
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andreas Stylianou | -- | 3590 | 2022-04-02 10:03:52 | | | |
| 2 | Amina Yu | -119 word(s) | 3471 | 2022-04-06 02:30:44 | | |
Video Upload Options
The molecules follow a quarter-staggered fashion packing, which leads to the formation of the so call D-band periodicity. This D-band periodicity is a repeating banding pattern of about 67 nm (depending on the different tissue) and includes gap and overlap regions. Collagen fibrils form bundles and fibers by appropriate alignment.
1. General
2. Collagen
2.1. Collagen Superfamily and Collagen-Related Pathological Conditions
2.2. Collagen Type I
2.3. Collagen D-Band Periodicity
2.4. Collagen-Based Biomaterials
2.5. Imaging Collagen and Collagen-Based Biomaterials
3. Atomic Force Microscopy
3.1. General
3.2. AFM Basic Principles
3.3. AFM Modes
3.4. AFM Limitations
3.5. AFM and Collagen
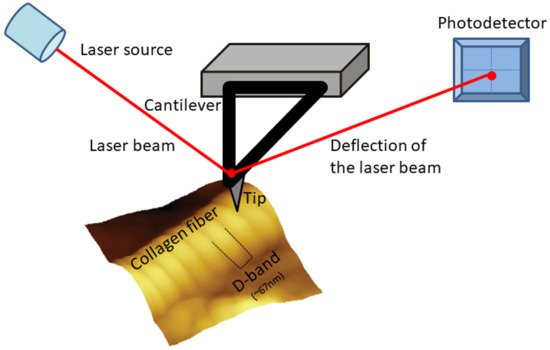
One of the major advantages of AFM in biology and bioengineering ones is the fact that it does not demand significant sample preparation. For example, for the AFM characterization, it is not necessary to coat or label the specimen with dyes/antibodies, while depending on the sample, dehydration is not mandatory [11][12][21]. Furthermore, AFM can operate both in air and liquid [86], while also experiments under vacuum conditions have been performed. Concerning the application of AFM on collagen-based samples both imaging and mechanical properties characterization have been applied in a wide range of samples, from pure collagen to collagen rich-tissues and biomaterials. AFM scanning does not affect or destroy the collagen structure, while AFM resolution can provide information from molecules to individual fibrils/fibers [6][107]. AFM has been applied for investigating different properties of collagen, including collagen structure, the role of collagen in a number of pathological conditions and collagen–cell interactions.
References
- Fratzl, P. Collagen Structure and Mechanics; Springer: New York, NY, USA, 2008.
- Tihan, G.T.; Rău, I.; Zgârian, R.G.; Ghica, M.V. Collagen-based biomaterials for ibuprofen delivery. Comptes Rendus Chim. 2016, 19, 389–393.
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501.
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978.
- Hulmes, D.J.S. Collagen diversity, synthesis and assembly. In Collagen: Structure and Mechanics; Springer: New York, NY, USA, 2008; pp. 15–47.
- Hasirci, V.; Vrana, E.; Zorlutuna, P.; Ndreu, A.; Yilgor, P.; Basmanav, F.B.; Aydin, E. Nanobiomaterials: A review of the existing science and technology, and new approaches. J. Biomater. Sci. Polym. Ed. 2006, 17, 1241–1268.
- Stylianou, A.; Yova, D. Surface nanoscale imaging of collagen thin films by Atomic Force Microscopy. Mater. Sci. Eng. C 2013, 33, 2947–2957.
- Stylianou, A.; Yova, D.; Politopoulos, K. Atomic force microscopy surface nanocharacterization of UV-irradiated collagen thin films. In Proceedings of the 12th IEEE International Conference on BioInformatics and BioEngineering, BIBE, Larnaca, Cyprus, 11–13 November 2012; pp. 602–607.
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930–933.
- Morris, V.J.; Kirby, A.R.; Gunning, A.P. Atomic Force Microscopy for Biologists; Imperial College Press: London, UK, 2008.
- Gadegaard, N. Atomic force microscopy in biology: Technology and techniques. Biotech. Histochem. 2006, 81, 87–97.
- Stylianou, A.; Yova, D.; Alexandratou, E. Nanotopography of collagen thin films in correlation with fibroblast response. J. Nanophotonics 2013, 7, 073590.
