
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcelo Soares | + 1976 word(s) | 1976 | 2020-01-03 04:03:58 | | | |
| 2 | Catherine Yang | + 6 word(s) | 1982 | 2020-10-30 07:02:49 | | |
Video Upload Options
The microbiome is able to modulate immune responses, alter the physiology of the human organism, and increase the risk of viral infections and development of diseases such as cancer. Herein, we address changes in the cervical microbiota as potential biomarkers to identify the risk of cervical intraepithelial neoplasia (CIN) development and invasive cervical cancer in the context of human papillomavirus (HPV) infection.
1. Introduction
Although HPV infection is a necessary cause, it is not determinant for cervical cancer development. HPV infects squamous epithelial basal cells, inducing lesions and even cervical cancer when it is not eliminated [1]. However, the majority of HPV infections are cleared and only a small fraction of infected women progress to premalignant lesions and cancer [1]. Pap smear has been used for cervical screening, which resulted in a decrease in deaths from cervical cancer. Nevertheless, the assay has low sensitivity (60–80%), high false-negative rates (30%), and significant false-positive rates, ranging from 15–50% [2][3]. On the other hand, introduction of HPV-DNA assays for screening has improved the results from equivocal cytology triage with Pap smear [4].
In many countries, Pap cytology is the primary screening test either alone or in conjunction with HPV DNA test (co-testing), although in some European countries a switch to primary HPV-DNA technique followed by cytology (Pap smear) has been recommended [5]. The American Cancer Society recommends the use of the HPV test as part of follow-up for an abnormal Pap result in women aged 21–29, while for women aged >31 co-testing screening is recommended every five years [6].
2. Microbiota as a Biomarker for HPV Infection and Cervical Dysplasia
The HPV-DNA test is a high-sensitive method, so the absence of high-risk HPV-DNA indicates low risk for cervical intraepithelial neoplasia grade 3 (CIN3) and cancer development, which may allow safe prolonging of cervicovaginal screening test intervals [5]. Additionally, even when HPV-DNA scores positive, the majority of HPV infections are eliminated and do not progress to cervical dysplasia. Nevertheless, since the risk of cancer development still exists, screening at short intervals is strongly recommended. Therefore, the characterization of novel biomarkers is important in order to decide precisely how each HPV-positive women will be treated (colposcopy) and whether HPV-DNA-negative women have high risk to acquire a new HPV infection and progress into CIN. They may function as secondary markers after HPV-DNA test and Pap smear to identify women under risk to HPV acquisition, persistent infection and cervical cancer, contributing to better follow-up/treatment strategies.
Acknowledging a biomarker potential, high diversity microbiota has been frequently shown to correlate with HPV status and different severities of cervical dysplasia, suggesting a potential in indicating vaginal health and disease (Figure 1). Therefore, we further discuss the cervicovaginal microbiome as a promising biomarker not only for HPV status but also for cytologic abnormalities.
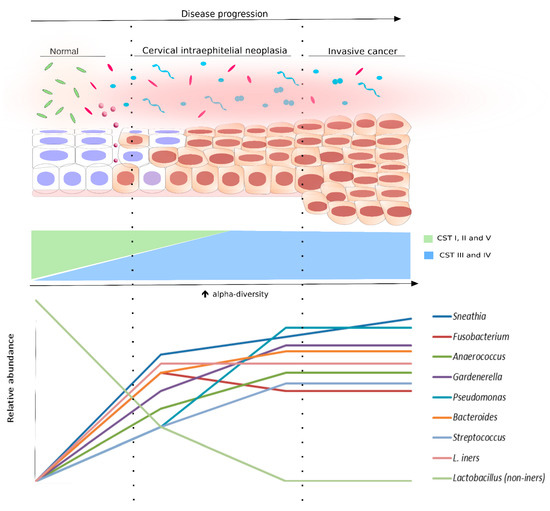
Figure 1. Bacterial diversity distribution in intraepithelial neoplasia progression. The scheme displays the progression of the cervical epithelium from normal to invasive cervical cancer, as well as the bacterial diversity (alpha-diversity) and the species abundance in the cervical microenvironment at each cytological stage. The normal cytology is commonly associated with community state types (CSTs) I, II, or V, which are Lactobacillus species (non-iners)-dominant (light green rods). However, following the cervical disease progression, the relative abundance of Lactobacillus non-iners species start to decrease. Concomitant to that, alpha-diversity increases and the microbiota is changed to CST III (pink rods and circles) or IV (light blue and pink shapes). Some bacterial species were found, in different studies, associated with cervical disease progression. They are also displayed in this figure in a representative graph of relative abundance (lower panel).
