The prevailing general view of acute-phase proteins (APPs) is that they are produced by the liver in response to the stress of the body as part of a systemic acute-phase response. A coordinated, local production of these proteins upon cell stress by the stressed cells has been demonstrated. The local, stress-induced APP production has been demonstrated in different tissues (kidney, breast cancer) and with different stressors (hypoxia, fibrosis and electromagnetic heat). Thus, this local acute-phase response (APR) seems to be a universal mechanism. APP production is an ancient defense mechanism observed in nematodes and fruit flies as well. Local APP production at the tissue level is also supported by sporadic literature data for single proteins.
1. Introduction
During the stress response of the body, the liver produces acute-phase proteins (APPs)
[1]. The known effect of stress on cells/organs is the heat shock response. A general stress response has been demonstrated of acute-phase proteins, termed the acute-phase response (APR), as the main proteomic response in different mouse models including acute kidney injury (AKI)-induced renal fibrosis and modulated electro-hyperthermia (mEHT)-induced death of triple-negative breast cancer (TNBC) by multiplex methods. It was observed that during renal scarring due to severe ischemic damage to the kidney and during heat therapy treatment of mouse breast cancer, the kidney and the tumor cells produce acute-phase proteins, and this is the leading response of these cells according to multiplex studies.
There is scarce information in the literature that APR proteins are produced outside of the liver and locally, in stressed cells. The APR was seen as independent of the model and tissue.
Better characterization of the local APR may lead to better diagnostic/prognostic or therapeutic possibilities at least in the fields of AKI-induced fibrosis and cancer. Possible clinical applications of the APR theory are: (1) Inhibition of the tumor stress response can increase the efficacy of various tumor treatment procedures. (2) Influencing the same stress response may slow renal scarring following ischemia. (3) The detection of stress proteins and their degradation products in the bloodstream and urine may be an effective new tool for clinical monitoring of these processes.
2. Common Pathways of Renal Fibrosis and Cancer Progression
Renal fibrosis and cancer progression have been linked through several common pathomechanistic pathways including tissue hypoxia, inflammation and oxidative stress. Furthermore, there are several common mediators, such as transforming growth factor beta (TGF-beta)—a key driver of malignant transformation—as well as tissue fibrosis, the involvement of fibroblasts in fibrosis and in the tumor microenvironment, or hypoxia-inducible factor-1 (HIF-1)
[2][3][4][5]. These factors (TGF-beta fibroblasts, HIF-1alpha) are the most important factors in wound healing and tissue repair; however, if they remain activated following healing of an acute injury such as hypoxia, they are the key factors of fibrosis progression. Furthermore, these factors are also regarded as important tumor promoters.
3. The Heat Shock Response and the Acute Phase Response
The heat shock response (HSR) is a well-described, general and ancient stress response of cells to several different types of stress
[6][7][8]. The acute-phase response (APR) is part of a general, systemic response to infections and tissue damage. According to the definition, proteins whose plasma concentration is changed by at least 25 percent in response to pro-inflammatory stimuli are termed acute-phase proteins (APPs)
[9]. They have a role in restoring homeostasis after inflammation
[10]. The generally held concept is that APPs are produced in the liver and triggered mainly by inflammatory interleukin-6 (IL-6) (as well as IL-1, IL-8 and TNF-alpha) and secreted into the blood. However, APPs are also synthesized in other organs. Thus, APPs can contribute to local defense responses and repair mechanisms
[11]. A complex local production of several APPs in different tissues upon injury have been demonstrated by proteomic analysis, such as electromagnetic heating of tumor cells
[12], or acute hypoxic
[13] or chronic fibrotic injury of the kidney
[14].
4. Modulated Electro-Hyperthermia (mEHT)
Modulated electro-hyperthermia (mEHT) is a newly emerging adjuvant cancer treatment used in human oncology. During mEHT, a focused electromagnetic field (EMF) is generated within the tumor by applying capacitive radiofrequency. Selective energy absorption by the tumor is the consequence of elevated oxidative glycolysis (Warburg effect) and conductivity of the tumor. The EMF induces cell death by thermal and non-thermal effects. Capacitive energy delivery and frequency modulation enable the application of non-thermal effects. The company inventing and producing mEHT devices for human therapy has developed the rodent mEHT device to enable the accurate, reproducible, standard and effective treatment of TNBC in the inguinal region of mice
[6].
5. Acute (AKI) and Chronic Kidney Disease (CKD)
Both acute (AKI) and chronic kidney disease (CKD) that can result in renal failure are common, significant, but underestimated problems regarding their relevance. CKD is becoming a major health care problem around the world for the aging population and due to the growing incidence of hypertension. AKI is a devastating common disease that can heal or be cured in some cases, but the acute injury can initiate unfavorable processes culminating in renal failure. Better understanding the AKI-induced CKD may lead to new therapeutic avenues for halting the progression of the presently incurable renal fibrosis. Following damage of tubular epithelial cells (TEC), the development of the highly differentiated form of TEC can be disturbed during the regenerative processes leading to pathological changes of intrinsic regulatory processes. Several factors, such as TGF-beta
[6][12][13][14], miR-21, HIF-1alpha, etc., are considered protective and stimulate regenerative processes following acute tubular injury, but sustained elevation of these factors is the major driving force of chronic scarring and fibrosis (CKD) leading to eventual renal failure.
