Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aashiq Hussain Bhat | -- | 2749 | 2022-03-31 12:04:27 | | | |
| 2 | Catherine Yang | Meta information modification | 2749 | 2022-04-06 08:13:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bhat, A.; Shakeel, F.; , .; Khan, A.; Alshahrani, S.; Alshehri, S.; Ghoneim, M.; Alam, P. Tricyclodecan-9-yl-Xanthogenate (D609). Encyclopedia. Available online: https://encyclopedia.pub/entry/21219 (accessed on 07 February 2026).
Bhat A, Shakeel F, , Khan A, Alshahrani S, Alshehri S, et al. Tricyclodecan-9-yl-Xanthogenate (D609). Encyclopedia. Available at: https://encyclopedia.pub/entry/21219. Accessed February 07, 2026.
Bhat, Aashiq, Faiyaz Shakeel, , Andleeb Khan, Saeed Alshahrani, Sultan Alshehri, Mohammed Ghoneim, Prawez Alam. "Tricyclodecan-9-yl-Xanthogenate (D609)" Encyclopedia, https://encyclopedia.pub/entry/21219 (accessed February 07, 2026).
Bhat, A., Shakeel, F., , ., Khan, A., Alshahrani, S., Alshehri, S., Ghoneim, M., & Alam, P. (2022, March 31). Tricyclodecan-9-yl-Xanthogenate (D609). In Encyclopedia. https://encyclopedia.pub/entry/21219
Bhat, Aashiq, et al. "Tricyclodecan-9-yl-Xanthogenate (D609)." Encyclopedia. Web. 31 March, 2022.
Copy Citation
Tricyclodecan-9-yl xanthogenate (D609) is a synthetic tricyclic compound possessing a xanthate group. This xanthogenate compound is known for its diverse pharmacological properties. Over the last three decades, many studies have reported the biological activities of D609, including antioxidant, antiapoptotic, anticholinergic, anti-tumor, anti-inflammatory, anti-viral, anti-proliferative, and neuroprotective activities.
tricyclodecan-9-yl xanthogenate (D609)
cellular proliferation
1. Introduction
Tricyclodecan-9-yl xanthogenate (D609) is a synthetic tricyclic compound featuring a xanthate group, known as a phosphate group analog (Figure 1). The first antiviral biological experiments did not commence until 1984, over fifty years after the initial synthesis was published [1][2]. The discovery of the various biological mechanisms that followed led to the detection of many other biological mechanisms, including anti-tumoral, antiviral, anti-apoptotic, and anti-inflammatory effects [3][4][5]. The D609 suppressed both acidic sphingomyelinase and phosphatidylcholine-specific phospholipase C (PC-PLC) activity due to its unique competitive inhibitory action on both these enzymes [6][7].
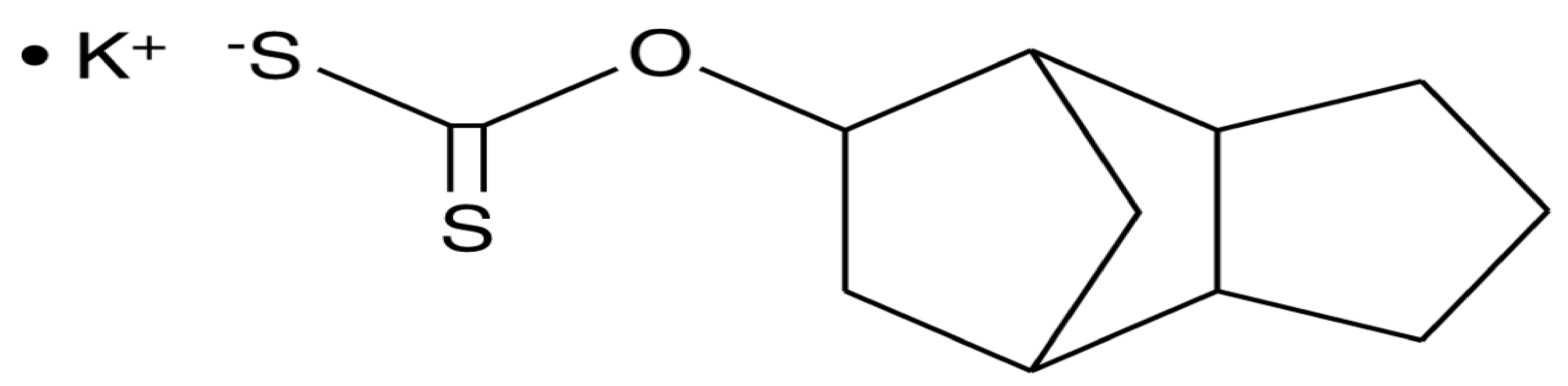
Figure 1. Structure of tricyclodecan-9-yl xanthogenate (D609).
