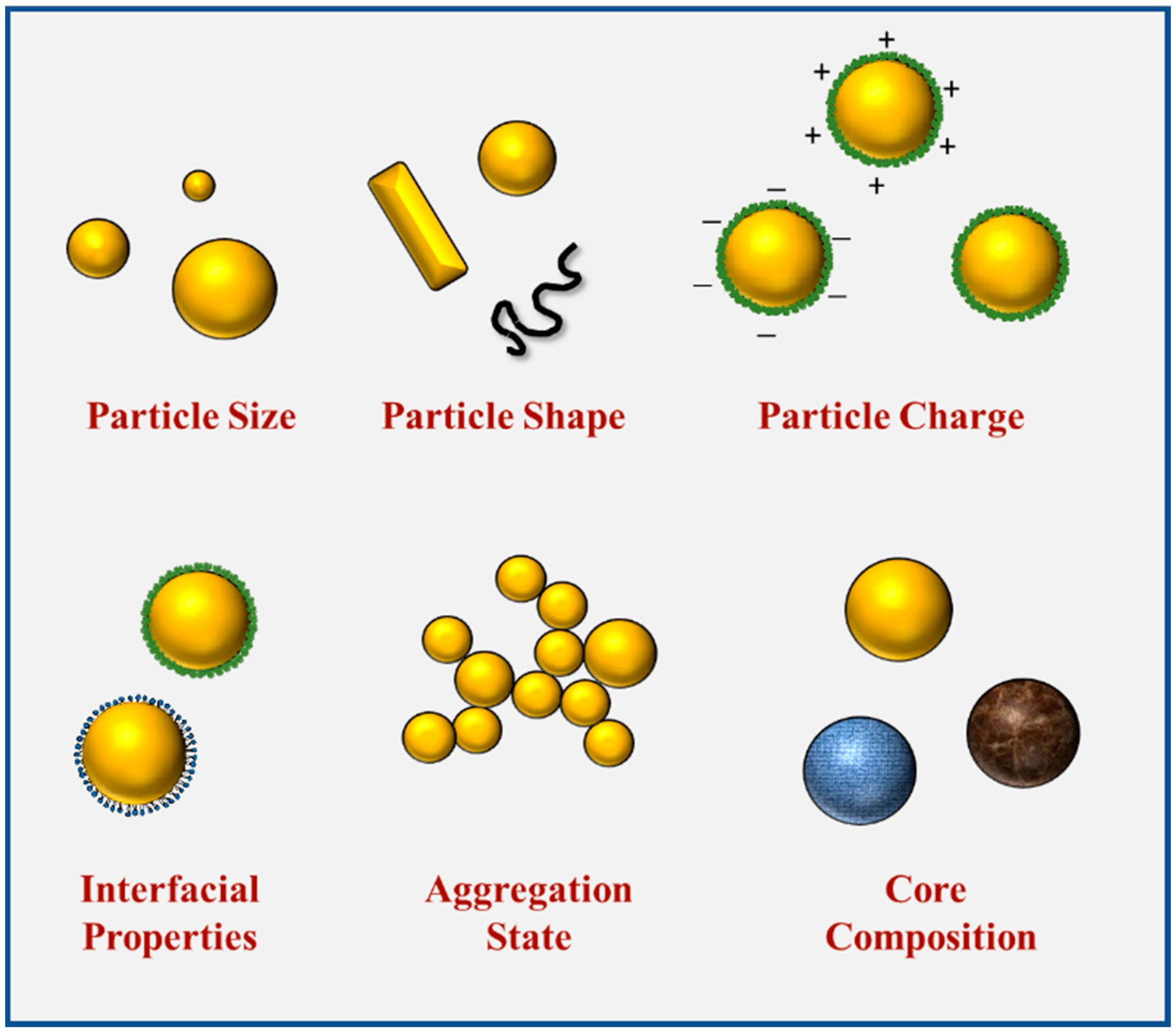
Figure 2. The characteristics of food-grade nanoparticles, including size, shape, composition, aggregation, and interfacial properties.
2.1. Size and Shape
The dimensions of the nanoparticles used in foods may vary from a few nanometers to a few hundred nanometers depending on their biological origin (for natural nanoparticles) or the ingredients and processing methods used to fabricate them (for engineered nanoparticles). Particle dimensions have a major influence on the physical, chemical, and biological properties of nanoparticles. The optical clarity of nanoparticle dispersions decreases as the particle dimensions decrease, which is important for developing transparent foods and drinks. Moreover, the resistance of nanoparticle dispersions to gravitational separation and aggregation increases as the particle dimensions are reduced, which is useful for increasing the shelf life of foods. After ingestion, the dimensions of the nanoparticles influence their gastrointestinal fate. The rate of digestion of digestible nanoparticles tends to increase as their dimensions decrease, because this increases the surface area exposed to the digestive enzymes in the gastrointestinal fluids [9][13]. Conversely, the ability of indigestible nanoparticles to pass through the pores in the mucus layer and be absorbed by the epithelium cells in the GIT tends to increase as their dimensions decrease [14]. The most common nanoparticles in foods are spherical, but other shapes are possible, such as fibers, ellipsoids, or cuboids [15]. The shape of ingested nanoparticles would also be expected to influence food properties and their biological fate. For instance, the ability of nanoparticles to increase the viscosity of foods tends to increase as their length-to-width ratio increases. Moreover, the ability of nanoparticles to penetrate through the mucus layer is typically easier for spheres than fibers.
