Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anton FICAI | -- | 2898 | 2022-04-03 18:39:38 | | | |
| 2 | Catherine Yang | Meta information modification | 2898 | 2022-04-06 04:55:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ficai, A.; Petrisor, G.; Motelica, L.; , .; Oprea, O.; Ficai, D. Melissa officinalis. Encyclopedia. Available online: https://encyclopedia.pub/entry/21335 (accessed on 07 February 2026).
Ficai A, Petrisor G, Motelica L, , Oprea O, Ficai D. Melissa officinalis. Encyclopedia. Available at: https://encyclopedia.pub/entry/21335. Accessed February 07, 2026.
Ficai, Anton, Gabriela Petrisor, Ludmila Motelica, , Ovidiu-Cristian Oprea, Denisa Ficai. "Melissa officinalis" Encyclopedia, https://encyclopedia.pub/entry/21335 (accessed February 07, 2026).
Ficai, A., Petrisor, G., Motelica, L., , ., Oprea, O., & Ficai, D. (2022, April 03). Melissa officinalis. In Encyclopedia. https://encyclopedia.pub/entry/21335
Ficai, Anton, et al. "Melissa officinalis." Encyclopedia. Web. 03 April, 2022.
Copy Citation
Melissa officinalis is a medicinal plant rich in biologically active compounds which is used worldwide for its therapeutic effects. Chemical studies on its composition have shown that it contains mainly flavonoids, terpenoids, phenolic acids, tannins, and essential oil. The main active constituents of Melissa officinalis are volatile compounds (geranial, neral, citronellal and geraniol), triterpenes (ursolic acid and oleanolic acid), phenolic acids (rosmarinic acid, caffeic acid and chlorogenic acid), and flavonoids (quercetin, rhamnocitrin, and luteolin).
Melissa officinalis
essential oil
polyphenolic compounds
biological activity
drug delivery
1. Introduction
Plants are the oldest health remedy, and have been known to people since antiquity. Over the centuries, various cultural groups have developed traditional herbal medical systems to improve health. From the beginning of the 19th century, the isolation of active compounds from plants began; due to the rapid developments in the field of chemistry, from the beginning of the 20th century the production of synthetic compounds increased [1]. Even though the use of synthetic compounds has grown in the drug industry, most developing countries continue to use drugs made from natural compounds [2]. Medicinal plants have a variety of biological properties, which means that they play an important role in preventing and treating various diseases [3]. These plants are a rich source of biological active agents, and can represent raw materials that can be used to develop new, semi-synthetic drugs.
The active substances are found in different parts of the plant, and can be extracted from different types of seeds, roots, leaves, fruits, skin, flowers, or even the whole plant. The active compounds extracted from most medicinal plants have direct or indirect therapeutic effects.
Melissa officinalis L. is a medicinal plant used in traditional medicine around the world. It is an aromatic perennial plant; it commonly grows in the Mediterranean region and Western Asia, being intensively cultivated in Europe [4][5] and, due to its chemical composition and numerous pharmacological effects, it is intensively studied. This plant is also called lemon balm, honey balm, or balm mint; it is an edible medicinal herb belonging to the mint family Lamiaceae and the subfamily Nepetoideae [5]. It is a plant that lives at least three years; it is bushy and upright, reaching a height between 60 and 100 cm [6]. The soft, hairy leaves are 2 to 8 cm long; they are dark green and heart shaped. The leaf surface is coarse and deeply veined, the leaf edge is scalloped or toothed [7], and it is rich in biological active agents; thus, the extracts highlight specific properties (Figure 1). Melissa officinalis has a hairy root system, which makes the plant more adaptable to different environmental conditions, but the upper parts of the plant die out in early winter and reappear in early spring [7]. It is one of the easiest herbs to grow, and spreads so readily that some gardeners consider it a weed [8].
