
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yvaine Wei | -- | 1495 | 2022-03-31 02:53:01 | | | |
| 2 | Ursula Panzner | + 819 word(s) | 2314 | 2022-09-26 05:10:38 | | | | |
| 3 | Yvaine Wei | -1 word(s) | 2313 | 2022-09-26 05:30:25 | | | | |
| 4 | Yvaine Wei | Meta information modification | 2313 | 2022-09-26 05:49:42 | | |
Video Upload Options
About 250 million people affected, 779 million people at risk of infection, and 440 million people with residual morbidity are globally attributable to schistosomiasis. Highly sensitive and specific, simple and fast to perform diagnostics are required for detecting trace infections, and applications in resource-poor settings and large-scale assessments. Research assessing isothermal diagnoses of S. japonicum, S. haematobium, S. mansoni, mixed infections, and schistosomal hybrids among clinical human specimens was investigated. Loop-mediated isothermal amplification (LAMP), recombinase polymerase amplification (RPA) and combined techniques were identified. Both, LAMP and RPA reached species-dependent 100% sensitivity, and detection levels within femtogram and nanogram amounts for pure and hybridale breeds. Cross-reactivity among Schistosoma species and co-endemic pathogens was rare though research on diagnostic markers and primer optimization should continue. Operating with ready-to-use lyophilized reagents, simplified and inexpensive nucleic acid extraction, tolerability to likely inhibitors, and enzyme stability at ambient temperature is advantageous. RPA performed optimal at 35–39ºC within 5–10 minutes while LAMP operated at 61–65ºC for up to 120 minutes; properties are preferable over assays requiring expensive laboratory equipment. DNA degradation could be prevented by stabilizing substances. A limitation throughout warranting future research is the small sample size reaching a few hundred participants at the maximum. Isothermal diagnostics are highly valuable in detecting trace infections seen subsequent to chemotherapeutic treatment, and among apparently healthy individuals, both constituting likely sources of ongoing pathogen transmission. Its expansion to the vaccine field for assessing parasitological trial endpoints could be considered.

References
- Avendaño, C.; Patarroyo, M. Loop-Mediated Isothermal Amplification as Point-of-Care Diagnosis for Neglected Parasitic Infections. Int. J. Mol. Sci. 2020, 21, 7981.
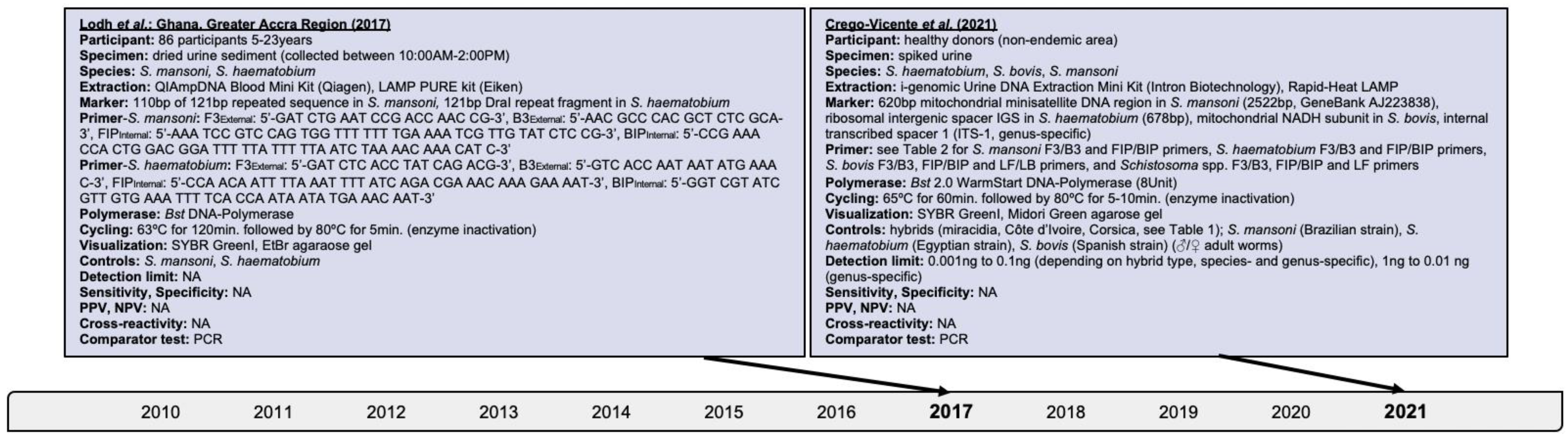
- Diego, J.G.-B.; Fernández-Soto, P.; Febrer-Sendra, B.; Crego-Vicente, B.; Muro, A. Loop-Mediated Isothermal Amplification in Schistosomiasis. J. Clin. Med. 2021, 10, 511.