2. Chemistry of D609
D609 comprises a total of eight stereoisomers in addition to their enantiomers, which are allocated among three asymmetric centers in D609 [2][8]. The initial reports of D609 date back to the year 2002 [9], and, currently, the drug is also available from several commercial sources. Until now, no comprehensive study has been carried out to examine the impact on the biological behavior of D609s. In fact, due to the enormous difficulties of assigning proper stereochemistry to modern chemical analytics, no chiral or relative stereochemistry information is generally present in commercially available D609s. Vibrational circular dichroism (VCD) spectroscopy with ab initio theoretical computations has been developed during the last decade to determine the underlying structure of D609. Through the cellular vibrational transition, VCD estimates the differential absorption of circularly polarized IR radiation on the chiral molecule [10].
3. Mechanism of Action of D609
3.1. PC Specific-PC-PLC
PC-PLC (66 kDa) hydrolyzes phosphocholine and 1,2-diacylglycerol PCs to produce DAG. Although scientists have purified bacterial PC-PLC, mammalian PC-PLC is yet to be cloned. Its sequence is still undetermined, thereby restricting the identification of D609 actions by a mammalian enzyme [11]. Rabbit polyclonal antibodies demonstrated cross-reactivity with mammalian PC-PLC to Bacillus cereus PC-PLCI in the occurrence or lack of an essential fibroblast growth factor (ßFGF). The suppression of PC-PLC by D609 blocked replication and permitted different cell systems to be distinguished (Figure 2). PC-PLC cells also have a role in vascular endothelial apoptosis and senesis. In a cell-cycle-dependent manner, the PC-PLC expression varied inversely with cell division cycle protein 20 (CDC20) homolog and CDC20 overexpression stimulated ubiquitin-proteasome pathway-mediated PC-PLC degradation [12]. A highly glycosylated transmembrane protein, cluster of differentiation-16 (CD16), also mediated PC-PLC expression in (natural killer) NK cells. D609 treatment caused a dramatic reduction in the expression of PC-PLC and CD16 receptor [13]. Acts of D609 due to PC-PLC inhibition include the inhibited post-stroke production of hypoxia-inducible factor 1-alpha (HIF-1 α), the protection of tumor necrosis factor (TNF-α) or lethal shock mediated by LPS in mice, diminished expression of cytokines in macrophages induced by lipopolysaccharide (LPS) and safeguarding immature neurons (non-expressed glutamate receptors) against oxidative toxic glutamate [14]. According to reports, inhibiting PC-PLC with D609 promotes phospholipase D (PLD) action in UMR-106 osteoblastic cells, which could be due to counterbalancing effects of D609 or direct enhancement of PLD [15].