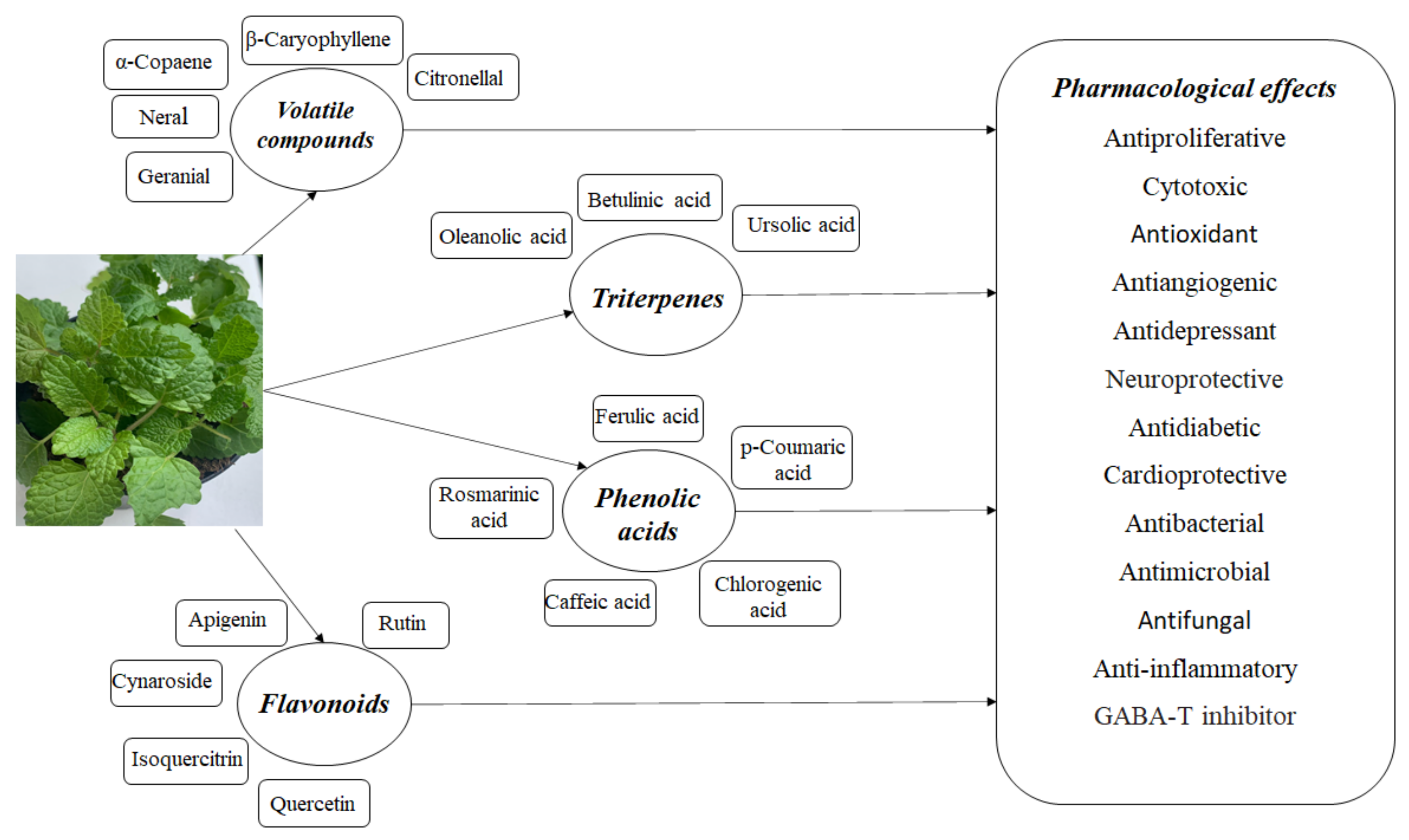
Figure 1. The composition of Melissa officinalis and its pharmacological effects.