- Panzner, U.; Boissier, J. Natural intra- and intercalde human hybrid schostosomes in Africa with considerations on prevention through vaccination Microorganisms. Microorganism 2021, 9, 1465.
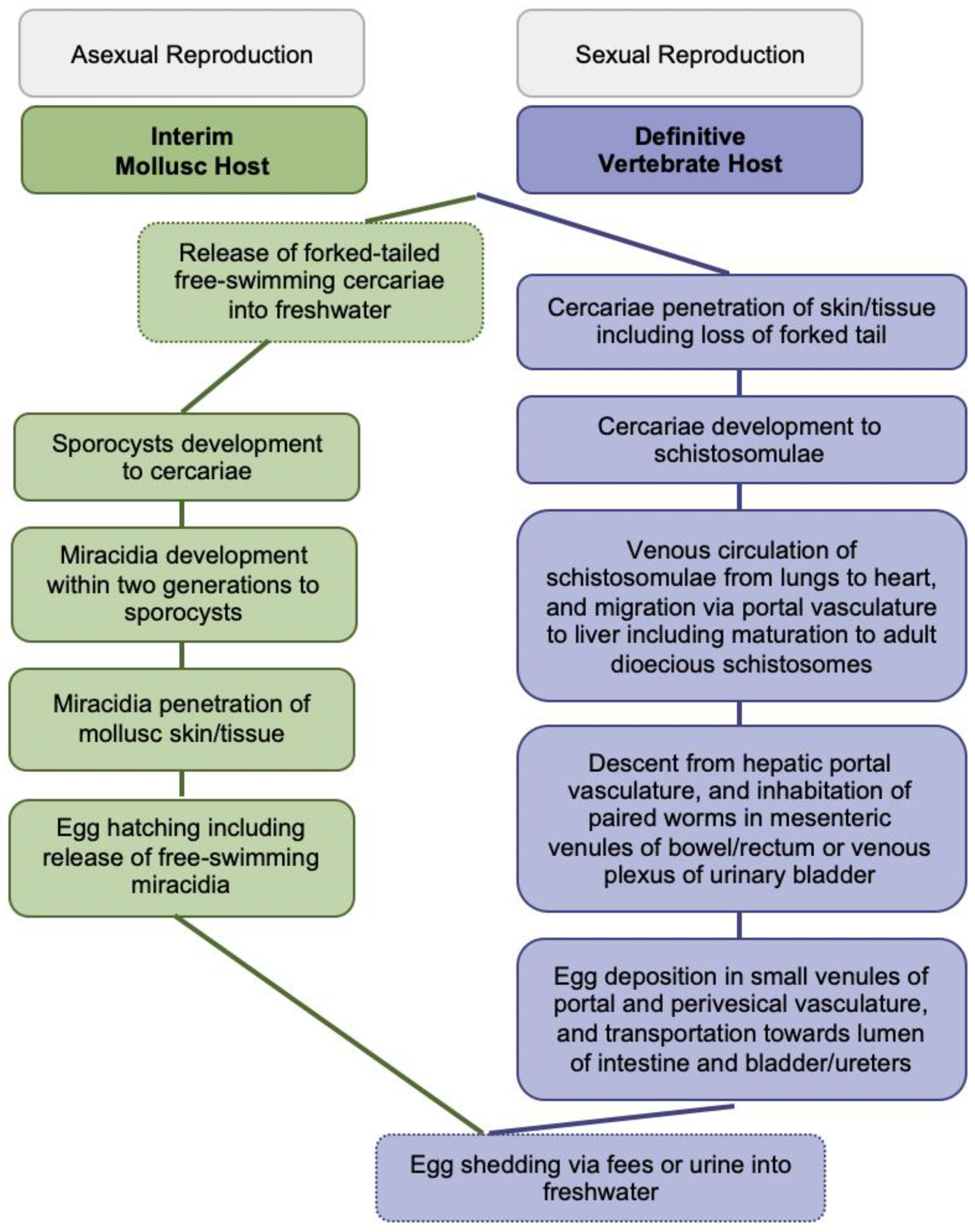
- Nelwan, M.L. Schistosomiasis: Life Cycle, Diagnosis, and Control. Curr. Ther. Res. 2019, 91, 5–9.