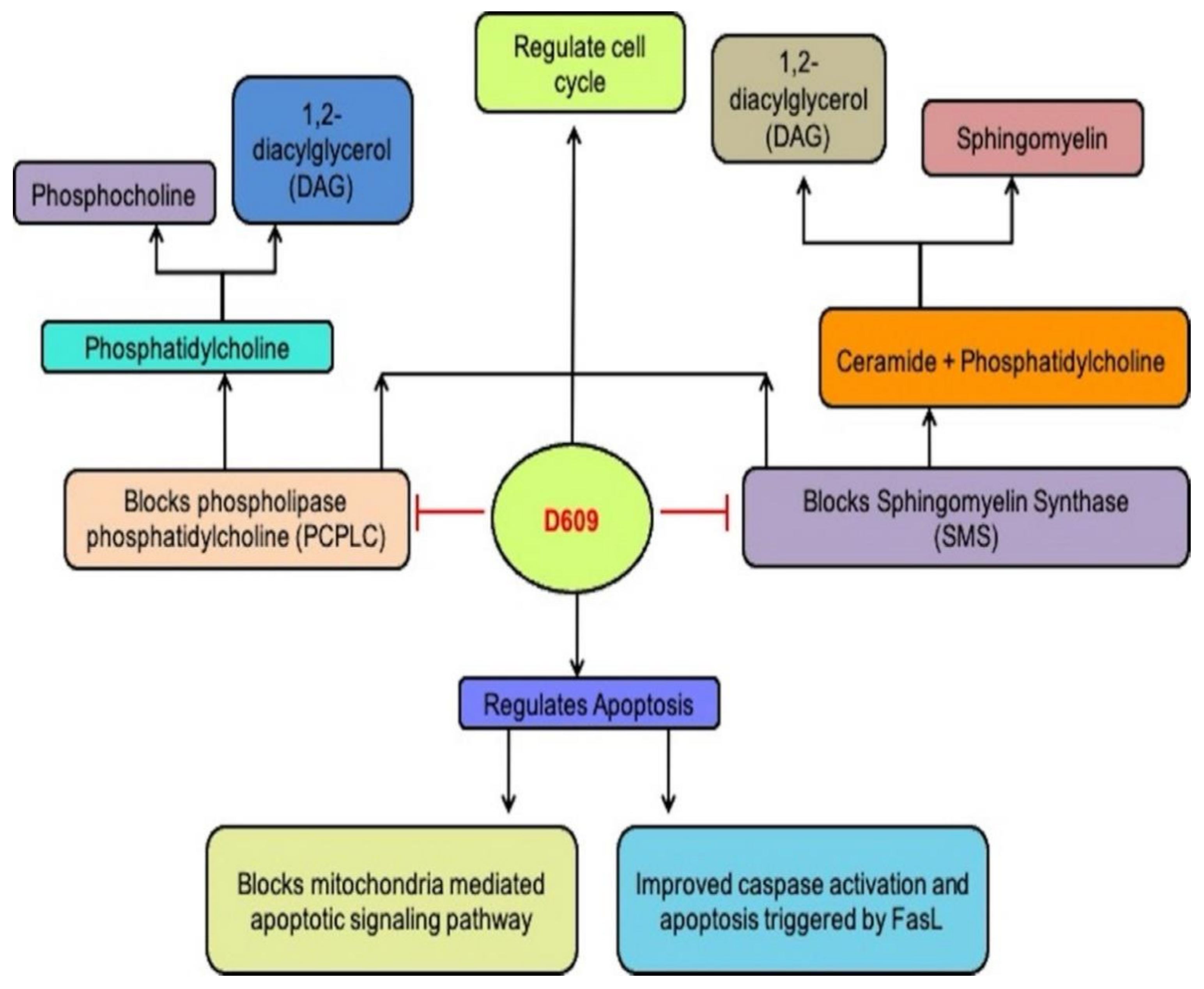
Figure 2. D609 blocks PCPLC and SMS and regulates cell cycle and apoptosis.