2. Phytochemical Composition
Chemical studies on the composition of the Melissa officinalis have shown that it contains mainly flavonoids, terpenoids, phenolic acids, tannins and essential oil [9]. The main active constituents of Melissa officinalis are volatile compounds (geranial, neral, citronellal and geraniol), triterpenes (ursolic acid and oleanolic acid), phenolic compounds (rosmarinic acid, caffeic acid, and protocatechuic acid) and flavonoids (quercetin, rhamnocitrin, luteolin) [8][10]. Essential oil is usually considered to be the responsible therapeutic principle for most biological activities, but polyphenols are also involved.
Melissa officinalis essential oil—obtained from the fresh or dried flower, leaf, and branches of this plant by water steam distillation or chemical extraction—is characterised by a fresh lemon odor and light yellow color [11].
2.1. Volatile Compounds
The volatile oil extracted from the leaves of Melissa officinalis is important due to its pharmacological effects, and is obtained in small quantities, unlike other plants in the Lamiaceae family. The major and minor components of the essential oil of the dried leaves of Melissa officinalis are volatile compounds that are found in different concentrations (Table 1).
Table 1. Components of the essential oil extracted from the dried leaves of Melissa officinalis.
| Component Name | Concentration of the Components of the Essential Oil, % | Reference |
|---|---|---|
| Majority components | ||
| (E)-Caryophyllene | 1.06–6.8 | [12][13][14] |
| Caryophyllene oxide | 1.3–43.55 | [12][13][14][15][16] |
| Citronellal | 0.4–20.3 | [12][13][15][16][17] |
| Geranial (citral A) | 6.22–51.21 | [14][15][16][17][18] |
| Geranyl acetate | 0.5–19.3 | [12][13][14][17] |
| Neral (citral B) | 4.28–35.02 | [12][13][14][15][16][17][18] |
| α-Cadinol | 5.64 | [14] |
| α-Copaene | 0.1–7.02 | [12][15][16] |
| β-Caryophyllene | 1.3–29.14 | [14][15][16][17] |
| Minority components (<5%) | ||
| (2E)-Nonen-1-al | 0.2 | [12] |
| (E)-Nerolidol | 0.2 | [12] |
| (E)-α-Bergamotene | 1.24 | [14] |
| (E)-β-Ionone | 0.9 | [12] |
| (E)-β-Ocimene | 0.1–0.5 | [12][13] |
| (E-E)-Geranyl linalool | 1.59 | [14] |
| (Z)-β-Ocimene | 0.1 | [15] |
| 1,2-Benzenedicarboxilic acid, butyl 2-methylopropyl ester | 0.6 | [13] |
| 1,8-Dehydro-cineol | 0.1 | [13] |
| 14-Hydroxy-9-epi-(E)-caryophyllene | 0.2 | [13] |
| 1-Octen-3-ol | 0.2–0.3 | [12][13][15] |
| 3,5-Heptadienal,2-ethylidene-6-methyl | 0.4 | [13] |
| 3-Octanone | 0.2 | [17] |
| 6-Methyl-5-hepten-2-ol | 0.2–1.7 | [12][13][15] |
| Benzene acetaldehyde | 0.3 | [12] |
| Camphene | 0.38–1.38 | [14][16] |
| Camphor | 0.1–0.4 | [13][15] |
| Carvacrol | 0.3–1 | [12][13] |
| Caryophyllenol | 0.5–2.23 | [14] |
| cis-2H-3a-Methyl-octahydro-Inden-2-one | 4.7 | [17] |
| Cis-Chrysanthenol | 0.7–1.7 | [12][13][15] |
| Cis-Rose oxide | 0.1–0.2 | [12][15] |
| Citronellol | 0.4–1.88 | [12][14] |
| Citronellyl acetate | 0.1 | [12] |
| Dihydrocitronellol acetate | 0.3 | [15] |
| Geraniol | 0.6–0.7 | [12][13][15] |