- Engel, J.; Bächinger, H.P. Structure, stability and folding of the collagen triple helix. Top. Curr. Chem. 2005, 247, 7–33.
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120, 1955–1958.
- Abou Neel, E.A.; Bozec, L.; Knowles, J.C.; Syed, O.; Mudera, V.; Day, R.; Hyun, J.K. Collagen—Emerging collagen based therapies hit the patient. Adv. Drug Deliv. Rev. 2013, 65, 429–456.
- Åsling, B.; Jirholt, J.; Hammond, P.; Knutsson, M.; Walentinsson, A.; Davidson, G.; Agreus, L.; Lehmann, A.; Lagerström-Fermer, M. Collagen type III alpha I is a gastro-oesophageal reflux disease susceptibility gene and a male risk factor for hiatus hernia. Gut 2009, 58, 1063–1069.
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014, 35, 2871–2882.
- Gkretsi, V.; Stylianou, A.; Papageorgis, P.; Polydorou, C.; Stylianopoulos, T. Remodeling components of the tumor microenvironment to enhance cancer therapy. Front. Oncol. 2015, 5, 214.
- Stylianou, A.; Gkretsi, V.; Louca, M.; Zacharia, L.; Stylianopoulos, T. Collagen Content and Extracellular Matrix Stiffness Remodels Pancreatic Fibroblasts Cytoskeleton. J. R. Soc. Interface 2019, 16, 20190226.
- Stylianou, A.; Gkretsi, V.; Stylianopoulos, T. Transforming Growth Factor-β modulates Pancreatic Cancer Associated Fibroblasts cell shape, stiffness and invasion. Biochim. Biophys. Acta 2018, 1862, 1537–1546.
- Stylianou, A.; Stylianopoulos, T. Atomic Force Microscopy Probing of Cancer Cells and Tumor Microenvironment Components. BioNanoScience 2016, 6, 33–46.
- Birk, D.E.; Bruckner, P. Collagen suprastructures. Top. Curr. Chem. 2005, 247, 185–205.
- Brinckmann, J. Collagens at a glance. In Collagen; Brinckmann, J., Mueller, P.K., Notbohm, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 247, pp. 1–6.
- Ricard-Blum, S.; Ruggiero, F.; van der Rest, M. The collagen superfamily. Top. Curr. Chem. 2005, 247, 35–84.
- Bozec, L.; van der Heijden, G.; Horton, M. Collagen Fibrils: Nanoscale Ropes. Biophys. J. 2007, 92, 70–75.
- Petruska, J.A.; Hodge, A.J. A Subunit Model for the Tropocollagen Macromolecule. Proc. Natl. Acad. Sci. USA 1964, 51, 871–876.
- Wallace, J.M.; Orr, B.G.; Marini, J.C.; Holl, M.M.B. Nanoscale morphology of Type I collagen is altered in the Brtl mouse model of Osteogenesis Imperfecta. J. Struct. Biol. 2011, 173, 146–152.
- Grant, C.A.; Phillips, M.A.; Thomson, N.H. Dynamic mechanical analysis of collagen fibrils at the nanoscale. J. Mech. Behav. Biomed. Mater. 2012, 5, 165–170.
- Fratzl, P. Collagen: Structure and mechanics, an introduction. In Collagen: Structure and Mechanics; Springer: Boston, MA, USA, 2008; pp. 1–13.
- Ivanova, V.P.; Krivchenko, A.I. A current viewpoint on structure and evolution of collagens. I. Fibrillar collagens. J. Evol. Biochem. Physiol. 2012, 48, 127–139.
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958.
- Gordon, M.K.; Hahn, R.A. Collagens. Cell Tissue Res. 2010, 339, 247–257.
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496.
- Bender, E.; Silver, F.H.; Hayashi, K. Model conformations of the carboxyl telopeptides in vivo based on type I collagen fibral banding patterns. Coll. Relat. Res. 1983, 3, 407–418.
- Bruns, R.R.; Gross, J. High-resolution analysis of the modified quarter-stagger model of the collagen fibril. Biopolymers 1974, 13, 931–941.
- Ortolani, F.; Giordano, M.; Marchini, M. A model for type II collagen fibrils: Distinctive D-band patterns in native and reconstituted fibrils compared with sequence data for helix and telopeptide domains. Biopolymers 2000, 54, 448–463.