Derivatives of xanthate fit into the active core of PC-PLC with a lipophilic chain, and the dithiocarbonate group possibly functions as a phosphate replacement and binds to the active site of Zn2+ ions [3][16][17][18]. Generally, Amplex Red assay is employed to monitor the operation of PC-PLC. This assay works on the theory that PC-PLC hydrolyzes DAG-PC and phosphocholine-PC. The enzyme alkaline phosphatase converts phosphocholine to choline. Choline oxidase is converted to betaine, which produces H2O2. The Amplex Red reagent is stoichiometrically oxidized by hydrogen peroxide in the presence of horseradish peroxidase (HRPO) to produce fluorescent resorufin, which can be measured spectrophotometrically or fluorometrically. [19][20].
3.2. Sphingolipid Metabolism
In de novo ceramide biosynthesis, serine palmitoyltransferase (SPT) catalyzes the first and rate-limiting step. In the form of SM and DAG, the phosphocholine group from PC is converted to ceramide in the PC-ceramide membrane framework. Golgi apparatus has SMS1, while plasma membrane has SMS2. These two types of SMS are inhibited by D609 [6][21][22][23][24]. D609′s effects on PC-PLC inhibition appear to include SMS inhibition, according to studies [25]. De novo ceramide production was similarly boosted by D609, which might be explained by SPT stimulation [26]. In one rat stroke model, D609′s neuroprotection was due to the inhibition of SMS, which induced the accumulation of ceramide and influenced cell-cycle events. Lipid rafts/microdomains and the transport of fatty acids to the cell are associated with scavenger CD36/fatty acid translocase. The translocation and function of CD36 can be impacted by the ceramide [6][27]. Levels of ceramide may be critical for its signaling and may induce retinoblastoma dephosphorylation, triggering the arrest of the cell cycle (Rb) [28][29][30]. D609 suppressed ßFGF-stimulated astrocyte spread, probably due to SMS inhibition and an elevation in levels of ceramide [31]. By inhibiting SMS and upregulating the cyclin-dependent kinase (Cdk) inhibitors p27 and p21, D609 can cause the cell-cycle arrest and increase ceramide levels [32][33][34]. Ceramide may stimulate p27 and p21 expression by activating c-myc regulating protein phosphatase 2A (PP2A), which suppresses p21 and p27 expression [35]. Since the expression of cellular myelocytomatosis oncogene (c-myc) is not blocked by D609, these downstream effects are likely to increase ceramide levels.
4. Pharmacological Properties of D609
4.1. Antioxidant
D609 is an effective antioxidant in numerous studies. As with Alzheimer’s disease (AD), many neurological disorders are linked to oxidative stress. Intracellular neurofibrillary tangles (NFTs), extracellular amyloid protein deposits (primarily consisting of hyperphosphorylated tau protein), mitochondrial malfunction, synapse degradation, and apoptosis or cell death are all symptoms of AD [36][37]. The reduced energy metabolism in AD could be due to the oxidative failure of some essential metabolic or mitochondrial enzymes, which would lead to increased ROS [38]. Mitochondria is well-known as a key cellular energy-producing organelle. The production of ROS, in turn, leads to neuronal oxidative damage, which is dependent on these organelles [39][40]. In mitochondria, specific anti-apoptotic signals and pro-apoptotic defenses unite. Protein factors such as the second mitochondria-derived activator of caspase (SMAC) protein, and direct inhibitor of apoptosis-binding protein with low pi (DIABLO), cytochrome-C, apoptosis-inducing factor (AIF), and apoptotic protease activating factor 1 (Apaf1) produced during mitochondrial oxidative stress trigger caspase-independent and caspase-dependent pathways, leading to programmed cell death [41]. Mitochondria regulate intracellular Ca2+ homeostasis, generate ATP, and produce endogenous ROS. A higher concentration of mitochondrial calcium leads to superoxide formation and pro-apoptotic mitochondrial protein release, culminating in cell death [42].