| Germacrene D | 0.2–2.0 | [12][13][14] |
| Humulene epoxide II | 0.2–1.29 | [13][14] |
| iso Aromadendren epoxide | 0.46 | [14] |
| Isogeranial | 1.4–2.0 | [13] |
| Isomenthol | 2.4 | [15] |
| Linalool | 0.3–0.5 | [12][15] |
| Linalool + trans-Sabinene hydrate | 0.5–0.8 | [13] |
| Menthol | 0.3 | [15] |
| Methyl citronellate | 0.5–2.78 | [12][13][16] |
| Methyl eugenol | 0.1 | [12] |
| Methyl geranate | 0.2–0.4 | [12][13][17] |
| Myrcene | 0.1–0.3 | [13][15] |
| n-Eicosane | 0.6 | [15] |
| Nerol | 0.2 | [15] |
| Neryl acetate | 0.1 | [12] |
| n-Heneicosane | 0.4 | [15] |
| n-Nonanal | 0.1–0.4 | [12][15] |
| para-Mentha-1(7),8-diene | 0.1 | [13] |
| p-Cymene | 0.1 | [12][13] |
| Phytol | 3.64 | [14] |
| Rosefuran epoxide | 0.6–0.7 | [13] |
| Sabinene | 0.4 | [13] |
| Thymol | 0.1–3.1 | [12][13][14] |
| t-Muurolol | 0.59 | [14] |
| trans-Limonene oxide | 0.6 | [13] |
| trans-para-Mentha-1(7),8-dien-2-ol | 2.3 | [17] |
| Trans-Rose oxide | 0.1 | [12][15] |
| Valencene | 0.1 | [15] |
| α-Humulene | 0.2–2.6 | [12][13][15][16] |
| α-Calacorene | 0.76 | [14] |
| α-Cubebene | 0.42–1.23 | [14] |
| β-Cubebene | 0.1 | [15] |
| β-Pinene oxide | 1.1 | [13] |
| β-sesquiphellandrene | 0.97 | [14] |
| γ-Cadinene | 0.76–1.77 | [14] |
| γ-Terpinene | 0.3–0.5 | [12][13] |
Traces of components were not taken into account (contents below 0.05%).
Kowalski et al. [19] reported a 0.17% essential oil content, which was very low, unlike plants in the same family. The study by Seidler-Łożykowska et al. [16] showed that the essential oil content ranged from 0.08 to 0.20% due to fluctuations in weather conditions during the research years. For the variability of the essential oil content and its composition, Kittler et al. [20] studied a set of 28 accessions of lemon balm. They obtained an essential oil content that varied between 0.01 and 0.72%, and found out—based on statistical analysis on the composition of the essential oil—that there are two chemotypes of essential oil: chemotype citral and chemotype germacrene D. As it is known from the literature, there are two subspecies of the plant Melissa officinalis: officinalis and altissima. The difference between the two subspecies is given by the composition of the essential oil, such that the subspecies officinalis contains major amounts of citral and/or neral, but the subspecies altissima contains only traces. Basta et al. [21] reported, in a study on the composition of the essential oil of Melissa officinalis in Greece, the lack of the major constituents citral and citronellal. Souihi et al. [14] reported, in a comparative study between Melissa officinalis from Tunisia (from two different cities), Germany, and France, the lack of citral but the major presence of germacrene D.
2.2. Triterpenes
Triterpenes are non-volatile components extracted from plants, and are one of the largest classes of natural plant products, with more than twenty thousand different triterpenes (Table 2) [22][23]. Some triterpenes contain a different number of sulfate groups bound to sugar or glucones [10].