2.2. Composition
The composition of nanoparticles has a major impact on their behavior in foods as well as their GIT fate. Nanoparticles that only contain inorganic substances may not be digested within the GIT, such as those comprised of TiO2. However, some inorganic nanoparticles do dissolve in the acidic gastric environment, such as those consisting of ZnO and Fe2O3 [16]. Organic nanoparticles are typically fabricated from lipids, proteins, and carbohydrates, which may be either digestible or indigestible depending on the precise nature of the ingredients present [6]. For instance, some lipids are digestible (such as triacylglycerols), whereas others are not (such as mineral oils, essential oils and flavor oils). Similarly, some carbohydrates are digestible (such as starch), whereas others are not (such as cellulose and chitosan). Moreover, the rate of digestion may vary depending on the type of macronutrients used and their organization within the nanoparticles.
2.3. Interfacial Properties
The interfacial properties of food-grade nanoparticles, including their composition, charge, polarity, thickness, and chemical reactivity, are also important because they influence their interactions with food matrix and GIT components [17]. The interfacial properties of engineered nanoparticles can be controlled by using different kinds of emulsifiers or other coating materials. It should be stressed that the interfacial properties of nanoparticles change appreciably after they are introduced into foods and as they pass through the GIT [13]. This phenomenon occurs because the original interfacial layers may be digested or displaced from the nanoparticle surfaces and/or because other components in the surroundings can adsorb to their surfaces. As a result, the composition, charge, polarity, thickness, and chemical reactivity of the interfacial layers of the nanoparticles changes, which alters their behavior in foods and the GIT. For instance, interfacial composition is likely to influence the ability of nanoparticles to penetrate through the mucus layer and be absorbed by epithelium cells [13]. Moreover, the relatively large specific surface area of ingested nanoparticles means that they may adsorb components within the gastrointestinal fluids to their surfaces, such as mucin, enzymes, bile salts, mineral ions, and proteins. As a result, the presence of the nanoparticles could interfere with normal digestion processes.
3. Types of Food Nanoparticles
3.1. Organic Nanoparticles
Organic nanoparticles are typically fabricated from lipids, proteins, and/or carbohydrates (Figure 3). These nanoparticles are usually incorporated into foods to provide desirable optical, textural, flavor, or nutritional attributes [15]. In general, the safety of organic nanoparticles is of less concern than that of inorganic ones because they are usually fully digested. For this reason, food scientists are typically more focused on their fabrication and utilization as functional ingredients in foods [9]. Nevertheless, there may be some potential toxicity concerns with digestible nanoparticles, such as their ability to greatly increase the bioavailability of encapsulated or co-ingested hydrophobic substances. In most cases, this is advantageous, but in some cases, it may lead to problems. For instance, they could increase the absorption of undesirable hydrophobic substances in foods, such as pesticides [18]. Alternatively, they could increase the bioavailability of food components into a region where they exhibit some toxicity.