4.2. Anti-Viral
D609 was created with the intention of turning it into an antiviral agent. D609 and other Xanthates were first described as broad-spectrum antiviral compounds [1]. D609 exists as an enantiomeric pair of four diastereomers, with unknown variable mixtures of these eight isomers in industrial preparations. In antiviral and PC-PLC inhibition assays, isomers have varying biochemical efficacy [43]. Lumavita (Basel, Switzerland) is working on an antiviral drug called Letermovir-601 (LMV-601), which is a pure enantiomeric isomer of D609. Over time, LMV-601, a PC-PLC inhibitor, decreased the high-risk Human Papillomavirus (HPV) expression in types 16, 18, and 31, and exacerbated defects of pre-cancer cervical cell [6]. Tricyclodecan-9-yl-xanthogenate, an antiviral xanthate compound, can inhibit RNA and DNA viruses in vitro [44]. Infectivity assays and Western blot analysis have shown that it can prevent infectious HIV from spreading to chronically infected lymphoma cell (KE37-III) tissue-culture medium [45]. HIV-specific proteins, on the other hand, have accumulated intracellularly. At D609 doses that allowed mitotic cell divisions, de novo HIV replication commencement after infection with permissive KE37-1 cells was entirely stopped. Furthermore, the loss of HIV replicative intermediate DNA demonstrated the xanthate compound′s selective anti-viral efficacy. Within these cells, the cellular gene expression of c-myc remains unchanged [45].
The human respiratory syncytial virus (RSV) is a cytoplasmic, enveloped virus with negative RNA polarity. In newborns and young children, paramyxovirus is among the common causes of respiratory tract infections [46]. In human epithelial cells, the antiviral compound D609 prevents the development of the respiratory syncytial (RS) virus (Hep 2) [47]. Viral protein aggregation, the viral phosphorylation of phosphoprotein, infectious particles levels, and extracellular antigens all decreased after treatment with D609. When the polarity was positive or negative, the viral proteins’ relative accumulation was also unbalanced, but there were no variations in viral RNA volume. Furthermore, nucleocapsid formation was not hampered [48]. HSV-1 (Herpes simplex virus-1) replication was prevented by D609 without causing cytotoxicity. It inhibited HSV-1 encoded protein kinase and decreased virus-infected cell polypeptide phosphorylation (US3 PK). At concentrations greater than 3.8 µM, virus production decreased by D609, with absolute inhibition at 75.2 µM at or below 1 PFU/cell MOI. D609 can be given up to 7 h after infection and still prevent virus replication. These findings indicate that D609′s antiviral activity is mediated by the protein kinase inhibition and phosphorylation of protein, which influences late HSV replication. As a result of the above research, it is clear that D609 prevents the development of many virus-related diseases [49]. The role of D609 in various diseases and its mechanisms are summarized in Figure 3.