Table 2. Triterpenes from Melissa officinalis extracts.
| Component Name | Content *, μg/g |
Part of Plant | Reference |
|---|---|---|---|
| Betulinic acid | 12.85–169.88 | aerial parts | [4] |
| Oleanolic acid | 915.03–6151.67 | aerial parts | [4] |
| Ursolic acid | 3577.00–11,234.97 | aerial parts | [4] |
| 23-Sulfate ester of niga-ichigoside F1 | n.a. | leaves and stems | [24] |
| 3β,16β,23-Trihydroxy-13,28-epoxyurs-11-ene-3-O-β-D-glucopyranoside | n.a. | dried leaves and stems | [23] |
| 3,23-Disulfate ester of 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oicacid | n.a. | dried leaves and stems | [23] |
| 3,23-Disulfate ester of 2α,3β,19α,23-tetrahydroxyurs-12-en-28-oicacid 28-O-β-D-glucopyranoside | n.a. | dried leaves and stems | [23] |
| 3,23-Disulfate ester of2α,3β,23,29-tetrahydroxyolean-12-en-28-oicacid | n.a. | dried leaves and stems | [23] |
| 3,23-disulfate ester of 3β-23,29-trihydroxyolean-12-en-28-oic acid | n.a. | dried leaves and stems | [23] |
| 3,23-Disulfate ester of 2α,3β-23,29-tetrahydroxyolean-12-ene-28-oicacid | n.a. | dried leaves and stems | [23] |
| 23-sulfate ester of 2α,3β,19 α,23-tetrahydroxyurs-12-en-28-oic acid | n.a. | fresh leaves and stems | [25] |
| 23-sulfate ester of 2α,3β,19 α,23-tetrahydroxyurs-12-en-28-oic acid 28-O-β- D-glucopyranoside |
n.a. | fresh leaves and stems | [25] |
| Melissioside A | n.a. | leaves and stems | [24] |
| Melissioside B | n.a. | leaves and stems | [24] |
| Melissioside C | n.a. | leaves and stems | [24] |
n.a. = not available; * expressed on a dry weight basis.
The most common triterpenes in Melissa officinalis extracts are ursolic acid and oleanolic acid. In the studies of Ghiulai et al. [4] and Ibarra et al. [26], ursolic acid and oleanolic acid were extracted together with polyphenolic compounds in order to show the biological properties of the plant. Using different extraction conditions, different extracts can be obtained with different compositions, and thus different activities. The first extraction was obtained by maceration for 9 days in 70% ethanol, the second was obtained by maceration in 96% ethanol for 24 h under continuous stirring, and the third was extracted by sonication in 80% methanol for one hour. In the three extractions, the largest amount of ursolic acid was obtained, followed by oleanolic acid and small amounts of betulinic acid. Comparing the values of the obtained concentrations, the extraction in methanol with sonication is the most favorable, obtaining the best results: ursolic acid (11,234.97 μg/g), oleanolic acid (6151.67 μg/g) and betulinic acid (169.88 μg/g).
2.3. Polyphenolic Compounds
Polyphenolic compounds are a group of secondary metabolites which includes flavonoids (e.g., anthocyanins, flavones, isoflavones) and phenolic acids, which have many biological properties (Table 3) [27].
Table 3. Major polyphenolic compounds from Melissa officinalis extracts.