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264.
- Patwary, F.K.; Archer, J.; Sturt, A.S.; Webb, E.L. Female Genital Schistosomiasis: Diagnostic Validation for Recombinant DNA-Polymerase-Amplification Assay using Cervicovaginal Lavage. Int. J. Obstet. Gynaecol. 2021, 128 (Suppl. S2), 248.
- Le, L.; Hsieh, M.H. Diagnosing Urogenital Schistosomiasis: Dealing with Diminishing Returns. Trends Parasitol. 2017, 33, 378–387.
- Panzner, U.; Excler, J.L.; Kim, H.J. Recent advances and methodological considerations on vaccine candidates for human schistosomiasis Front. Trop. Dis. 2021, 2, 719369.
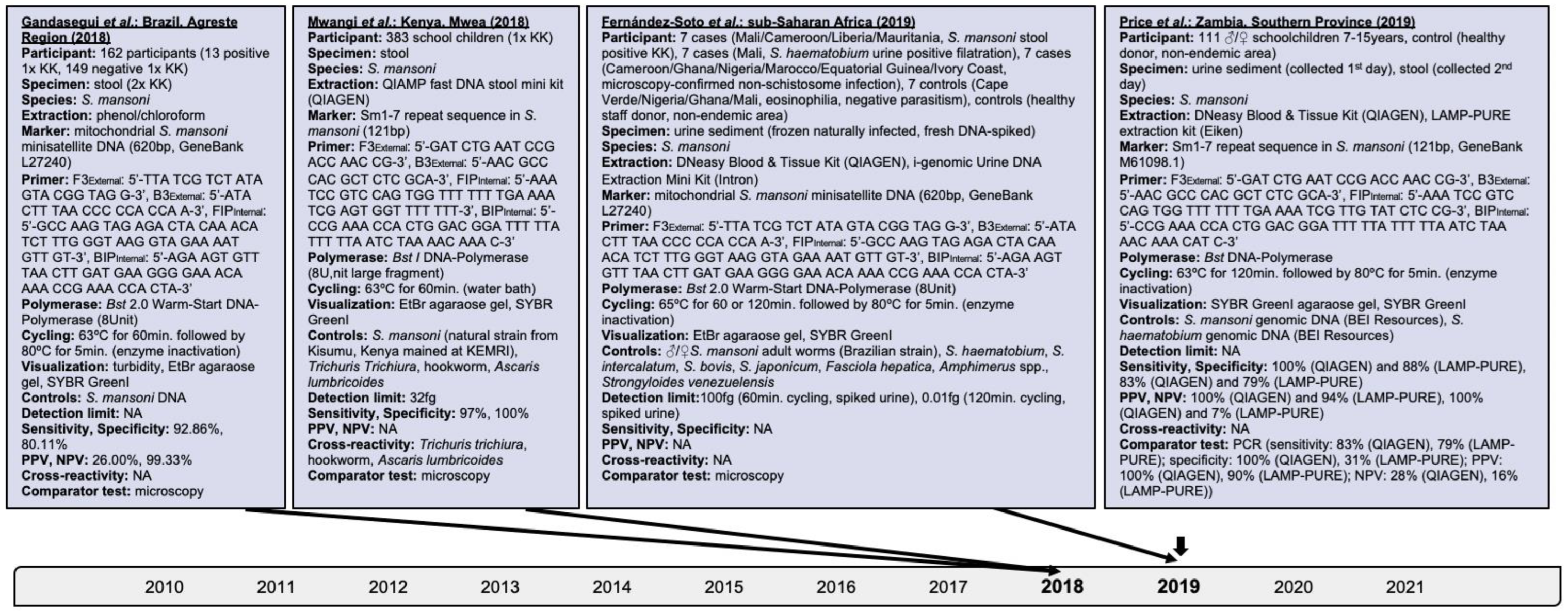
- Gandasegui, J.; Fernández-Soto, P.; Carranza-Rodríguez, C.; Perez-Arellano, J.-L.; Vicente, B.; López-Abán, J.; Muro, A. The Rapid-Heat LAMPellet Method: A Potential Diagnostic Method for Human Urogenital Schistosomiasis. PLoS Negl. Trop. Dis. 2015, 9, e0003963.