6. The Potential of Degradomic Analysis
Pathological states such as cancer, ischemia and inflammation can affect various proteolytic pathways, which usually lead to increased general proteolysis within the organism. Identification of the affected proteolytic substrates can significantly improve the understanding of specific pathological mechanisms. Furthermore, since proteolytic peptides and protein fragments diffuse from the primary tissues to body fluids, such as blood and urine, such fluids can serve as good sources for protease substrate identification and their utilization as diagnostic, prognostic or therapeutic biomarkers. Thus, the effect of physiological stress on general proteolysis can be determined by performing the peptidomic analysis of urine and/or blood samples collected from subjects with cancer or postischemic renal fibrosis. APPs have been proposed already as potential biomarkers of cancer and renal ischemia/fibrosis
[11].
7. Known Roles of Major Acute-Phase Proteins Detected by NGS in Different Models
7.1. Fibrinogens
Fibrin(ogen) (FN) is an abundant protein, present in human blood at concentrations of 1.5–4 g/L
[15]. The physiological functions of FN are: besides providing a scaffold for blood clotting, FN is important for the assembly of (extracellular) matrices to enhance host defense
[16]. FN has been implicated in pathological processes including renal diseases and cancer. Fg-deficient mice (Fg
−/−)
[17] were protected from endotoxemia
[18], renal fibrosis
[19] and ischemia-reperfusion injury
[20]. Coagulation factors have been linked with malignancy for over 100 years and high plasma fibrinogen, in particular, has been associated with cancer progression. FN can be produced by cancer cells, such as breast cancer
[16], and binds to and surrounds cancer cells, forming a protective structure (
Figure 1A). Furthermore, by interacting with endothelial cells, FN contributes to the extravasation of cancer cells
[15]. A hallmark of breast carcinoma is the local synthesis and deposition of fibrinogen (precipitation without conversion to fibrin)
[21]. FN in the ECM augments the innate immune response to tissue injury or cancer
[21]. FN deposition is a predominant component in breast tumor stroma
[21]. All three FN chains were within the top upregulated genes on all three multiplex screens in TNBC and renal models. The multiplex data do not distinguish between fibrinogen and fibrin and do not indicate the involvement of thrombin or other coagulation-related factors. Besides FN upregulation, fibrinolysis inhibitors Serpin A3 (alpha-1-Antichymotrypsin) and alpha-2-macroglobulin were also upregulated on both the tumor and at least one renal screen (
Figure 1B).
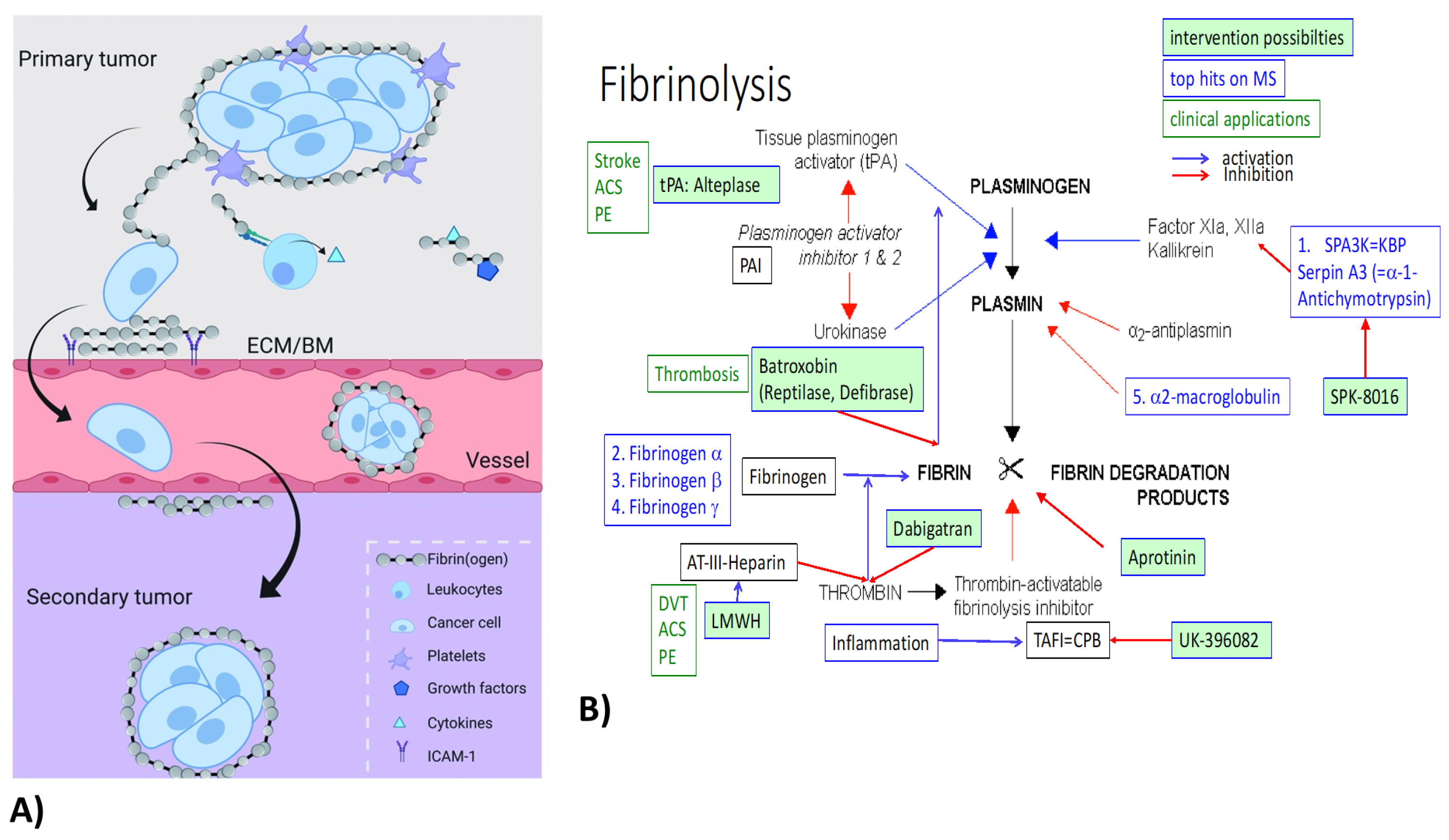
7.2. Haptoglobin
Haptoglobin (Hp) is a well-characterized glycoprotein found in all mammals but also, in its simpler form, in fish. However, its exact physiological function is still uncertain
[26]. Hp functions as an antioxidant to prevent oxidative damage to tissues, including the kidney
[27]. Further, already-described Hp functions include pro-angiogenic and immunomodulatory
[28] functions through inhibition of cathepsin-B
