
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peter Hamar | -- | 1915 | 2022-03-31 16:08:32 | | | |
| 2 | Yvaine Wei | -1 word(s) | 1914 | 2022-04-01 05:01:38 | | |
Video Upload Options
The prevailing general view of acute-phase proteins (APPs) is that they are produced by the liver in response to the stress of the body as part of a systemic acute-phase response. A coordinated, local production of these proteins upon cell stress by the stressed cells has been demonstrated. The local, stress-induced APP production has been demonstrated in different tissues (kidney, breast cancer) and with different stressors (hypoxia, fibrosis and electromagnetic heat). Thus, this local acute-phase response (APR) seems to be a universal mechanism. APP production is an ancient defense mechanism observed in nematodes and fruit flies as well. Local APP production at the tissue level is also supported by sporadic literature data for single proteins.
1. Introduction
2. Common Pathways of Renal Fibrosis and Cancer Progression
3. The Heat Shock Response and the Acute Phase Response
4. Modulated Electro-Hyperthermia (mEHT)
5. Acute (AKI) and Chronic Kidney Disease (CKD)
6. The Potential of Degradomic Analysis
7. Known Roles of Major Acute-Phase Proteins Detected by NGS in Different Models
7.1. Fibrinogens
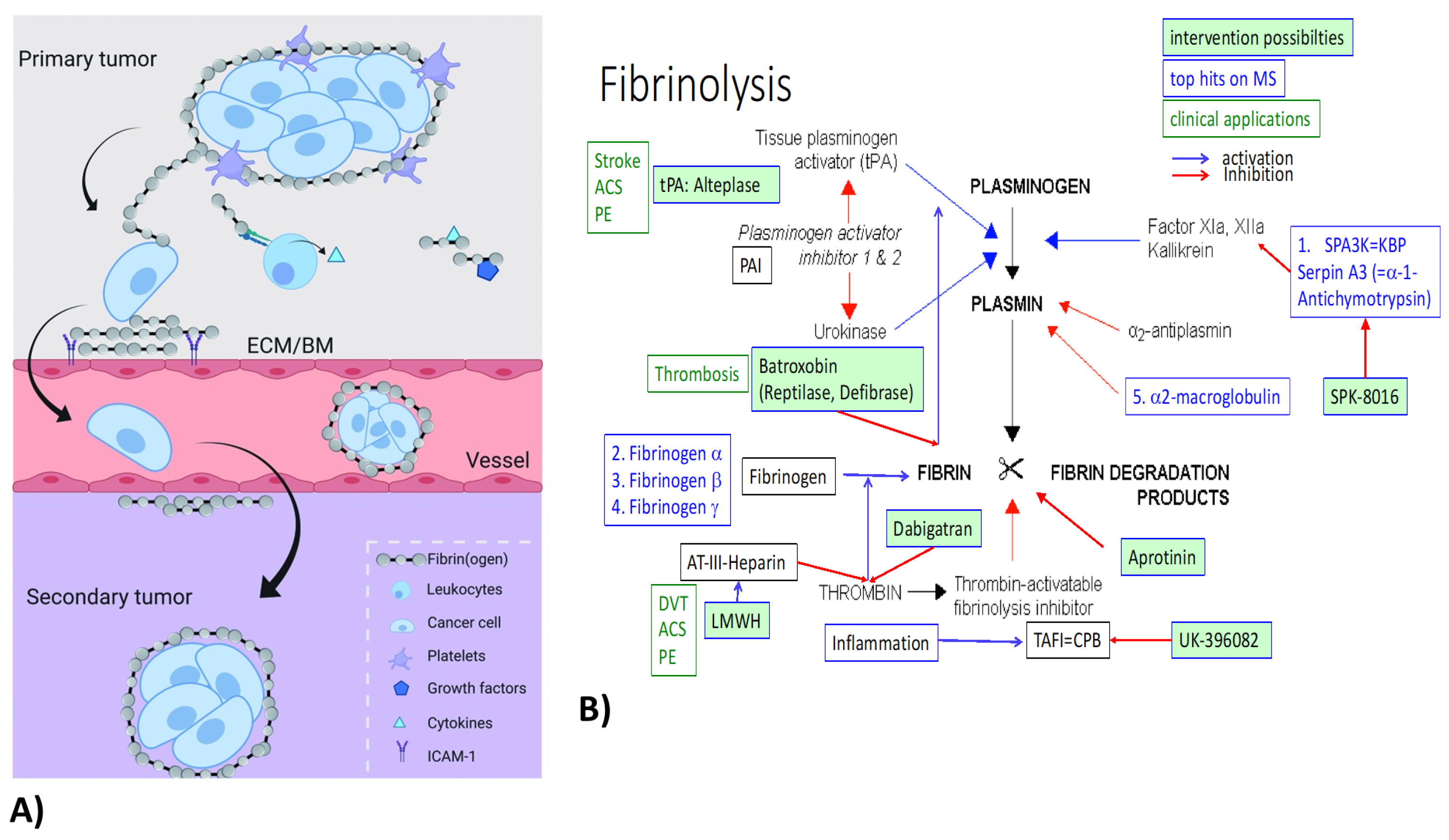
7.2. Haptoglobin
7.3. Protease Inhibitors (Serpins, ITI)
7.4. Alpha-2-Macroglobulin (a2-MG)
7.5. Complement Factors (C4B)
8. Conclusions
References
- Marcos-Perez, D.; Sanchez-Flores, M.; Proietti, S.; Bonassi, S.; Costa, S.; Teixeira, J.J.; Fernández-Tajes, J.; Pasaro, E.; Laffon, B.; Valdiglesias, V. Association of inflammatory mediators with frailty status in older adults: Results from a systematic review and meta-analysis. Geroscience 2020, 42, 1451–1473.
- Gasparics, A.; Kokeny, G.; Fintha, A.; Bencs, R.; Mozes, M.M.; Agoston, E.I.; Buday, A.; Ivics, Z.; Hamar, P.; Gyorffy, B.; et al. Alterations in SCAI Expression during Cell Plasticity, Fibrosis and Cancer. Pathol. Oncol. Res. 2018, 24, 641–651.