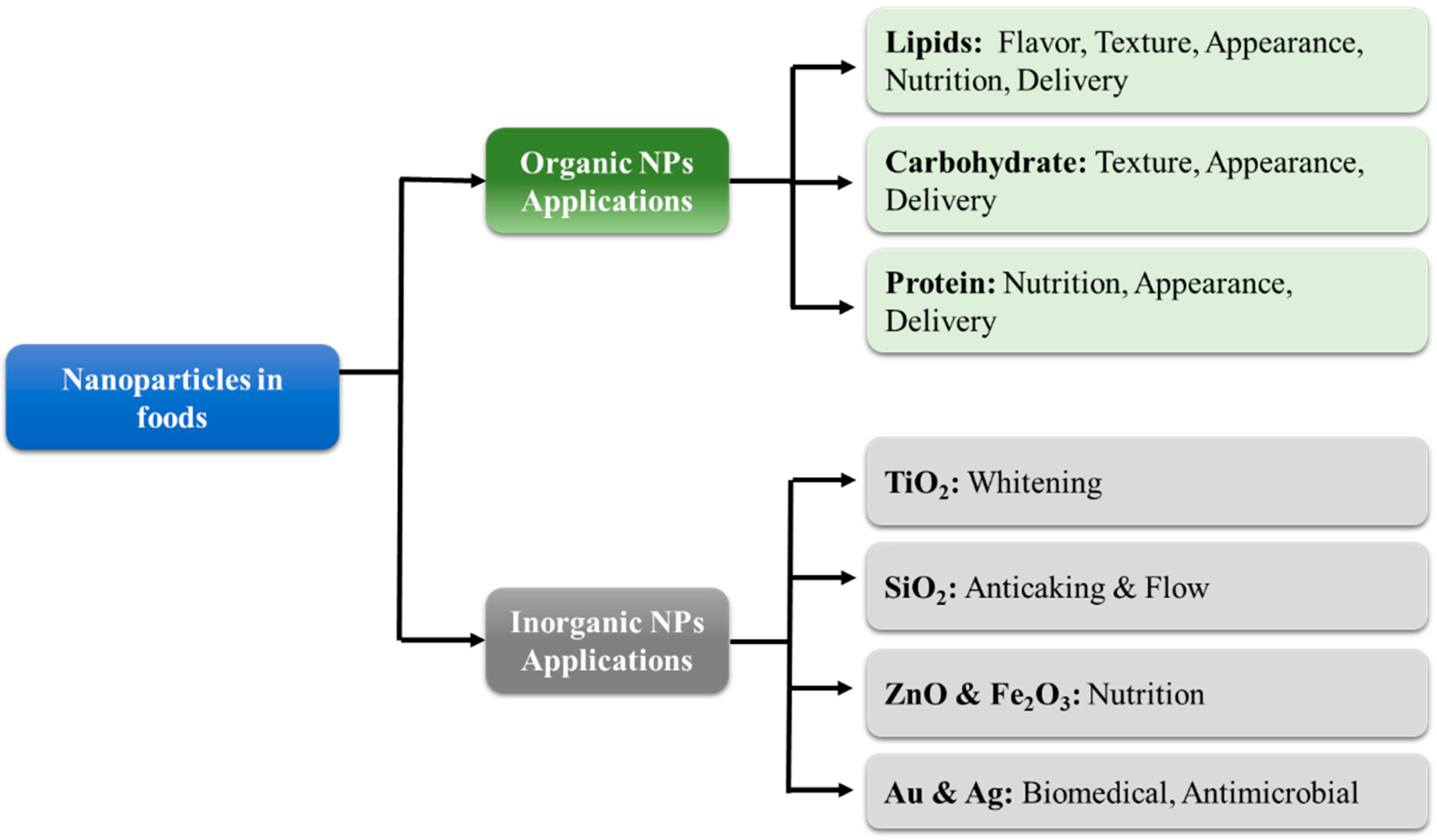
Figure 3. The categories and applications of organic and inorganic nanoparticles in foods.
3.1.1. Carbohydrate Nanoparticles
The main structural components used to fabricate carbohydrate-based nanoparticles are polysaccharides. This type of nanoparticle can be fabricated using various approaches. For example, carbohydrate nanoparticles can be assembled by bottom–up methods that are based on the self-assembly of polysaccharides under appropriate environmental conditions, e.g., pectin, alginate, carrageenan, agar, starch, and chitosan [19][20][21]. Typically, solution or environmental conditions, such as solvent quality, pH, ionic strength, enzyme activity, or temperature, are changed to promote the formation of physical or chemical crosslinks between the polysaccharide molecules. Conversely, nanocrystals or nanofibers can be obtained using top–down methods that utilize the controlled disintegration of bulk polysaccharide materials, such as acid or alkaline treatment of cellulose or chitin [22][23]. Carbohydrate nanoparticles are often used as carriers for nutraceuticals, vitamins, or other bioactive agents, but they may also be used as texture modifiers, film formers, lightening agents, and UV-light blockers. After oral intake, the gastrointestinal fate of carbohydrate nanoparticles is strongly related to their digestibility. Nanoparticles assembled from digestible polysaccharides, such as some types of starch, are hydrolyzed in the mouth and small intestine by amylases. Conversely, nanoparticles fabricated from indigestible polysaccharides, such as cellulose, chitin, or gums, are not hydrolyzed until they reach the colon, where they may be fermented by colonic bacteria. These characteristics also influence the pharmacokinetics and bioavailability of other bioactive components encapsulated within carbohydrate nanoparticles [24]. For instance, if a bioactive component needs to be delivered to the mouth, then it can be encapsulated within digestible starch nanoparticles. If it needs to be delivered into the small intestine, then it can be encapsulated inside protein nanoparticles. Finally, if it needs to be delivered into the colon, then it can be encapsulated within dietary fiber-based nanoparticles.