Different reports consistently demonstrated the relationship between HPV infection and/or persistence and microbiome composition. In fact, a study reported that HPV+ women exhibited a more complex microbiome diversity than HPV- counterparts [7]. Similarly, a work with Korean twins reported that HPV+ patients had a high-diversity vaginal microbiome with reduced proportion of Lactobacillus spp. when compared to their HPV- matches. Moreover, bacteria of the Sneathia genus was remarkably associated with HPV positivity and suggested as a biomarker for viral status [8]. Taken together, those reports strongly suggest a relationship between microbiome increased diversity and HPV infection. On the other hand, they do not demonstrate a causality link between a shift in microbiota composition and HPV acquisition, since those studies were not longitudinal with a patient’s follow-up through time.
In this scenario, a study following women for 16 weeks showed that a vaginal microbiota dominated by L. gasseri was related to a faster HPV clearance, while microbiomes with lower Lactobacillus levels and higher abundance of Atopobium, Gardnerella, and Prevotella were associated with a slower infection resolution [9]. Further, L. crispatus was the most prevalent Lactobacillus spp. in Italian women that cleared HPV infection or were consistently HPV-, while a microbiome composition characterized for lower Lactobacillus spp. counts and higher abundance of Gardnerella, Prevotella, Atopobium, and Sneathia (a combination commonly associated with bacterial vaginosis) was the most frequent among women with persistent hr-HPV infection during one year. Also, among patients with persistent HPV infection, L. iners was the most abundant Lactobacillus species [9]. Accordingly, a longitudinal observation of African/Caribbean women living in Canada showed that a microbiome with lower abundance of Lactobacillus spp. and greater representation of anaerobic bacteria was more frequent in HPV+ than in HPV- subjects [10]. Altogether, those studies are important to elucidate the cause-effect relationship between vaginal microbiota and HPV status. Since they report a temporal dynamics in which infection resolution and persistence are associated with different bacterial compositions, microbiome alteration is suggested as a factor that could occur before HPV acquisition, modulating, and facilitating viral maintenance. Moreover, a possible protective role for Lactobacillus spp. dominated microbiota is noteworthy, while its paucity and increased amounts of other bacterial genera is related with higher HPV risk. Nevertheless, a study has recently provided some insights into possible interactions between viruses and the vaginal microbiome. The authors demonstrated that cervicovaginal samples with CSTs I or IV that were positive for oncogenic viruses (HPV and/or polyomaviruses) showed increased abundance of L. crispatus as well as P. timonensis and S. sanguinegens, respectively, when compared to its counterparts without any virus detected [11]. That suggested that the presence of viruses may also exert influence in the cervicovaginal microbiome composition. Ethnicity is another important factor that must be considered, since L. gasseri (together with G. vaginalis) was more frequent in HPV+ subjects compared to HPV- in a Chinese cohort [7] as well as L. mucosae and Enterococcus faecalis were the most dominant species among women from Northeast India [12], which opposes previous findings.
It is also important to highlight that microbiome composition has also been related to other viral infections. For example, African women with L. crispatus-dominated microbiota registered significantly less frequent HIV, HSV-2, and HPV infections and bacterial STIs when compared to other compositions [13]. A report studying Caucasian Italian women demonstrated higher rates of HPV infection among samples belonging to CSTs III or IV, as well as a higher frequency of polyomaviruses in women with CSTs III or I. Although the L. crispatus-dominated environment has been associated with the presence of polyomaviruses, a longitudinal analysis of those patients revealed that CST I was associated with increased rates of viral clearance, while women harboring CSTs III or IV commonly progressed to persistent infections [11].
As discussed above, the vaginal microbiota composition has been extensively associated with HPV positivity and suggested as a promising biomarker for HPV risk. Accordingly, HPV infection is strongly recognized as a necessary, but not sufficient, cause for cervical carcinogenesis. Therefore, since specific bacterial compositions are linked with increased viral infections, the microbial community could also be associated with cervical dysplasia development and inform about cytological abnormalities. Indeed, different studies have already described a cervicovaginal microbiome shift with increased proportions of bacteria such as Gardnerella, Prevotella, Atopobium, and decreased abundance of Lactobacillus spp. occurring together with CIN and cancer. However, again, the cause–effect relationship between microbiome and cervical dysplasia has not been elucidated throughly [14].