- Rosser, A.; Rollinson, D.; Forrest, M.S.; Webster, B.L. Isothermal Recombinase Polymerase amplification (RPA) of Schistosoma haematobium DNA and oligochromatographic lateral flow detection. Parasites Vectors 2015, 8, 446.
- Archer, J.; Barksby, R.; Pennance, T.; Rostron, P.; Bakar, F.; Knopp, S.; Allan, F.; Kabole, F.; Ali, S.M.; Ame, S.M.; et al. Analytical and Clinical Assessment of a Portable, Isothermal Recombinase Polymerase Amplification (RPA) Assay for the Molecular Diagnosis of Urogenital Schistosomiasis. Molecules 2020, 25, 4175.
- Bayoumi, A.; Al-Refai, S.A.; Badir, M.S.; El-Aal, A.A.A.; El Akkad, D.M.H.; Saad, N.; Elesaily, K.M.; Aziz, I.Z.A. Loop-Mediated Isothermal Amplification (Lamp): Sensitive and Rapid Detection of Schistosoma Haematobium DNA in Urine Samples of Egyptian Suspected Cases. J. Egypt Soc. Parasitol. 2016, 46, 299–308.
- Eyoh, E.; McCallum, P.; Killick, J.; Amanfo, S.; Mutapi, F.; Astier, A.L. The anthelmintic drug praziquantel promotes human Tr1 differentiation. Immunol. Cell Biol. 2019, 97, 512–518.
- Song, J.; Liu, C.; Mauk, M.G.; Rankin, S.C.; Lok, J.B.; Greenberg, R.M.; Bau, H.H. Two-Stage Isothermal Enzymatic Amplification for Concurrent Multiplex Molecular Detection. Clin. Chem. 2017, 63, 714–722.
- Zhao, G.-H.; Li, J.; Blair, D.; Li, X.-Y.; Elsheikha, H.M.; Lin, R.-Q.; Zou, F.-C.; Zhu, X.-Q. Biotechnological advances in the diagnosis, species differentiation and phylogenetic analysis of Schistosoma spp. Biotechnol. Adv. 2012, 30, 1381–1389.
- Mosquera-Romero, M.; Zuluaga-Idárraga, L.; Tobón-Castaño, A. Challenges for the diagnosis and treatment of malaria in low transmission settings in San Lorenzo, Esmeraldas, Ecuador. Malar. J. 2018, 17, 440.
- Cavalcanti, M.G.; Cunha, A.F.A.; Peralta, J.M. The Advances in Molecular and New Point-of-Care (POC) Diagnosis of Schistosomiasis Pre- and Post-praziquantel Use: In the Pursuit of More Reliable Approaches for Low Endemic and Non-endemic Areas. Front. Immunol. 2019, 10, 858.
- Wang, C.; Chen, L.; Yin, X.; Hua, W.; Hou, M.; Ji, M.; Yu, C.; Wu, G. Application of DNA-based diagnostics in detection of schistosomal DNA in early infection and after drug treatment. Parasites Vectors 2011, 4, 164.
- Deng, M.-H.; Zhong, L.-Y.; Kamolnetr, O.; Limpanont, Y.; Lv, Z.-Y. Detection of helminths by loop-mediated isothermal amplification assay: A review of updated technology and future outlook. Infect. Dis. Poverty 2019, 8, 20.
- Poulton, K.; Webster, B. Development of a lateral flow recombinase polymerase assay for the diagnosis of Schistosoma mansoni infections. Anal. Biochem. 2018, 546, 65–71.
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. TrAC Trends Anal. Chem. 2018, 98, 19–35.
- Li, H.-M.; Qin, Z.-Q.; Bergquist, R.; Qian, M.-B.; Xia, S.; Lv, S.; Xiao, N.; Utzinger, J.; Zhou, X.-N. Nucleic acid amplification techniques for the detection of Schistosoma mansoni infection in humans and the intermediate snail host: A structured review and meta-analysis of diagnostic accuracy. Int. J. Infect. Dis. 2021, 112, 152–164.