- Stark, M.; Miller, E.J.; Kühn, K. Comparative electron-microscope studies on the collagens extracted from cartilage, bone, and skin. Eur. J. Biochem. 1972, 27, 192–196.
- Wiedemann, H.; Chung, E.; Fujii, T.; Miller, E.J.; Kühn, K. Comparative electron-microscope studies on type-III and type-I collagens. Eur. J. Biochem. 1975, 51, 363–368.
- Kühn, K. Segment-long-spacing crystallites, a powerful tool in collagen research. Coll. Relat. Res. 1982, 2, 61–80.
- Mallinger, R.; Schmut, O. Reaggregation behavior of different types of collagen in vitro: Variations in the occurrence and structure of dimeric segment long-spacing collagen. J. Ultrastruct. Mol. Struct. Res. 1988, 98, 11–18.
- Kobayashi, K.; Hashimoto, Y.; Hayakawa, T.; Hoshino, T. Further evidence for the correlation between the primary structure and the stain exclusion banding pattern of the segment-long-spacing crystallites of collagen. J. Ultrastruct. Mol. Struct. Res. 1988, 100, 255–262.
- Ortolani, F.; Marchini, M. Cartilage type II collagen fibrils show distinctive negative-staining band patterns differences between type II and type I unfixed or glutaraldehyde-fixed collagen fibrils. J. Electron Microsc. 1995, 44, 365–375.
- Adachi, E.; Hayashi, T. Comparison of axial banding patterns in fibrils of type V collagen and type I collagen. Coll. Relat. Res. 1987, 7, 27–38.
- Brodsky, B.; Eikenberry, E.F.; Cassidy, K. An unusual collagen periodicity in skin. Biochim. Biophys. Acta 1980, 621, 162–166.
- Marchini, M.; Morocutti, M.; Ruggeri, A.; Koch, M.H.; Bigi, A.; Roveri, N. Differences in the fibril structure of corneal and tendon collagen. An electron microscopy and X-ray diffraction investigation. Connect. Tissue Res. 1986, 15, 269–281.
- Chapman, J.A.; Armitage, P.M. An analysis of fibrous long spacing forms of collagen. Connect. Tissue Res. 1972, 1, 31–37.
- Loo, R.W.; Goh, J.B.; Cheng, C.C.H.; Su, N.; Cynthia Goh, M. In vitro synthesis of native, fibrous long spacing and segmental long spacing collagen. J. Vis. Exp. 2012, 67, e4417–e4425.
- Nakanishi, I.; Masuda, S.; Kitamura, T.; Moriizumi, T.; Kajikawa, K. Distribution of fibrous long-spacing fibers in normal and pathological lymph nodes. Pathol. Int. 1981, 31, 733–745.
- Wen, C.K.; Goh, M.C. Fibrous long spacing type collagen fibrils have a hierarchical internal structure. Proteins Struct. Funct. Genet. 2006, 64, 227–233.
- Highberger, J.H.; Gross, J.; Schmitt, F.O. Electron microscope observations of certain fibrous structures obtained from connective tissue extracts. J. Am. Chem. Soc. 1950, 72, 3321–3322.
- Jakus, M.A. Studies on the cornea. II. The fine structure of Descement’s membrane. J. Biophys. Biochem. Cytol. 1956, 2, 243–252.
- Cauna, N.; Ross, L.L. The fine structure of Meissner’s touch corpuscles of human fingers. J. Biophys. Biochem. Cytol. 1960, 8, 467–482.
- Luse, S.A. Electron microscopic studies of brain tumors. Neurology 1960, 10, 881–905.
- Poole, K.; Khairy, K.; Friedrichs, J.; Franz, C.; Cisneros, D.A.; Howard, J.; Mueller, D.; Baumeister, W. Molecular-scale topographic cues induce the orientation and directional movement of fibroblasts on two-dimensional collagen surfaces. J. Mol. Biol. 2005, 349, 380–386.
- Stamov, D.R.; Müller, A.; Wegrowski, Y.; Brezillon, S.; Franz, C.M. Quantitative analysis of type I collagen fibril regulation by lumican and decorin using AFM. J. Struct. Biol. 2013, 183, 394–403.