3.1.2. Protein Nanoparticles
Protein nanoparticles have also been explored for many years for their potential utilization as functional ingredients in foods and beverages [25]. Casein micelles are natural nanoparticles found in milk that are assembled from protein, calcium, and phosphate, which are held together by physical forces such as electrostatic and hydrophobic attraction. Engineered protein nanoparticles have also being widely explored for their utilization as carrier systems, texture modifiers, fat replacers, and lightening agents [25]. A number of different preparation methods have been developed, with the most suitable one depending on the nature of the proteins. For example, protein nanoparticles can be formed from hydrophobic proteins, such as zein or gliadin, using an antisolvent precipitation method [26]. In this method, the hydrophobic proteins are first dissolved within a good solvent, such as a concentrated ethanol solution. Then, this solution is added to a bad solvent, such as water, which leads to the spontaneous formation of protein nanoparticles because the hydrophobic protein molecules do not want to be in contact with water and so interact with each other [15]. The hydrophobic interior of this kind of nanoparticle is particularly suitable for the encapsulation of lipophilic bioactives, such as oil-soluble vitamins and nutraceuticals [27]. Protein nanoparticles can also be assembled from hydrophilic proteins by heating them to a temperature above their thermal denaturation temperature, which causes them to unfold and expose hydrophobic groups. As a result, the protein molecules self-assemble into nanoparticles to reduce the number of hydrophobic groups exposed to water. It is more difficult to entrap bioactives into nanoparticles assembled from hydrophilic proteins using this method, but the nanoparticles can be used as texture modifiers, lightening agents, or fat replacers. The charge on bare protein nanoparticles tends to move from positive to negative as the pH is raised from below to above their isoelectric points. Around their isoelectric point, they tend to aggregate because the weak electrostatic repulsion between them is not strong enough to overcome the van der Waals or hydrophobic attraction. For this reason, protein nanoparticles are often coated with surfactants or charged biopolymers to increase the repulsive forces or decrease the attractive forces acting between them, thereby improving their resistance to aggregation.
The GIT fate of protein nanoparticles depends on their size, composition, and surface characteristics. They are typically digested within the stomach and small intestine due to the presence of gastric (pepsin) and pancreatic (trypsin and chymotrypsin) proteases. However, the rate of digestion may depend on the type of proteins used, their conformation, and any coating materials used. Consequently, it is possible to control the retention and release of bioactives inside protein nanoparticles.
3.1.3. Lipid Nanoparticles
Lipid nanoparticles have been the focus of many studies on the application of nanotechnology within foods and beverages and are already widely utilized in some food and beverage products. This kind of nanoparticle is formed during the homogenization of milk and beverages, including many soft drinks. They may also be produced using various other high-energy (mechanical) and low-energy (physicochemical) methods [28]. For instance, they can be produced using sonicators or microfluidizers (high-energy) or using spontaneous emulsification or phase inversion temperature methods (low-energy). Lipid nanoparticles are often utilized as carriers for lipophilic nutraceuticals and oil-soluble vitamins, which can help solve challenges associated with their poor water solubility, chemical stability, and bioavailability characteristics [15]. Numerous kinds of lipid nanoparticles have been developed as carriers, including micelles, nanoliposomes, nanoemulsions, and solid lipid nanoparticles [2]. Polar lipids such as phospholipids that contain a hydrophilic “head” and two hydrophobic “tails” can form liposomes, whereas non-polar lipids such as triacylglycerols can be used to form the core of oil droplets or solid fat particles.
In the GIT, digestible lipid nanoparticles, such as those fabricated from triacylglycerols, are digested by lipase in the stomach and small intestine, whereas indigestible ones, such as those fabricated from essential, flavor, or mineral oils, are not. Lipid nanoparticles have relatively large specific surface areas, which means that lipase can rapidly adsorb to their surfaces, leading to rapid lipid digestion and bioactive release [29]. However, the emulsifier used to coat the lipid nanoparticles also plays an important role in determining their GIT fat because it can alter their aggregation state in the GIT or inhibit the adsorption of bile salts or lipase [30][31]. Consequently, it is important to select an appropriate emulsifier type and oil phase for the specific application.
3.1.4. Composite Nanoparticles
Carbohydrates, proteins, and lipids can be used in combination to create composite nanoparticles [32]. Many proteins and carbohydrates have hydrophilic and lipophilic areas on their surfaces and so can act as emulsifiers that promote the formation and stabilization of lipid nanoparticles [33]. Polysaccharides and proteins that have opposite charges can be made to assemble into composite nanoparticles via electrostatic attraction. Composite nanoparticles have great potential within the food industry because their functional properties can be tailored for particular applications.