- Song, J.; Liu, C.; Bais, S.; Mauk, M.G.; Bau, H.H.; Greenberg, R.M. Molecular Detection of Schistosome Infections with a Disposable Microfluidic Cassette. PLoS Negl. Trop. Dis. 2015, 9, e0004318.
- Wong, Y.-P.; Othman, S.; Lau, Y.-L.; Radu, S.; Chee, H.-Y. Loop-mediated isothermal amplification (LAMP): A versatile technique for detection of micro-organisms. J. Appl. Microbiol. 2018, 124, 626–643.
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63.
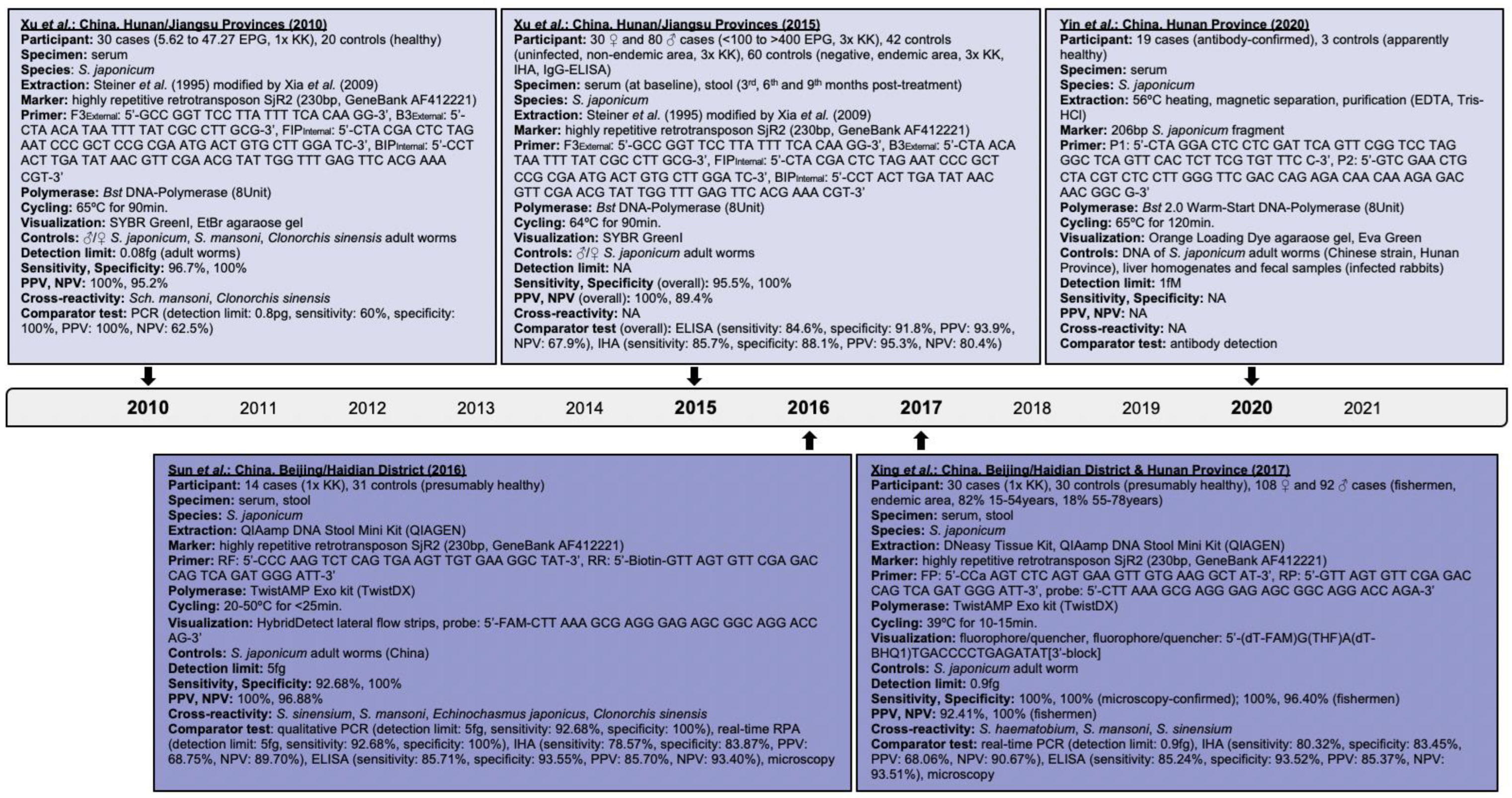
- Xu, J.; Rong, R.; Zhang, H.; Shi, C.; Zhu, X.; Xia, C. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP). Int. J. Parasitol. 2010, 40, 327–331.