Differences in microbiota composition were found between normal cytology, cervical lesions and cancer. That is, while L. crispatus and L. iners were respectively the predominant species for HPV- and HPV+ women without cytologic alterations, Sneathia spp. and Fusobacterium spp. were predominant in squamous intraepithelial lesions and cervical cancer, respectively [15]. Similarly, CST IV (high diversity microbiome lacking Lactobacillus spp.) frequency was shown to be directly proportional to cervical abnormalities severity: the cluster was gradually more abundant in low-grade squamous intraepithelial lesions (LSIL), high-grade SIL (HSIL) and cervical cancer. Moreover, HSIL samples had greater abundance of Sneathia sanguinegens, Anaerococcus tetradius, and Peptostreptococcus anaerobius than LSIL, suggesting shifts in microbiome composition according to disease severity [16]. Likewise, Lactobacillus dominance decreased together with cervical dysplasia severity while Sneathia spp. were increased in low/ high grade precancerous lesions and invasive cervical carcinoma [17]. In this context, different analyses suggest a paucity of Lactobacillus and increased relative abundance of other species as a factor associated with cervical abnormality development. Those reports also indicate that each stage of cervical dysplasia is associated with a corresponding microbial pattern. For example, while Lactobacillus spp. are more associated with normal cytology and absence of HPV, a higher abundance of Sneathia spp. was observed throughout cancer development and, therefore, could be used as indicative of vaginal disease together with other species mentioned above.
Additional evidence corroborates the findings described above. First, the presence of A. vaginae, G. vaginalis, and L. iners together with L. crispatus in low levels was suggested as the most hazardous combination for CIN development, with an odds ratio of 34.1 for CIN in the presence of hr-HPV [18]. Moreover, a report indicated that B. fragilis, L. delbrueckii, and S. agalactiae had an indirect effect on cervical cancer mediated by HPV infection, while A. vaginae and P. stutzeri also exhibited a direct effect on cervical carcinogenesis independently of HPV status [19]. Of note, a study evaluated the impact of loop electrosurgical excision procedure (LEEP), a method to treat CIN 2/3 to avoid cancer development, in the microbiome composition. The authors noticed that a microbiota containing Prevotella and lacking a consistent dominant species shifted significantly to an L. iners dominated community after three months of LEEP intervention [20]. However, in sharp contrast to those findings, it has been reported that a microbiome dominated by unclassified Lactobacillus spp. and L. iners was significantly associated with CIN 2 and CIN 3 in women with hr-HPV [21]. Although L. iners was already described to be related with CIN [18] or HPV positivity [15], the dominance by other Lactobacillus species was previously reported as a protective factor, but this cohort showed a different observation for unclassified Lactobacillus spp. In this scenario, Hispanic ethnicity by itself was associated with a decrease in Lactobacillus dominance and Sneathia spp. enrichment when compared to non-Hispanic women living in the U.S [17].
HIV-positive women show increased risk of HPV acquisition and CIN development [22][23][24]. A study that analyzed the cervicovaginal microbiome in the postpartum period of HIV-positive women showed a high frequency of L. iners, Moryella, Schlegelella, and Gardnerella associated with CIN with significant odds ratios of 40 for Moryella and of 3.5 for Schlegelella [25]. In a longitudinal analysis, when comparing the bacterial microbiome of women that showed CIN regression to normal cytology, Gardnerella appeared with a higher frequency in CIN when compared to normal status [25]. Similarly, a report from HIV+ pregnant women in Zambia showed that they had higher microbiome diversity, greater abundance of G. vaginalis and A. vaginae and lack of L. crispatus when compared to HIV- pregnant participants. Also, L. iners enrichment was observed in HIV- individuals and in HIV+ subjects with preconceptional ART exposure [26]. These findings corroborate the association of specific bacteria with cervical premalignant lesions. Despite such association, it is important to highlight that little is known about the longitudinal nature of changes in microbiome in the development of CIN and cervical cancer in HPV+ women. However, the association seen in cross-sectional and in some longitudinal studies indicates that the microbiome could be used as a sensor for cervical alteration and risk for CIN progression.