- Fintha, A.; Gasparics, A.; Fang, L.; Erdei, Z.; Hamar, P.; Mozes, M.M.; Kokeny, G.; Rosivall, L.; Sebe, A. Characterization and role of SCAI during renal fibrosis and epithelial-to-mesenchymal transition. Am. J. Pathol. 2013, 182, 388–400.
- Hamar, P.; Song, E.; Kokeny, G.; Chen, A.; Ouyang, N.; Lieberman, J. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2004, 101, 14883–14888.
- Wu, L.L.; Xu, G.G.; Zhao, Q.; Zhou, L.L.; Wang, D.; Chen, W.W. The association between hypoxia inducible factor 1 subunit alpha gene rs2057482 polymorphism and cancer risk: A meta-analysis. BMC Cancer 2019, 19, 1123.
- Danics, L.; Schvarcz, C.C.; Viana, P.; Vancsik, T.; Krenacs, T.; Benyo, Z.; Kaucsar, T.; Hamar, P. Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers 2020, 12, 2581.
- Gomez, C.C. Role of heat shock proteins in aging and chronic inflammatory diseases. Geroscience 2021, 43, 2515–2532.
- Smith-Sonneborn, J. Telomerase Biology Associations Offer Keys to Cancer and Aging Therapeutics. Curr. Aging Sci. 2020, 13, 11–21.
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454.
- Moshage, H. Cytokines and the hepatic acute phase response. J. Pathol. 1997, 181, 257–266.
- Schrodl, W.; Buchler, R.; Wendler, S.; Reinhold, P.; Muckova, P.; Reindl, J.; Rhode, H. Acute phase proteins as promising biomarkers: Perspectives and limitations for human and veterinary medicine. Proteomics Clin. Appl. 2016, 10, 1077–1092.
- Schvarcz, C.C.; Danics, L.; Krenacs, T.; Viana, P.; Beres, R.; Vancsik, T.; Nagy, A.; Gyenesei, A.; Kun, J.; Fonovic, M.; et al. Modulated Electro-Hyperthermia Induces a Prominent Local Stress Response and Growth Inhibition in Mouse Breast Cancer Isografts. Cancers 2021, 13, 1744.
- Roka, B.; Tod, P.; Kaucsar, T.; Vizovisek, M.; Vidmar, R.; Turk, B.; Fonovic, M.; Szenasi, G.; Hamar, P. The Acute Phase Response Is a Prominent Renal Proteome Change in Sepsis in Mice. Int. J. Mol. Sci. 2019, 21, 200.