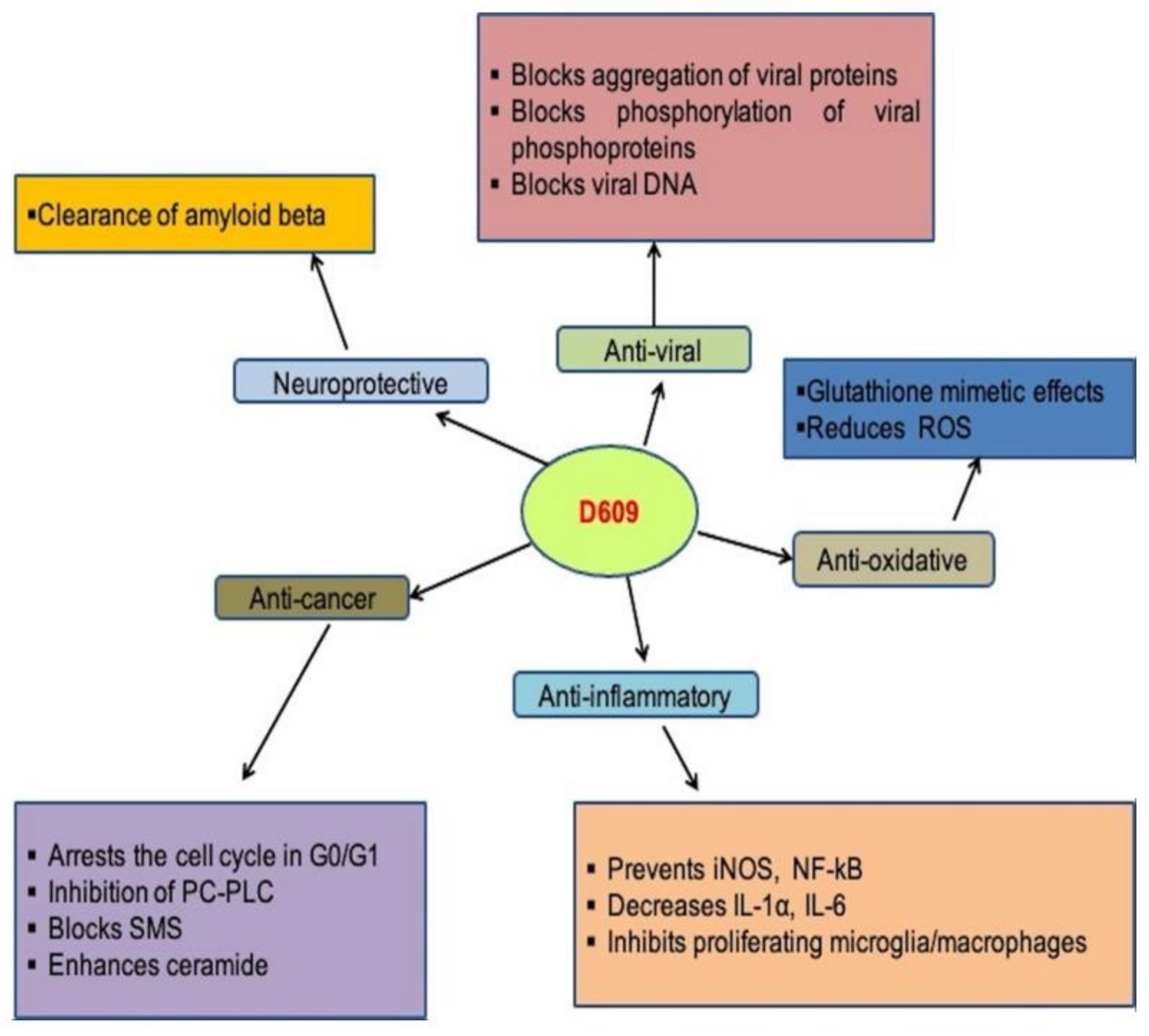
Figure 3. Role of D609 in human diseases and mechanisms involved.