| Group Name | Compound Name | Content *, μg/g |
Part of Plant | Reference |
|---|---|---|---|---|
| Phenolic acids | Caffeic acid | 39.38–860.72 | Dried leaves | [4] |
| Caftaric acid | 1.85–344.34 | Dried leaves | [4] | |
| Chlorogenic acid | 0.62–75.529 | Dried leaves | [4][28] | |
| Ferulic acid | 1.03–45.489 | Dried leaves | [4][28] | |
| Gentisic acid | 10.40–60.48 | Dried leaves | [4] | |
| p-Coumaric acid | 1.06–20.72 | Dried leaves | [4] | |
| 13.37 ± 2.84 | Aerialparts | [29] | ||
| Rosmarinic acid | 3515.60–86,637.60 | Dried leaves | [4] | |
| 6914.1 ± 779 | Aerial parts | [29] | ||
| Flavonoids | Apigenin | 0.66–84.53 | Dried leaves | [4][28] |
| 41.71 ± 20.6 | Aerial parts | [29] | ||
| Cynaroside | 408.13 ± 30.0 | Aerial parts | [29] | |
| Daidzein | 51.25 ± 8.07 | Aerial parts | [29] | |
| Hyperoside | 3.30–16.240 | Dried leaves | [4] | |
| Isoquercetin | 6.82–162.40 | Dried leaves | [4] | |
| Kaempherol | 21.84 | Dried leaves | [4] | |
| Luteolin | 0.81–26.32 | Dried leaves | [4] | |
| Myricetin | 3.45–17.92 | Dried leaves | [4] | |
| Quercetin | 153.46 | Dried leaves | [28] | |
| Quercetrol | 5.72–33.60 | Dried leaves | [4] | |
| Rutin | 8.11–1462.99 | Dried leaves | [4][28] |
* expressed on a dry weight basis.
Phenolic acids compose a large group of natural compounds, which exhibit a wide range of biological activities. Flavonoids are a class of polyphenolic compounds, classified according to their chemical structures into flavonols, flavones, flavanones, isoflavones, catechins, anthocyanidins and chalcones [30]. Phenolic acids and flavonoids contain chemical structural elements that are responsible for the antioxidant process, and their antioxidant activities have been well established biochemically. Phenolic acids are important bioactive constituents of Melissa officinalis, among which are rosmarinic, caffeic, chlorogenic and ferulic acids. Following the phenolic profiles of the three types of extracts from the study performed by Ghiulai et al. [4], it was observed that rosmarinic acid was obtained in the highest amount (86,637.60 μg/g). The extraction with methanol, by sonication, favored the extraction of all of the phenolic compounds sought; thus, it can be observed that, depending on the extraction technique, some compounds can be in high concentrations and others cannot be detected.
2.4. Other Components
Melissa officinalis is a source of active biocompounds such as volatile compounds, triterpenes and polyphenolic compounds, but, in addition to these, it also contains other important biological active agents. Ashori et al. [31] conducted a study on the chemical composition of the stalk of Melissa officinalis. The content of extractive agents, lignin, polysaccharides and ash was determined, with the observation that the results showed a high content of alpha-cellulose and a low content of lignin. Komes et al. [32] reported the content of tannins, phenolic acids and flavonoids in non-hydrolyzed and hydrolyzed extracts from various plants, including Melissa officinalis. Dias et al. [33] performed a comparative study between two commercial samples, an in vitro culture sample and a normal culture sample. After the analysis of all of the samples, they observed the highest levels of proteins (8 g/100 g dw) and ash (12 g/100 g dw) in the in vitro cultured lemon balm content; the highest levels of carbohydrates were found in the granulate commercial sample (85 g/100 g dw), and a bag of commercial lemon balm had the highest energetic value (377 kcal/100 g dw) due to its higher fat content (3 g/100 g dw).
Overall, Melissa officinalis has a complex chemical composition, with many active biocompounds, which differ depending on the way in which the extraction is performed and the part of the plant that is subjected to extraction.
3. Pharmacological Studies
According to many biological studies, plant extracts and volatile oils are known for many beneficial activities for the human body. Melissa officinalis is considered to be a medicinal plant due to the numerous pharmacological effects associated with its chemical composition (Table 4).