References
- John Doorbar; Nagayasu Egawa; Heather Griffin; Christian Kranjec; Isao Murakami; Human papillomavirus molecular biology and disease association. Reviews in Medical Virology 2015, 25, 2-23, 10.1002/rmv.1822.
- Yim, E.-K.; Park, J.-S; Biomarkers in cervical cancer.. Biomark. Insights 2007, 1, 215–225.
- Vikrant V Sahasrabuddhe; Patricia Luhn; Nicolas Wentzensen; Human papillomavirus and cervical cancer: biomarkers for improved prevention efforts. Future Microbiology 2011, 6, 1083-1098, 10.2217/fmb.11.87.
- Maria Lina Tornesello; Luigi Buonaguro; Paolo Giorgi-Rossi; Franco M. Buonaguro; Viral and Cellular Biomarkers in the Diagnosis of Cervical Intraepithelial Neoplasia and Cancer. BioMed Research International 2013, 2013, 1-10, 10.1155/2013/519619.
- Joakim Dillner; Matejka Rebolj; Philippe Birembaut; Karl-Ulrich Petry; Anne Szarewski; Christian Munk; Silvia De Sanjose; Pontus Nauclér; Belén Lloveras; Susanne Kjaer; et al.Jack CuzickMarjolein Van BallegooijenChristine ClavelThomas Iftner Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008, 337, a1754-a1754, 10.1136/bmj.a1754.
- The American Cancer Society Guidelines for the Prevention and Early Detection of Cervical Cancer. . The American Cancer Society Guidelines for the Prevention and Early Detection of Cervical Cancer.. Retrieved 2020-1-3
- Weijiao Gao; Jinlong Weng; Yunong Gao; Xiaochi Chen; Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infectious Diseases 2013, 13, 271-271, 10.1186/1471-2334-13-271.
- Jung Eun Lee; Sunghee Lee; Heetae Lee; Yun-Mi Song; Kayoung Lee; Min Ji Han; Joohon Sung; GwangPyo Ko; Association of the Vaginal Microbiota with Human Papillomavirus Infection in a Korean Twin Cohort. PLOS ONE 2013, 8, e63514, 10.1371/journal.pone.0063514.
- Rebecca M. Brotman; Michelle D. Shardell; Pawel Gajer; J. Kathleen Tracy; Jonathan M. Zenilman; Jacques Ravel; Patti E. Gravitt; Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. The Journal of Infectious Diseases 2014, 210, 1723-1733, 10.1093/infdis/jiu330.
- B Shannon; T J Yi; S Perusini; P Gajer; B Ma; M S Humphrys; J Thomas-Pavanel; L Chieza; P Janakiram; M Saunders; et al.W TharaoS HuibnerK ShahabiJ RavelA RebbapragadaR Kaul Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota.. Mucosal Immunology 2017, 10, 1310-1319, 10.1038/mi.2016.129.
- Giuseppina Campisciano; Tarik Gheit; Francesco De Seta; Carolina Cason; Nunzia Zanotta; Serena Delbue; Giuseppe Ricci; Pasquale Ferrante; Massimo Tommasino; Manola Comar; et al. Oncogenic Virome Benefits from the Different Vaginal Microbiome-Immune Axes.. Microorganisms 2019, 7, 414, 10.3390/microorganisms7100414.
- Sumi Das Purkayastha; Mrinal Kanti Bhattacharya; Himanshu Kishore Prasad; Hrishikesh Upadhyaya; Suparna Das Lala; Kunal Pal; Meenakshi Das; Gauri Dutt Sharma; Maloyjo Joyraj Bhattacharjee; Contrasting diversity of vaginal lactobacilli among the females of Northeast India.. BMC Microbiology 2019, 19, 198-10, 10.1186/s12866-019-1568-6.
- Hanneke Borgdorff; Evgeni Tsivtsivadze; Rita Verhelst; Massimo Marzorati; Suzanne Jurriaans; Gilles F Ndayisaba; Frank H Schuren; Janneke Hhm Van De Wijgert; Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. The ISME Journal 2014, 8, 1781-1793, 10.1038/ismej.2014.26.
- Anita Mitra; David A. MacIntyre; Julian R. Marchesi; Yun S. Lee; Phillip R. Bennett; Maria Kyrgiou; The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next?. Microbiome 2016, 4, 58, 10.1186/s40168-016-0203-0.