4.3. Anti-Inflammatory
D609 has also demonstrated significant potential anti-inflammatory activities [50]. Immunization of uveal melanin protein causes experimental melanin–protein induced uveitis (EMIU), which is a form of chronic autoimmune uveitis. The induction of inductive nitric oxide synthase (iNOS) is prevented by D609, a basic inhibitor of phosphatidylcholine-specific phospholipase C [51][52]. Thirty-five (Lewis) rats with EMIU were given either PBS or D609 in two separate experiments. D609 had a significant inhibitory effect on EMIU during this study [53]. D609-treated eyes had lower peroxide and nitrite levels, higher SOD levels, and lower iNOS mRNA expressions than PBS-treated eyes. The Fas and FasL levels in D609-injected animals′ eyes and lymph nodes increased significantly. D609-treated rats had increased DNA fragmentation in their lymph nodes. D609 inhibited EMIU by quenching NO and initiating programmed cell death, which had formerly been inhibited by NO, as well as displaying anti-inflammatory properties [52]. D609, a NO scavenger, was recently shown to be effective at preventing allergic encephalomyelitis in animals [54][55]. D609, in addition to inhibiting NO, can also inhibit other inflammatory mediators. By preventing the PC-PLC pathway, D609 inhibits the development of NOS activity. In response to inflammation, the PC-PLC pathway, which plays an important role, is activated [56][57]. D609 reduces IL-1α, IL-6, and NO release in endotoxin shock mice, according to Tschaikowsky et al. D609′s inhibition of NO synthesis has a much greater protective effect than D609′s inhibition of other inflammatory mediators. It has also been shown that D609, a PC-PLC and NOS inhibitor, inhibited NO expression in EMIU [58]. N9 and BV-2 microglia, RAW 264.7 macrophages, and DITNC1 astrocytes were all significantly inhibited by 100-μM D609 treatment without affecting cell viability [32]. D609 may perform an anti-inflammatory function by preventing the proliferation of macrophages/microglia, which are the principal sources of IL-1 and TNF and other pro-inflammatory cytokines. TNF-induced PAR formation is inhibited in D609-treated cultures, according to studies. D609 reduced microglial morphological change, NF-kB transcriptional activation, and PARP-1 activation [59].
4.4. Anti-Tumor and Anti-Proliferative
Ceramide and DAG, linked by the sphingomyelin (SM) synthase pathway, are two-second messengers that control cell proliferation and growth arrest in opposing ways [60]. In SM and DAG, SMS transports the phosphocholine group from the PC to the ceramide [61]. SMS comes in two varieties: SMS1 is located in the Golgi apparatus, while SMS2 is found in the plasma membrane [62][63]. D609 inhibits both forms of SMS. By preventing ceramide injection into SM, inhibiting SMS elevates ceramide levels. Thanks to SMS inhibition, D609 prevented the proliferation of ßFGF-stimulated astrocyte [64][65]. According to one study, D609 decreased non-neuronal cell-line proliferation without triggering cell death [66]. In BV-2 microglia, D609 therapy elevated the expression of ceramide and p21 levels [32][67]. D609 also hypophosphorylated Rb, causing cell-cycle inhibition in the G0/G1 step, and a decrease in proportion of cells in the S-Phase [32]. Ceramide′s significance in D609-induced cell-cycle arrests is supported by the exogenous C8-ceramide investigations [32].
D609 regulates ceramide formation and cell death caused by death receptors [68][69]. Nontoxic concentrations of D609 inhibit sphingomyelin synthase and glucosylceramide synthase in Jurkat cells [70]. FasL-induced caspase activation and apoptosis were significantly enhanced by D609 [71][72]. Since the authors claim that Bcl-xL overexpression caused D609 outcomes, mitochondrial events were likely involved. The authors conclude that D609 causes cell death in T lymphocyte cells by acting downstream of caspases 8. They believe that in Fasl-induced cell death, a rise occurs due to D609 inhibition of ceramide transfer to complex sphingolipids [73]. Anti-HER2 medicines, which block the tumorigenic actions of HER2, do not significantly affect the treatment of HER2-positive EOC (epithelial ovarian cancer) [74][75]. Preclinical trial models can be utilized to assess the molecular processes causing HER2 overexpression and oncogenicity, giving rise to new EOC treatments. The enhanced HER2 expression in breast cancer cells is regulated by PC-PLC [16]. Researchers found that the inhibition of PC-PLC may be a good target for the tumorigenic effects of increased HER2 expression in EOC41 cells [26].