- Fang, M.; Holl, M.M.B. Variation in type I collagen fibril nanomorphology: The significance and origin. Bonekey Rep. 2013, 2, 394.
- Gross, J.; Schmitt, F.O. The structure of human skin collagen as studied with the electron microscope. J. Exp. Med. 1948, 88, 555–568.
- Beniash, E.; Traub, W.; Veis, A.; Weiner, S. A transmission electron microscope study using vitrified ice sections of predentin: Structural changes in the dentin collagenous matrix prior to mineralization. J. Struct. Biol. 2000, 132, 212–225.
- Habelitz, S.; Balooch, M.; Marshall, S.J.; Balooch, G.; Marshall, G.W., Jr. In situ atomic force microscopy of partially demineralized human dentin collagen fibrils. J. Struct. Biol. 2002, 138, 227–236.
- Meek, K.M. The cornea and sclera. In Collagen: Structure and Mechanics; Springer: Boston, MA, USA, 2008; pp. 359–396.
- Plant, A.L.; Bhadriraju, K.; Spurlin, T.A.; Elliott, J.T. Cell response to matrix mechanics: Focus on collagen. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 893–902.
- Loesberg, W.A.; te Riet, J.; van Delft, F.C.M.J.M.; Schön, P.; Figdor, C.G.; Speller, S.; van Loon, J.J.W.A.; Walboomers, X.F.; Jansen, J.A. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 2007, 28, 3944–3951.
- Lisboa, P.; Villiers, M.B.; Brakha, C.; Marche, P.N.; Valsesia, A.; Colpo, P.; Rossi, F. Fabrication of bio-functionalised polypyrrole nanoarrays for bio-molecular recognition. Micro Nanosyst. 2011, 3, 83–89.
- Brouwer, K.M.; van Rensch, P.; Harbers, V.E.; Geutjes, P.J.; Koens, M.J.; Wijnen, R.M.; Daamen, W.F.; van Kuppevelt, T.H. Evaluation of methods for the construction of collagenous scaffolds with a radial pore structure for tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 501–504.
- Silver, F.H.; Freeman, J.W.; Seehra, G.P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003, 36, 1529–1553.
- Phong, H.Q.; Wang, S.L.; Wang, M.J. Cell behaviors on micro-patterned porous thin films. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 169, 94–100.
- Tay, C.Y.; Irvine, S.A.; Boey, F.Y.C.; Tan, L.P.; Venkatraman, S. Micro-/nano-engineered cellular responses for soft tissue engineering and biomedical applications. Small 2011, 7, 1361–1378.
- Stylianou, A. Atomic force microscopy for collagen-based nanobiomaterials. J. Nanomater. 2017, 2017, 9234627.
- Stylianou, A.; Kontomaris, S.V.; Yova, D. Assessing Collagen Nanoscale Thin Films Heterogeneity by AFM Multimode Imaging and Nanoindetation for NanoBioMedical Applications. Micro Nanosyst. 2014, 6, 95–102.
- Stylianou, A.; Kontomaris, S.B.; Kyriazi, M.; Yova, D. Surface characterization of collagen films by atomic force microscopy. In Proceedings of the 12th Mediterranean Conference on Medical and Biological Engineering and Computing, MEDICON, Chalkidiki, Greece, 23–30 May 2010; Volume 29, pp. 612–615.
- Garcia, A.M.; Magalhes, F.L.; Soares, J.S.; Junior, E.P.; de Lima, M.F.R.; Mamede, M.; de Paula, A.M. Second harmonic generation imaging of the collagen architecture in prostate cancer tissue. Biomed. Phys. Eng. Express 2018, 4, 025026.
- Kim, B.M.; Eichler, J.; Reiser, K.M.; Rubenchik, A.M.; Da Silva, L.B. Collagen structure and nonlinear susceptibility: Effects of heat, glycation, and enzymatic cleavage on second harmonic signal intensity. Lasers Surg. Med. 2000, 27, 329–335.
- Han, M.; Giese, G.; Bille, J.F. Second harmonic generation imaging of collagen fibrils in cornea and sciera. Opt. Express 2005, 13, 5791–5797.
- Tuer, A.E.; Krouglov, S.; Prent, N.; Cisek, R.; Sandkuijl, D.; Yasufuku, K.; Wilson, B.C.; Barzda, V. Nonlinear optical properties of type i collagen fibers studied by polarization dependent second harmonic generation microscopy. J. Phys. Chem. B 2011, 115, 12759–12769.