3.2. Inorganic Nanoparticles
Several kinds of inorganic nanoparticles can also be used in foods for their functional attributes (Figure 3), such as their ability to alter the appearance, texture, stability, or nutritional profile of foods or packaging materials. However, as mentioned earlier, there is often more concern about the potential toxicity of inorganic nanoparticles in foods than organic ones because they are not digested in the GIT but may still be absorbed by the human body [34]. In this section, a brief overview of some of the inorganic nanoparticles that are commonly used in foods or food packaging materials is given.
3.2.1. Titanium Dioxide (TiO2) Nanoparticles
Titanium dioxide is commonly used in food products as a brightening or whitening agent because the crystalline material is white and has a high refractive index so that TiO2 particles scatter light strongly. For this reason, it has been widely in foods to improve their visual appearance, most notably candies, chewing gums, bakery goods, and milk powders. The powdered TiO2 used as an additive in the food industry (E171) contains a broad range of differently sized particles, with an appreciable fraction falling into the nano-range. Indeed, analysis of a commercial TiO2 food additive found that more than 36% of the particles had diameters below 100 nm [35]. These nanoparticles are likely to behave differently in the human gut than the larger particles because of their small dimensions and high surface areas, which has raised some health concerns [36][37]. Indeed, the French government recently banned the use of this kind of nanoparticles in foods due to these concerns [38]. However, it should be noted that the potential toxicity exhibited by TiO2 nanoparticles in cell culture and animal feeding experiments varies considerably from study to study. Some studies suggest that TiO2 nanoparticles alter the gut microbiota or accumulate in the tissues of mammals and other vertebrates with a low elimination rate, whereas others indicated low toxicity and accumulation [39][40][41]. Part of the variations in findings between studies may be because the impact of food matrix effects and GIT conditions on the gastrointestinal fate of TiO2 nanoparticles is often ignored as well as due to differences in the nature of the TiO2 nanoparticles used.
3.2.2. Silicon Dioxide (SiO2) Nanoparticles
Powdered silicon dioxide is commonly used as an additive in the food industry because it is capable of strongly absorbing water, thereby acting as an anticaking agent [42]. For this reason, it is often incorporated into powdered foods such as salts, dried milk, and icing sugar to prevent clumping and to enhance their flow properties. Most of the particles in commercial food-grade SiO2 additives (E551) fall between 100 and 1000 nm in diameter, but an appreciable proportion may fall into the nano-range [43]. Some cell culture studies have indicated that high levels of SiO2 nanoparticles caused cytotoxic and genotoxic effects [44]. However, another study reported no accumulation or toxicity of this type of nanoparticle when it was fed to rats [45]. Again, there appear to be large variations in the potential toxicity of this kind of inorganic nanoparticle reported in different studies. This apparent discrepancy may be because of differences in the nature of the SiO2 additives tested (such as dose, size, aggregation state, and surface characteristics), as well as differences in food matrix and GIT effects.
3.2.3. Zinc Oxide (ZnO) and Iron Oxide (Fe2O3) Nanoparticles
Zinc and iron are important micronutrients that are often lacking from the human diet, and so foods are often fortified with bioavailable forms of these essential minerals [46]. For this reason, powdered ZnO and Fe2O3 additives are sometimes used as a source of these minerals in functional foods and nutritional supplements. As with other inorganic additives, these powders are engineered particles, which likely contain a range of different particle sizes with some falling within the nano-scale range [47]. These additives may also be incorporated into food packaging materials because of their strong antimicrobial activity. ZnO nanoparticles can penetrate through microbial cell walls and generate reactive oxygen species (ROS) inside the cells, thereby damaging critical cellular components and interfering with key biochemical pathways, ultimately leading to cytotoxicity [48]. Fe2O3 nanoparticles have also been reported to generate ROS and promote oxidative stress in human lymphocytes [49]. There are also concerns that these nanoparticles could penetrate into human cells after ingestion and have similar effects [50]. However, the extent of these effects depends on the dose used. It has been estimated that people typically consume around 0.45 mg/day of iron oxide, but the amount taken from dietary supplements may range from 10 to 32 mg/day [51]. Nevertheless, a rat feeding study reported no appreciable tissue accumulation or toxicity for this type of nanoparticle, even when it was administered by relatively high doses (250–10,000 mg/kg body weight) [52]. Unlike TiO2 and SiO2 particles, ZnO and Fe2O3 particles may be dissolved in the acidic gastric fluids, thereby altering their gastrointestinal fate [53].