4.5. Neuroprotective
Evidence suggests that oxidative stress has a role in the pathogenesis of Alzheimer′s disease [76]. Amyloid-peptide accumulation causes a cascade of oxidative neuron damage and eventually leads to neuronal death, one of the hallmarks of Alzheimer′s disease [77][78]. Amyloid-peptide is senile plaque’s major component. It causes damage to nucleic acids, proteins, and membrane lipids in neurons by generating free radicals [79][80]. As a result, interest is growing in antioxidant chemicals as they play a protective role in the treatment of AD and other disorders of oxidative stress [81][82]. Amid the various antioxidant medications, “thiol-delivering” compounds have received much attention. PC-PLC is inhibited by D609, a compound that mimics the action of glutathione. Recent studies have shown that it can inhibit phosphatidylcholine-specific phospholipase C [83][84]. Another study assesses the efficiency of D609 to protect synaptosomes in vivo from amyloid-peptide caused by oxidative stress [85][86]. After gerbils were fed D609 or saline solution, synaptosomes were extracted from their brains. The ex vivo treatment of gerbils with synaptosomal preparations derived from D609-injected gerbils with amyloid peptide (1–42) significantly decreased oxidative stress parameters, such as reactive oxygen species, lipid peroxidation (4-hydroxy-2-nonenal), protein oxidation (carbonyl and 3-nitrotyrosine protein), and levels [85]. These findings support the idea that amyloid-peptide-induced free-radical control may be a useful therapeutic tool for treating AD and other related oxidative stress diseases [87]. Based on the above evidence, D609 is a potent antioxidant that may help cure AD and other related oxidative stress diseases [83][85][88][89].
4.6. Cholinergic Neuron Differentiation
A cholinergic neuron is a nerve cell that sends signals primarily via acetylcholine (ACh). Cholinergic neurons, which primarily use the neurotransmitter acetylcholine (Ach) for message transmission, play a crucial role in memory, locomotion, and behavioral response [90][91]. Cholinergic neuron loss causes a decline in choline acetyltransferase (ChAT) function, which causes motor nerve degeneration and cognitive dysfunction, as seen in AD [92][93]. A new treatment for cholinergic neuron loss is yet to be found, despite the use of cell-based therapies and nerve transplants to treat a variety of neurological disorders [94]. Several stem-cell-based therapies, including motor nerve disorders and Alzheimer′s disease, have recently been proposed as experimental therapies to alleviate the pathophysiology of cholinergic nerve disorders [94][95]. The tricyclodecane-9-yl-xanthogenate (D609) neuronal induction approach was used to successfully differentiate hDPSCs-cryo into cholinergic neurons (DF-chN) [96]. Motor nerve regeneration on an unprecedented scale was observed when DF-chN was inserted in vivo into experimental rats with sciatic nerve defects [96]. The simple inhibitor of phosphatidylcholine-specific C phospholipase, D609, has previously been shown to distinguish bone marrow MSCs (BMSCs) from neuron-like cells [97]. In BMSCs, D609 induces neuron-like cells with cholinergic neuronal properties; however, the mechanism underlying D609 neurogenic induction is unknown [98]. There is another belief that the treatment of D609 in BMSCs blocks PC-PLC activity, while elevated levels of HSP70 trigger the activation of transcription regulator B-cell translocation gene 2 (BTG2), resulting in cholinergic neuronal differentiation based on the number of responsive neuronal-specific genes [99].
D609 therapy has also been shown to reduce mesodermal and endodermal differentiation gene expression while increasing neuroprotection, neuronal differentiation, and cholesterol synthesis gene expression [96]. The formation and maintenance of the myelin sheath require cholesterol. In addition, differentiated cholinergic neuron relocation increases functional neuron regeneration and protection in animals with spinal cord injuries in vivo models. As a result, treating D609 with BMSCs is a quick and easy way to induce a cholinergic neuron induction [98]. According to Soomi Jang et al. (2018), treatment with D609 caused stem cells (derived from cryopreserved dental pulp) to appropriately transform into cholinergic neurons [98]. These cholinergic neurons, differentiated from dental pulp stem cells, had morphological properties similar to those of neurons, such as a neuron body and axonal fiber, as well as positive mRNA and protein expression of cholinergic neuronal markers [96].
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
06 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