- Psilodimitrakopoulos, S.; Filippidis, G.; Kouloumentas, C.; Alexandratou, E.; Yova, D. Combined two photon excited fluorescence and second harmonic generation imaging microscopy of collagen structures. Proc. SPIE 2006, 6089, 60891P.
- Drifka, C.R.; Loeffler, A.G.; Mathewson, K.; Mehta, G.; Keikhosravi, A.; Liu, Y.; Lemancik, S.; Ricke, W.A.; Weber, S.M.; Kao, W.J.; et al. Comparison of Picrosirius Red Staining With Second Harmonic Generation Imaging for the Quantification of Clinically Relevant Collagen Fiber Features in Histopathology Samples. J. Histochem. Cytochem. 2016, 64, 519–529.
- Rittié, L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. Methods Mol. Biol. 2017, 1627, 395–407.
- Stylianou, A.; Voutouri, C.; Mpekris, F.; Stylianopoulos, T. Pancreatic cancer collagen-based optical signatures. In Proceedings of the Polarized Light and Optical Angular Momentum for Biomedical Diagnostics, online, 6–12 March 2021; International Society for Optics and Photonics (SPIE): Bellingham, WA, USA, 2021; Volume 11646.
- Starborg, T.; Kalson, N.S.; Lu, Y.; Mironov, A.; Cootes, T.F.; Holmes, D.F.; Kadler, K.E. Using transmission electron microscopy and 3View to determine collagen fibril size and three-dimensional organization. Nat. Protoc. 2013, 8, 1433–1448.
- Starborg, T.; Lu, Y.; Kadler, K.E.; Holmes, D.F. Chapter 17 Electron Microscopy of Collagen Fibril Structure In Vitro and In Vivo Including Three-Dimensional Reconstruction. In Methods in Cell Biology; Academic Press: Cambridge, MA, USA, 2008; Volume 88, pp. 319–345.
- Maurer, T.; Stoffel, M.H.; Belyaev, Y.; Stiefel, N.G.; Vidondo, B.; Küker, S.; Mogel, H.; Schäfer, B.; Balmer, J. Structural characterization of four different naturally occurring porcine collagen membranes suitable for medical applications. PLoS ONE 2018, 13, e0205027.
- Hodge, A.J.; Schmitt, F.O. The charge profile of the tropocollagen macromolecule and the packing arrangement in native-type collagen fibrils. Proc. Natl. Acad. Sci. USA 1960, 46, 186–197.
- Ushiki, T. Collagen Fibers, Reticular Fibers and Elastic Fibers. A Comprehensive Understanding from a Morphological Viewpoint. Arch. Histol. Cytol. 2002, 65, 109–126.
- Ruprecht, J.; Nield, J. Determining the structure of biological macromolecules by transmission electron microscopy, single particle analysis and 3D reconstruction. Prog. Biophys. Mol. Biol. 2001, 75, 121–164.
- Baumeister, W.; Grimm, R.; Walz, J. Electron tomography of molecules and cells. Trends Cell Biol. 1999, 9, 81–85.
- Allison, D.P.; Mortensen, N.P.; Sullivan, C.J.; Doktycz, M.J. Atomic force microscopy of biological samples. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 618–634.
- Stylianou, A.; Yova, D.; Politopoulos, K. Atomic force microscopy quantitative and qualitative nanoscale characterization of collagen thin films. In Proceedings of the 5th International Conference on Emerging Technologies in Non-Destructive Testing, NDT, Ioannina, Greece, 10–21 September 2011; pp. 415–420.
- Stylianou, A.; Politopoulos, K.; Yova, D. Atomic force microscopy imaging of the nanoscale assembly of type i collagen on controlled polystyrene particles surfaces. In Proceedings of the 5th European Conference of the International Federation for Medical and Biological Engineering, Budapest, Hungary, 14–18 September 2011; Volume 37, pp. 1058–1061.
- Kontomaris, S.V.; Stylianou, A.; Yova, D.; Politopoulos, K. Mechanical properties of collagen fibrils on thin films by Atomic Force Microscopy nanoindentation. In Proceedings of the 12th IEEE International Conference on BioInformatics and BioEngineering, BIBE, Lancarna, Cyprus, 11–13 November 2012; pp. 608–613.