3.2.4. Gold and Silver Nanoparticles
Colloidal forms of gold or silver nanoparticles have been used in a diverse range of applications within foods and biomedicines [54][55]. The applications of golden nanoparticles in the biomedical field have been reviewed in detail in several recent articles, including drug delivery, bioimaging, and cancer therapy [56][57][58]. They have also been used to formulate food packaging materials, which may also lead to oral exposure, e.g., due to the migration of silver nanoparticles from packages into foods [59]. In principle, any silver nanoparticles present in packaging materials that are in contact with foods may diffuse into the foods themselves and therefore be ingested. However, the extent of this effect depends on the nanoparticles, packaging materials, and storage conditions used. Nevertheless, it has been estimated that the amount of silver consumed by human adults (20 to 80 μg/day) is relatively low, and only a fraction of this is actually silver nanoparticles [60]. Some studies have reported that a small fraction (<1%) of ingested silver nanoparticles can accumulate in tissues but that most of them are excreted in the feces or urine [61]. Thus, the potential adverse effects of silver nanoparticles still remain inconclusive, and more studies are needed, especially on their long-term chronic toxicity. It should be noted that silver can undergo chemical reactions in foods and the GIT that could alter its gastrointestinal fate. For instance, silver may be oxidized to silver oxide in air, which can dissolve under acidic conditions, or silver ions can precipitate when they come into contact with chloride ions, thereby leading to the possible formation of new nanoparticles [62][63].
References
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45.
- Luo, Y. Perspectives on important considerations in designing nanoparticles for oral delivery applications in food. J. Agric. Food Res. 2020, 2, 100031.
- Shah, S.; Nene, S.; Rangaraj, N.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Bridging the gap: Academia, industry and FDA convergence for nanomaterials. Drug Dev. Ind. Pharm. 2020, 46, 1735–1746.
- Xavier, M.; Parente, I.A.; Rodrigues, P.M.; Cerqueira, M.A.; Pastrana, L.; Gonçalves, C. Safety and fate of nanomaterials in food: The role of in vitro tests. Trends Food Sci. Technol. 2021, 109, 593–607.
- Prajitha, N.; Athira, S.S.; Mohanan, P.V. Bio-interactions and risks of engineered nanoparticles. Environ. Res. 2019, 172, 98–108.
- Liu, X.; Zhang, B.; Sohal, I.S.; Bello, D.; Chen, H. Chapter Eight—Is “nano safe to eat or not”? A review of the state-of-the art in soft engineered nanoparticle (sENP) formulation and delivery in foods. In Advances in Food and Nutrition Research; Lim, L.-T., Rogers, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 88, pp. 299–335.
- Cao, Y.; Li, J.; Liu, F.; Li, X.Y.; Jiang, Q.; Cheng, S.S.; Gu, Y.X. Consideration of interaction between nanoparticles and food components for the safety assessment of nanoparticles following oral exposure: A review. Environ. Toxicol. Pharmacol. 2016, 46, 206–210.
- McClements, D.J.; DeLoid, G.; Pyrgiotakis, G.; Shatkin, J.A.; Xiao, H.; Demokritou, P. The role of the food matrix and gastrointestinal tract in the assessment of biological properties of ingested engineered nanomaterials (iENMs): State of the science and knowledge gaps. NanoImpact 2016, 3–4, 47–57.
- McClements, D.J.; Xiao, H.; Demokritou, P. Physicochemical and colloidal aspects of food matrix effects on gastrointestinal fate of ingested inorganic nanoparticles. Adv. Colloid Interface Sci. 2017, 246, 165–180.
- Moustafa, H.; Darwish, N.A.; Youssef, A.M. Rational formulations of sustainable polyurethane/chitin/rosin composites reinforced with ZnO-doped-SiO2 nanoparticles for green packaging applications. Food Chem. 2022, 371, 131193.
- Moustafa, H.; El-Sayed, S.M.; Youssef, A.M. Synergistic impact of cumin essential oil on enhancing of UV-blocking and antibacterial activity of biodegradable poly(butylene adipate-co-terephthalate)/clay platelets nanocomposites. J. Thermoplast. Compos. Mater. 2021.
- Moustafa, H.; Karmalawi, A.M.; Youssef, A.M. Development of dapsone-capped TiO2 hybrid nanocomposites and their effects on the UV radiation, mechanical, thermal properties and antibacterial activity of PVA bionanocomposites. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100482.
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 6.
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185.
- Pan, K.; Zhong, Q. Organic Nanoparticles in Foods: Fabrication, Characterization, and Utilization. Annu. Rev. Food Sci. Technol. 2016, 7, 245–266.
- Sohal, I.S.; Cho, Y.K.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Dissolution behavior and biodurability of ingested engineered nanomaterials in the gastrointestinal environment. ACS Nano 2018, 12, 8115–8128.
- Moradi, M.; Razavi, R.; Omer, A.K.; Farhangfar, A.; McClements, D.J. Interactions between nanoparticle-based food additives and other food ingredients: A review of current knowledge. Trends Food Sci. Technol. 2022, 120, 75–87.
- Zhang, R.; Zhang, Z.; Li, R.; Tan, Y.; Lv, S.; McClements, D.J. Impact of Pesticide Type and Emulsion Fat Content on the Bioaccessibility of Pesticides in Natural Products. Molecules 2020, 25, 1466.