Table 4. Pharmacological effects reported from Melissa officinalis extracts.
| Effect | Model | Dosage or Concentration | Tested Systems | Results | Type of Extract | Reference |
|---|---|---|---|---|---|---|
| Antiproliferative | in vitro | 20, 100, 250 μg/mL | Breast cancer cells MDA-MB- 231 and healthy HaCat cells |
Inhibitory effect on migration and proliferation of both types of cells | ethanolic extract | [34] |
| in vitro | 50% | Human Colon Cancer Cell Line (HCT-116) | The 50 % ethanolic extract showed significant differences after 72 h of treatment, reducing cell proliferation to values close to 40% | ethanolic and aqueous extracts | [35] | |
| Antitumor | in vitro | Different concentration | Human tumor cell lines: MCF-7, AGS and NCI-H460 |
Obtained revealed that the ethanolic extract presented the highest cell growth inhibitory potential in all the human tumor cell lines tested | ethanolic, methanolic, hydro-methanolic, hydro-ethanolic and aqueous extracts) |
[36] |
| Antioxidant | in vitro | Different concentration | Encephalic tissue from male Wistar rats | Effective agent in the prevention of various neurological diseases associated with oxidative stress | ethanolic, methanolic and aqueous extracts | [37] |
| in vitro | 1, 2.5, 5 and 10 mg/mL | DPPH radical scavenging activity assay, β-carotene bleaching test and ABTS assay | Good antioxidant activity | essential oil | [38] | |
| Antiangiogenic | in vitro, in ovo | 50 μg/mL | Two breast cancer cell lines, MCF-7 And MDA-MB-231 |
Highest cell inhibitory activity was exhibited by the 96% ethanolic extract | ethanolic extracts and methanolic extracts | [4] |
| Cardioprotective | in vivo | 25, 50 and 100 mg/kg b.w. * (4.23/8.46/16.91 mg/kg b.w. *) |
Rats | Antioxidant and cardio-protective effects against arrhythmias induced by ischemia and ischemia-reperfusion | ethanolic leaf extract | [39] |
| Antinociceptive Antihyperglycemic |
in vivo | 0.01, 0.02 and 0.04 mg/day (0.0063/0.0126/0.0252 mg/kg b.w. *) |
Male adult Wistar rats | Long-term oral administration of essential oil (at an effective dose of 0.04 mg/day) can suppress chemical hyperalgesia in diabetic rats | essential oil | [40] |
| Anxiolytic Antidepressant |
in vivo | 50, 75 and 150 mg/kg b.w./day * (3.91/5.86/11.72 mg/kg b.w. *) |
Albino BALB/c male mice | Hydro-alcoholic extract (75 and 150 mg/kg) significantly reversed anxiety- and depressive-like behaviors | hydro-alcoholic extract | [41] |
| Neuroprotective | in vitro | 5, 10, 50, 100, 500 μg/mL | Cortical neuronal Culture system |
Protective effects on neurons in the brain |
balm oil | [42] |
| in vivo | 50, 100, 200 and 400 mg/kg b.w. * (8.35/16.71/33.41/66.83 mg/kg b.w. *) |
Male rats | Treatment with 100 mg/kg of oil attenuated the increased caspase-3 like protease activity significantly |
balm oil | [42] | |
| GABA-T inhibitor | in vitro | 0–4 mg/mL | Rat brain | Phytochemical characterization of the crude extract determined rosmarinic acid as the major compound responsible for activity (40% inhibition at 100 μg/mL) since it represented approximately 1.5% of the dry mass of the leaves | methanol extract | [43] |
| Anti-kinetoplastidae | in vitro | 31.25, 62.5, 125, 250 μg/mL | T. cruzi, L. brasiliensi, L. infantum |
A potential source of natural product featuring anti-Leishmania and anti-Trypanosoma activity | ethanol extract | [44] |
| Analgesic | in vivo | 5, 10, 20 mg/kg b.w. * (0.87/1.73/3.46 mg/kg b.w. *) |
Male Wistar rats | Intrathecal administration could significantly improve hot-water and formalin-induced pain in male Wistar rats | hydro-alcoholic extract | [45] |
| Hypnotic | in vivo | 200, 400 and 800 mg/kg b.w. * (14.81/29.61/59.23 mg/kg b.w. *) |
Male Swiss mice | Extracts may be useful for insomnia | hydro-alcoholic extracts | [46] |
| Antidiabetic | in vivo | 0.0125 mg/d | db/db mice | Anti-hyperglycaemic agent | essential oil | [47] |