- Astride Audirac-Chalifour; Kirvis Torres-Poveda; Margarita Bahena-Román; Juan Tellez-Sosa; Jesús Martínez-Barnetche; Bernardo Cortina-Ceballos; Guillermina López-Estrada; Karina Delgado-Romero; Ana I. Burguete-García; David Cantú; et al.Alejandro García-CarrancáVicente Madrid-Marina Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLOS ONE 2016, 11, e0153274, 10.1371/journal.pone.0153274.
- A. Mitra; D. A. MacIntyre; Y. S. Lee; A. Smith; J. R. Marchesi; B. Lehne; R. Bhatia; D. Lyons; E. Paraskevaidis; J. V. Li; et al.E. HolmesJ. K. NicholsonP. R. BennettM. Kyrgiou Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Scientific Reports 2015, 5, 16865, 10.1038/srep16865.
- Paweł Łaniewski; Dominique Barnes; Alison Goulder; Haiyan Cui; Denise J. Roe; Dana M. Chase; Melissa M. Herbst-Kralovetz; Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women.. Scientific Reports 2018, 8, 7593, 10.1038/s41598-018-25879-7.
- H.Y. Oh; B.-S. Kim; S.-S. Seo; J.-S. Kong; J.-K. Lee; S.-Y. Park; K.-M. Hong; H.-K. Kim; M.K. Kim; The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clinical Microbiology and Infection 2015, 21, 674.e1-674.e9, 10.1016/j.cmi.2015.02.026.
- Chaoting Zhang; Ying Liu; Weijiao Gao; Yaqi Pan; Yunong Gao; Jing Shen; Hongchao Xiong; The direct and indirect association of cervical microbiota with the risk of cervical intraepithelial neoplasia. Cancer Medicine 2018, 7, 2172-2179, 10.1002/cam4.1471.
- Hongwei Zhang; Jiaqi Lu; Yingying Lu; Qingqing Cai; Haiou Liu; Congjian Xu; Cervical microbiome is altered in cervical intraepithelial neoplasia after loop electrosurgical excision procedure in china.. Scientific Reports 2018, 8, 4923, 10.1038/s41598-018-23389-0.
- Chandrika J. Piyathilake; Nicholas J. Ollberding; Ranjit Kumar; Maurizio Macaluso; Ronald D. Alvarez; Casey D. Morrow; Cervical Microbiota Associated with Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses.. Cancer Prevention Research 2016, 9, 357-66, 10.1158/1940-6207.CAPR-15-0350.
- David Adler; Melissa Wallace; Thola Bennie; Beau Abar; Rokhsanna Sadeghi; Tracy Meiring; Anna-Lise Williamson; Linda-Gail Bekker; High risk human papillomavirus persistence among HIV-infected young women in South Africa.. International Journal of Infectious Diseases 2015, 33, 219-21, 10.1016/j.ijid.2015.02.009.
- Hugo De Vuyst; Flavia Lillo; Nathalie Broutet; Jennifer S. Smith; HIV, human papillomavirus, and cervical neoplasia and cancer in the era of highly active antiretroviral therapy. European Journal of Cancer Prevention 2008, 17, 545-554, 10.1097/cej.0b013e3282f75ea1.
- Diego Serraino; Patrizia Carrieri; Christian Pradier; Ettore Bidoli; Maria Dorrucci; Elisa Ghetti; Antonella Schiesari; Roberta Zucconi; Patrizio Pezzotti; Pierre Dellamonica; et al.Silvia FranceschiGiovanni Rezza Risk of invasive cervical cancer among women with, or at risk for, HIV infection. International Journal of Cancer 1999, 82, 334-337, 10.1002/(sici)1097-0215(19990730)82:3<334::aid-ijc5>3.3.co;2-3.
- Gislaine Curty; Raquel L. Costa; Juliana D. Siqueira; Angela I. Meyrelles; Elizabeth S. Machado; Esmeralda A. Soares; Marcelo A. Soares; Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Scientific Reports 2017, 7, 17364, 10.1038/s41598-017-17351-9.
- Joan T. Price; Bellington Vwalika; Marcia Hobbs; Julie A. E. Nelson; Elizabeth M. Stringer; Fei Zou; Katelyn J. Rittenhouse; Andrea Azcarate-Peril; Margaret P. Kasaro; Jeffrey S. A. Stringer; et al. Highly diverse anaerobe-predominant vaginal microbiota among HIV-infected pregnant women in Zambia.. PLOS ONE 2019, 14, e0223128, 10.1371/journal.pone.0223128.