- Bukosza, E.E.; Kornauth, C.; Hummel, K.; Schachner, H.; Huttary, N.; Krieger, S.; Nobauer, K.; Oszwald, A.; Razzazi Fazeli, E.; Kratochwill, K.; et al. ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS. Int. J. Mol. Sci. 2020, 21, 2095.
- Vilar, R.; Fish, R.R.; Casini, A.; Neerman-Arbez, M. Fibrin(ogen) in human disease: Both friend and foe. Haematologica 2020, 105, 284–296.
- Simpson-Haidaris, P.P.; Rybarczyk, B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann. N. Y. Acad. Sci. 2001, 936, 406–425.
- Degen, J.J.; Drew, A.A.; Palumbo, J.J.; Kombrinck, K.K.; Bezerra, J.J.; Danton, M.M.; Holmback, K.; Suh, T.T. Genetic manipulation of fibrinogen and fibrinolysis in mice. Ann. N. Y. Acad. Sci. 2001, 936, 276–290.
- Cruz-Topete, D.; Iwaki, T.; Ploplis, V.V.; Castellino, F.F. Delayed inflammatory responses to endotoxin in fibrinogen-deficient mice. J. Pathol. 2006, 210, 325–333.
- Sorensen, I.; Susnik, N.; Inhester, T.; Degen, J.J.; Melk, A.; Haller, H.; Schmitt, R. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011, 80, 1035–1044.
- Petzelbauer, P.; Zacharowski, P.P.; Miyazaki, Y.; Friedl, P.; Wickenhauser, G.; Castellino, F.F.; Groger, M.; Wolff, K.; Zacharowski, K. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ische-mia-reperfusion injury. Nat. Med. 2005, 11, 298–304.
- Rybarczyk, B.B.; Simpson-Haidaris, P.P. Fibrinogen assembly, secretion, and deposition into extracellular matrix by MCF-7 human breast carcinoma cells. Cancer Res. 2000, 60, 2033–2039.
- Baskin, J.J.; Pui, C.C.; Reiss, U.; Wilimas, J.J.; Metzger, M.M.; Ribeiro, R.R.; Howard, S.S. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet 2009, 374, 159–169.
- Gomez-Outes, A.; Terleira-Fernandez, A.A.; Calvo-Rojas, G.; Suarez-Gea, M.M.; Vargas-Castrillon, E. Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups. Thrombosis 2013, 2013, 640723.
- Spark Therapeutics, I. Available online: https://sparktx.com/pipelines/spk-8016-hemophilia-a-inhibitor-market/ (accessed on 6 November 2018).
- Pfizer, A. Study In Healthy People of Multiple Doses of UK-396,082 Given By Mouth, to Investigate the Safety, Toleration and Time Course of Blood Concentration of UK-396,082 and Its Effects. 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT01091532 (accessed on 24 March 2010).
- Saeed, S.S. Denning-Kendall, A. McDonald-Gibson, W.J. Collier, H.O.J. Human haptoglobin: An endogenous inhibitor of prostaglandin synthase. In Inflammation: Mechanisms and Treatment; Springer: Dordrecht, The Netherlands, 1980; Volume 4, pp. 285–300.
- NCBI HP Haptoglobin [Homo Sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene/3240 (accessed on 27 February 2022).
- Quaye, I.I. Haptoglobin, inflammation and disease. Trans. R Soc. Trop. Med. Hyg. 2008, 102, 735–742.
- Snellman, O.; Sylvén, B. Haptoglobin acting as a Natural Inhibitor of Cathepsin B Activity. Nature 1967, 216, 1033.
- Huber-Lang, M.; Ekdahl, K.K.; Wiegner, R.; Fromell, K.; Nilsson, B. Auxiliary activation of the complement system and its importance for the pathophysiology of clinical conditions. Semin Immunopathol. 2018, 40, 87–102.
- Wessels, M.R.; Butko, P.; Ma, M.; Warren, H.B.; Lage, A.L.; Carroll, M.C. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc. Natl. Acad. Sci. USA 1995, 92, 11490–11494.
- Schubart, A.; Anderson, K.; Mainolfi, N.; Sellner, H.; Ehara, T.; Adams, C.M.; Mac Sweeney, A.; Liao, S.M.; Crowley, M.; Littlewood-Evans, A.; et al. Small-molecule factor B inhibitor for the treatment of complement-mediated diseases. Proc. Natl. Acad. Sci. USA 2019, 116, 7926–7931.