- Mwangi, I.N.; Agola, E.L.; Mugambi, R.M.; Shiraho, E.A.; Mkoji, G.M. Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for Diagnosis of Schistosoma mansoni Infection in Faecal Samples. J. Parasitol. Res. 2018, 2018, 1267826.
- Gandasegui, J.; Fernández-Soto, P.; Muro, A.; Barbosa, C.S.; De Melo, F.L.; Loyo, R.; Gomes, E.C.D.S. A field survey using LAMP assay for detection of Schistosoma mansoni in a low-transmission area of schistosomiasis in Umbuzeiro, Brazil: Assessment in human and snail samples. PLoS Negl. Trop. Dis. 2018, 12, e0006314.
- Yansouni, C.P.; Bottieau, E.; Lutumba, P.; Winkler, A.S.; Lynen, L.; Buscher, P.; Jacobs, J.; Gillet, P.; Lejon, V.; Alirol, E.; et al. Rapid diagnostic tests for neurological infections in central Africa. Lancet Infect. Dis. 2013, 13, 546–558.
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69.
- Notomi, T.; Mori, Y.; Tomita, N.; Kanda, H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J. Microbiol. 2015, 53, 1–5.
- Yin, Y.; Wu, Z.; Li, G.; Huang, J.; Guo, Q.; Meng, X. A DNA molecular diagnostic technology with LAMP-like sensitivity based on one pair of hairpin primers-mediated isothermal polymerization amplification. Anal. Chim. Acta 2020, 1134, 144–149.
- Kaiglová, A.; Beňo, P.; Changoma, M.J.S. Detection of schistosomiasis applicable for primary health care facilities in endemic regions of Africa. Biologia 2017, 72, 1113–1120.
- Nagamine, K.; Hase, T.; Notomi, T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol. Cell. Probes 2002, 16, 223–229.
- Mori, Y.; Nagamine, K.; Tomita, N.; Notomi, T. Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation. Biochem. Biophys. Res. Commun. 2001, 289, 150–154.
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882.
- Zhang, X.; Lowe, S.B.; Gooding, J.J. Brief review of monitoring methods for loop-mediated isothermal amplification (LAMP). Biosens. Bioelectron. 2014, 61, 491–499.
- Weerakoon, K.; Gobert, G.N.; Cai, P.; McManus, D.P. Advances in the Diagnosis of Human Schistosomiasis. Clin. Microbiol. Rev. 2015, 28, 939–967.
- Kaneko, H.; Kawana, T.; Fukushima, E.; Suzutani, T. Tolerance of loop-mediated isothermal amplification to a culture medium and biological substances. J. Biochem. Biophys. Methods 2007, 70, 499–501.
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.-J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48.
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin. Chem. 2016, 62, 947–958.
- Chen, C.; Guo, Q.; Fu, Z.; Liu, J.; Lin, J.; Xiao, K.; Sun, P.; Cong, X.; Liu, R.; Hong, Y. Reviews and advances in diagnostic research on Schistosoma japonicum. Acta Trop. 2020, 213, 105743.
- Tani, H.; Teramura, T.; Adachi, K.; Tsuneda, S.; Kurata, S.; Nakamura, K.; Kanagawa, T.; Noda, N. Technique for Quantitative Detection of Specific DNA Sequences Using Alternately Binding Quenching Probe Competitive Assay Combined with Loop-Mediated Isothermal Amplification. Anal. Chem. 2007, 79, 5608–5613.
- Xing, W.; Yu, X.; Feng, J.; Sun, K.; Fu, W.; Wang, Y.; Zou, M.; Xia, W.; Luo, Z.; He, H.; et al. Field evaluation of a recombinase polymerase amplification assay for the diagnosis of Schistosoma japonicum infection in Hunan province of China. BMC Infect. Dis. 2017, 17, 164.