- Koshani, R.; Madadlou, A. A viewpoint on the gastrointestinal fate of cellulose nanocrystals. Trends Food Sci. Technol. 2018, 71, 268–273.
- Mackie, A.; Gourcy, S.; Rigby, N.; Moffat, J.; Capron, I.; Bajka, B. The fate of cellulose nanocrystal stabilised emulsions after simulated gastrointestinal digestion and exposure to intestinal mucosa. Nanoscale 2019, 11, 2991–2998.
- Qiu, C.; Wang, C.; Gong, C.; McClements, D.J.; Jin, Z.; Wang, J. Advances in research on preparation, characterization, interaction with proteins, digestion and delivery systems of starch-based nanoparticles. Int. J. Biol. Macromol. 2020, 152, 117–125.
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum alkaline treatment parameters for the extraction of cellulose and production of cellulose nanocrystals from apple pomace. Carbohydr. Polym. 2019, 215, 330–337.
- Lv, S.; Zhou, H.; Bai, L.; Rojas, O.J.; McClements, D.J. Development of food-grade Pickering emulsions stabilized by a mixture of cellulose nanofibrils and nanochitin. Food Hydrocoll. 2021, 113, 106451.
- Qin, D.; Yang, X.; Gao, S.; Yao, J.; McClements, D.J. Influence of dietary fibers on lipid digestion: Comparison of single-stage and multiple-stage gastrointestinal models. Food Hydrocoll. 2017, 69, 382–392.
- Cho, Y.-H.; Jones, O.G. Chapter Two—Assembled protein nanoparticles in food or nutrition applications. In Advances in Food and Nutrition Research; Lim, L.-T., Rogers, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 88, pp. 47–84.
- Wang, Y.; Yan, W.; Li, R.; Jia, X.; Cheng, Y. Impact of deamidation on gliadin-based nanoparticle formation and curcumin encapsulation. J. Food Eng. 2019, 260, 30–39.
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82.
- Safaya, M.; Rotliwala, Y.C. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Mater. Today Proc. 2020, 27, 454–459.
- McClements, D.J. Advances in edible nanoemulsions: Digestion, bioavailability, and potential toxicity. Prog. Lipid Res. 2021, 81, 101081.
- Tan, Y.; Zhang, Z.; Muriel Mundo, J.; McClements, D.J. Factors impacting lipid digestion and nutraceutical bioaccessibility assessed by standardized gastrointestinal model (INFOGEST): Emulsifier type. Food Res. Int. 2020, 137, 109739.
- Borreani, J.; Leonardi, C.; Moraga, G.; Quiles, A.; Hernando, I. How do Different Types of Emulsifiers/Stabilizers Affect the In Vitro Intestinal Digestion of O/W Emulsions? Food Biophys. 2019, 14, 313–325.
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology for increased micronutrient bioavailability. Trends Food Sci. Technol. 2014, 40, 168–182.
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33.
- De Matteis, V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation. Toxics 2017, 5, 29.
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250.
- Sanches, P.L.; Geaquinto, L.R.d.O.; Cruz, R.; Schuck, D.C.; Lorencini, M.; Granjeiro, J.M.; Ribeiro, A.R.L. Toxicity Evaluation of TiO2 Nanoparticles on the 3D Skin Model: A Systematic Review. Front. Bioeng. Biotechnol. 2020, 8, 575.
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129.
- Boutillier, S.; Fourmentin, S.; Laperche, B. Food additives and the future of health: An analysis of the ongoing controversy on titanium dioxide. Futures 2020, 122, 102598.
- Kose, O.; Tomatis, M.; Leclerc, L.; Belblidia, N.-B.; Hochepied, J.-F.; Turci, F.; Pourchez, J.; Forest, V. Impact of the Physicochemical Features of TiO2 Nanoparticles on Their In Vitro Toxicity. Chem. Res. Toxicol. 2020, 33, 2324–2337.
- Cao, X.; Zhang, T.; DeLoid, G.M.; Gaffrey, M.J.; Weitz, K.K.; Thrall, B.D.; Qian, W.-J.; Demokritou, P. Evaluation of the cytotoxic and cellular proteome impacts of food-grade TiO2 (E171) using simulated gastrointestinal digestions and a tri-culture small intestinal epithelial model. NanoImpact 2020, 17, 100202.
- Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of health safety aspects of titanium dioxide nanoparticles in food application. NanoImpact 2020, 18, 100224.
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gottet, D.; et al. Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA J. 2018, 16, e05088.
- Yang, Y.; Faust, J.J.; Schoepf, J.; Hristovski, K.; Capco, D.G.; Herckes, P.; Westerhoff, P. Survey of food-grade silica dioxide nanomaterial occurrence, characterization, human gut impacts and fate across its lifecycle. Sci. Total Environ. 2016, 565, 902–912.