- Stylianou, A.; Kontomaris, S.V.; Yova, D.; Balogiannis, G. AFM Multimode Imaging and Nanoindetation Method for Assessing Collagen Nanoscale Thin Films Heterogeneity. IFMBE Proc. 2014, 41, 407–410.
- Kontomaris, S.V.; Stylianou, A.; Yova, D.; Balogiannis, G. The effects of UV irradiation on collagen D-band revealed by atomic force microscopy. Scanning 2015, 37, 101–111.
- Keysight-Technologies Keysight 5500 Scanning Probe Microscope-User’s Guide. Available online: http://nano.em.keysight.com/PDFs/5500%20User%20Guide%20Dec%202015%20REV%20H.pdf (accessed on 16 October 2021).
- Han, W.; Serry, F.M. Force Spectroscopy with the Atomic Force Microscope-Application Note; Agilent Technologies: Santa Clara, CA, USA, 2008.
- Stolz, M.; Raiteri, R.; Daniels, A.U.; VanLandingham, M.R.; Baschong, W.; Aebi, U. Dynamic Elastic Modulus of Porcine Articular Cartilage Determined at Two Different Levels of Tissue Organization by Indentation-Type Atomic Force Microscopy. Biophys. J. 2004, 86, 3269–3283.
- Stolz, M.; Gottardi, R.; Raiteri, R.; Miot, S.; Martin, I.; Imer, R.; Staufer, U.; Raducanu, A.; Düggelin, M.; Baschong, W.; et al. Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat. Nanotechnol. 2009, 4, 186–192.
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20.
- Darling, E.M. Force scanning: A rapid, high-resolution approach for spatial mechanical property mapping. Nanotechnology 2011, 22, 175707.
- Braunsmann, C.; Seifert, J.; Rheinlaender, J.; Schäffer, T.E. High-speed force mapping on living cells with a small cantilever atomic force microscope. Rev. Sci. Instrum. 2014, 85, 073703.
- Kontomaris, S.V.; Yova, D.; Stylianou, A.; Politopoulos, K. The significance of the percentage differences of young’s modulus in the AFM nanoindentation procedure. Micro Nanosyst. 2015, 7, 86–97.
- Hertz, H. Ueber die Berührung fester elastischer Körper. J. Für Die Reine Und Angew. Math. 1882, 1882, 156–171.
- Mackay, J.L.; Kumar, S. Measuring the elastic properties of living cells with atomic force microscopy indentation. Methods Mol. Biol. 2013, 931, 313–329.
- Kontomaris, S.V.; Stylianou, A. Atomic force microscopy for university students: Applications in biomaterials. Eur. J. Phys. 2017, 38, 033003.
- Graham, H.K.; Hodson, N.W.; Hoyland, J.A.; Millward-Sadler, S.J.; Garrod, D.; Scothern, A.; Griffiths, C.E.M.; Watson, R.E.B.; Cox, T.R.; Erler, J.T.; et al. Tissue section AFM: In situ ultrastructural imaging of native biomolecules. Matrix Biol. 2010, 29, 254–260.
- Ushiki, T.; Hitomi, J.; Ogura, S.; Umemoto, T.; Shigeno, M. Atomic force microscopy in histology and cytology. Arch. Histol. Cytol. 1996, 59, 421–431.
- Strange, A.P.; Aguayo, S.; Ahmed, T.; Mordan, N.; Stratton, R.; Porter, S.R.; Parekh, S.; Bozec, L. Quantitative nanohistological investigation of scleroderma: An atomic force microscopy-based approach to disease characterization. Int. J. Nanomed. 2017, 12, 411–420.
- Gisbert, V.G.; Benaglia, S.; Uhlig, M.R.; Proksch, R.; Garcia, R. High-Speed Nanomechanical Mapping of the Early Stages of Collagen Growth by Bimodal Force Microscopy. ACS Nano 2021, 15, 1850–1857.
- Cisneros, D.A.; Friedrichs, J.; Taubenberger, A.; Franz, C.M.; Muller, D.J. Creating ultrathin nanoscopic collagen matrices for biological and biotechnological applications. Small 2007, 3, 956–963.