[29] and prostaglandin synthase
[26]. Moreover, especially haptoglobin but also fibrinogen beta were indicative of metastasis in a human triple-negative MDA-MB-231 breast cancer model .
7.3. Protease Inhibitors (Serpins, ITI)
Proteases are involved in many, if not all, acute diseases and are found in all living organisms, including plants
[30]. Within the serine protease group, the chymotrypsin family includes numerous proteases within the complement system, the contact activation (kallikrein)/coagulation system, and the fibrinolytic system
[30].
7.4. Alpha-2-Macroglobulin (a2-MG)
Alpha-2-macroglobulin is a well-known acute-phase protein produced by the liver, but local production by macrophages and fibroblasts has also been described. Both in mEHT-treated tumors and in hypoxic kidneys, a2-MG was upregulated greater than two-fold both at the mRNA and protein levels. A2-MG is an antiprotease: it inactivates an enormous variety of proteinases. It inhibits fibrinolysis by inhibiting both plasmin and kallikrein.
7.5. Complement Factors (C4B)
Several complement components (C2, C3, C4B, C7, C8 alpha and gamma chains), complement regulators (CD59A glycoprotein - MAC inhibitor), complement factors B, D, H, I and P (properidin) and the pentraxin-related gene (PTX3) were upregulated on the multiplex screens. From these, C4B was three-fold (protein-MS) and four-fold (mRNA-NGS, nanostring) upregulated in mEHT-treated tumors and three-fold (mRNA-NGS) in fibrotic kidneys. Furthermore, CFB, D, I and P appeared on these two screens. The complement system is the central effector of the humoral arm of innate immunity: the more ancient part of the immune system. However, both C3 and C4 are essential parts of both the innate and the acquired immune system
[31]. C4B is the proteolytically active component of the C3 and C5 convertases. The alternative pathway is triggered by the activation of C4B and acts as an amplification loop of the complement system
[32]. A small-molecule factor B inhibitor has been described recently
[32].
8. Conclusions
In conclusion, the presently prevailing concept of the acute-phase response (APR) may be only part of the truth. Besides the well-known systemic acute-phase response, where in response to tissue injury and consequent inflammation, locally produced inflammatory cytokines (IL-6, IL-1b, etc.) induce acute-phase protein (APP) production by the liver, there is a local APR at the cellular level: a coordinated local production of several acute-phase proteins with the aim of protecting the cells from environmental stress, similarly to the also well-described heat shock response. A future perspective includes the possible utilization of this novel stress response for diagnostic and therapeutic purposes. In the case of cancers, the cancer cells may produce APPs to protect themselves from conventional (chemotherapy, radiotherapy) or adjuvant (heat therapy) treatments. Local, targeted inhibition of the production of these APPs in the tumor cells may substantially enhance the anti-tumor effects of presently avialable anti-cancer treatments.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23062972