| in vivo | 0.4%, 0.8% (w/w) | Otsuka Long-Evans Tokushima fatty rats | An effective therapeutic strategy to treat human obesity and type 2 diabetes | herbal extract | [48] | |
| Anti-Alzheimer | in vitro | 8.8 mg/mL | GSK-3Β, CK-1δ, and BACE-1 | Best activity for ck-1δ inhibitory activity with maximum inhibitory concentration values at half (IC50) below 250 μg/mL | methanol extract | [49] |
| Antispasmodic | ex vivo | 1, 5, 10, 25, and 50 mg/mL | Different segments of the gastrointestinal tract of mice | Site- and dose-dependent effects on the contractile activity of the gastrointestinal tract | hydro-ethanolic leaf extract | [50] |
| Antiviral | in vitro | 1.5–150 μg/mL | RC-37 cells | High virucidal activity against HSV-1 |
aqueous extract | [51] |
| Antifungal | in vitro | 15.5–2000 μg/mL | Human Pathogenic fungi |
Good antifungal activity | ethanol extracts | [52] |
| 0.25–2 μL/mL | Phytopathogenic fungi in apples | essential oil | [53] | |||
| Antibacterial | in vitro | 10 and 15 mg/mL | E. coli, L. monocytogenes, S. aureus and S. typhimurium | A significant antimicrobial effect | essential oil | [38] |
* estimated human equivalent dose. b.w. = body weight.
Some of the pharmacological activities may be connected with the polyphenolic compounds occurring in Melissa officinalis, which include phenolic acids and flavonoids [41][54]. Most studies have focused on Melissa officinalis leaf extracts, obtaining phenolic profiles correlated with antiproliferative [35], antiangiogenic [4], antiviral [51][55], antioxidant [37][38], anti-anxiety [41], antidepressant [41], anti-Alzheimer [49], neuroprotective [42], cardioprotective [39], antifungal [52][53] and antibacterial [38] effects. Moaca et al. [34] performed a comparative study between extractions from stems and leaves in order to evaluate the antioxidant activity, the total phenolic contents, and the cytotoxic and antiproliferative effects. In this study, a good antioxidant activity was observed to be correlated with the high total polyphenol content of the leaf ethanolic extract (32.76 mg gallic acid equivalents/g) as opposed to the seed ethanolic extract (8.4 mg gallic acid equivalents/g). The extracts obtained showed a different profile of cytotoxic effects on breast cancer cells, MDA-MB-231, but with significant antitumor activity for future in vitro studies. Ghiulai et al. [4] investigated the potential for angioprevention and chemoprevention in breast cancer from various extracts of Melissa officinalis. The antioxidant activity and in vitro effect on cell viability were evaluated on two breast cancer cell lines, MCF7 and MDA-MB-231. Based on the evaluation in ovo, using the chorioallantoic membrane test, it was found that 96% ethanolic extract is the strongest chemopreventive agent.
Melissa officinalis and other plant extracts (Iberis amara, Silybum marianum and a mixture of Angelica archangelica and Carum carvi) were evaluated in association with STW5, a well-known fixed herbal multicomponent preparation recommended, at least, by the German treatment guideline for some gastrointestinal diseases under non-inflammatory and inflammatory conditions. It was found that only Melissa officinalis plant extract manifested a strong synergic effect when intestinal smooth muscle cells were considered [56]. The oral administration of a Melissa officinalis infusion can be also beneficial for the radiology staff, as the oxidative stress and DNA damage are reduced [57]. At present, only a few studies have been reported on the synergies between Melissa officinalis and other plants. Due to the volatile compounds in Melissa officinalis essential oil, the following effects have been reported: antifungal, antioxidant, antidiabetic, antibacterial and antimicrobial effects. These effects have also been reported in various extracts of Melissa officinalis.
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
06 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