- Liman, R.; Acikbas, Y.; Ciğerci, İ.H.; Ali, M.M.; Kars, M.D. Cytotoxic and Genotoxic Assessment of Silicon Dioxide Nanoparticles by Allium and Comet Tests. Bull. Environ. Contam. Toxicol. 2020, 104, 215–221.
- Yun, J.W.; Kim, S.H.; You, J.R.; Kim, W.H.; Jang, J.J.; Min, S.K.; Kim, H.C.; Chung, D.H.; Jeong, J.; Kang, B.C.; et al. Comparative toxicity of silicon dioxide, silver and iron oxide nanoparticles after repeated oral administration to rats. J. Appl. Toxicol. 2015, 35, 681–693.
- Palanog, A.D.; Calayugan, M.I.C.; Descalsota-Empleo, G.I.; Amparado, A.; Inabangan-Asilo, M.A.; Arocena, E.C.; Sta. Cruz, P.C.; Borromeo, T.H.; Lalusin, A.; Hernandez, J.E.; et al. Zinc and Iron Nutrition Status in the Philippines Population and Local Soils. Front. Nutr. 2019, 6, 81.
- Sohal, I.S.; DeLoid, G.M.; O’Fallon, K.S.; Gaines, P.; Demokritou, P.; Bello, D. Effects of ingested food-grade titanium dioxide, silicon dioxide, iron (III) oxide and zinc oxide nanoparticles on an in vitro model of intestinal epithelium: Comparison between monoculture vs. a mucus-secreting coculture model. NanoImpact 2020, 17, 100209.
- Shen, C.; James, S.A.; de Jonge, M.D.; Turney, T.W.; Wright, P.F.A.; Feltis, B.N. Relating Cytotoxicity, Zinc Ions, and Reactive Oxygen in ZnO Nanoparticle–Exposed Human Immune Cells. Toxicol. Sci. 2013, 136, 120–130.
- Assadian, E.; Dezhampanah, H.; Seydi, E.; Pourahmad, J. Toxicity of Fe2O3 nanoparticles on human blood lymphocytes. J. Biochem. Mol. Toxicol. 2019, 33, e22303.
- Petters, C.; Irrsack, E.; Koch, M.; Dringen, R. Uptake and Metabolism of Iron Oxide Nanoparticles in Brain Cells. Neurochem. Res. 2014, 39, 1648–1660.
- Fulgoni III, V.L.; Keast, D.R.; Bailey, R.L.; Dwyer, J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J. Nutr. 2011, 141, 1847–1854.
- Hilty, F.M.; Arnold, M.; Hilbe, M.; Teleki, A.; Knijnenburg, J.T.N.; Ehrensperger, F.; Hurrell, R.F.; Pratsinis, S.E.; Langhans, W.; Zimmermann, M.B. Iron from nanocompounds containing iron and zinc is highly bioavailable in rats without tissue accumulation. Nat. Nanotechnol. 2010, 5, 374–380.
- Voss, L.; Hoché, E.; Stock, V.; Böhmert, L.; Braeuning, A.; Thünemann, A.F.; Sieg, H. Intestinal and hepatic effects of iron oxide nanoparticles. Arch. Toxicol. 2021, 95, 895–905.
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93.
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844.
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556.
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282.
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681.
- Simbine, E.O.; Rodrigues, L.d.C.; Lapa-GuimarÃEs, J.; Kamimura, E.S.; Corassin, C.H.; Oliveira, C.A.F.d. Application of silver nanoparticles in food packages: A review. Food Sci. Technol. 2019, 39, 793–802.
- Fröhlich, E.E.; Fröhlich, E. Cytotoxicity of nanoparticles contained in food on intestinal cells and the gut microbiota. Int. J. Mol. Sci. 2016, 17, 509.
- Hendrickson, O.D.; Klochkov, S.G.; Novikova, O.V.; Bravova, I.M.; Shevtsova, E.F.; Safenkova, I.V.; Zherdev, A.V.; Bachurin, S.O.; Dzantiev, B.B. Toxicity of nanosilver in intragastric studies: Biodistribution and metabolic effects. Toxicol. Lett. 2016, 241, 184–192.
- Sundaresan, V.; Monaghan, J.W.; Willets, K.A. Visualizing the Effect of Partial Oxide Formation on Single Silver Nanoparticle Electrodissolution. J. Phys. Chem. C 2018, 122, 3138–3145.
- Pinďáková, L.; Kašpárková, V.; Kejlová, K.; Dvořáková, M.; Krsek, D.; Jírová, D.; Kašparová, L. Behaviour of silver nanoparticles in simulated saliva and gastrointestinal fluids. Int. J. Pharm. 2017, 527, 12–20.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
07 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




