
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sabrin R. M. Ibrahim | -- | 1533 | 2022-03-30 12:10:31 | | | |
| 2 | Peter Tang | -9 word(s) | 1524 | 2022-03-31 03:30:25 | | |
Video Upload Options
Dactylospongia elegans Thiele (Thorectidae) is a wealth pool of various classes of sesquiterpenes, including hydroquinones, quinones, and tetronic acid derivatives. These metabolites possessed a wide array of potent bioactivities such as antitumor, cytotoxicity, antibacterial, and anti-inflammatory.
1. Introduction
|
Sponge Class |
Compounds Classes |
|---|---|
|
Calcarea |
C27 to C29 ∆5,7,9(11),22 and C27 to C29 ∆5,7,22 sterols Amino alcohols |
|
Hexactinellida |
5α(H)-Cholestan-3β-ol/cholest-5-en-3β-ol Ceramide glycosides |
|
Homoscleromorpha |
Steroidal alkaloids Peroxy-polyketides |
|
Demospongiae |
Pyrroloquinoline, azetidine, pyrrole-2-aminoimidazole, and pentacyclic guanidine alkaloids Norditerpene and norsesterterpene peroxides Tetramic acids Steroidal saponins and glycosides Isomalabaricane triterpenoids Bengamide and bengazoles Hydroxyimino- and 3β-hydroxymethyl-A-nor-sterols 3-Alkylpyridines/3-alkylpiperidines Renieramycins and polyacetylenes Pentacyclic hydroquinones/polyprenylated benzoquinones Adenine- and cyanthiwigin diterpenes Hypotaurocyamine (Sesquiterpene derivatives) Diterpene thio/iso/cyanides and formamides Sesquiterpene thio/iso/cyanides and formamides Aaptamines and bromotyrosines Suberitane-derived sesterterpenes Diterpene, sesquiterpene, and sesterterpenefurans/lactones Scalarane sesterterpenes/sesterterpene hydroquinones Thiazole polyketides Polybrominated diphenyl ethers |
2. Secondary Metabolites of D. elegans
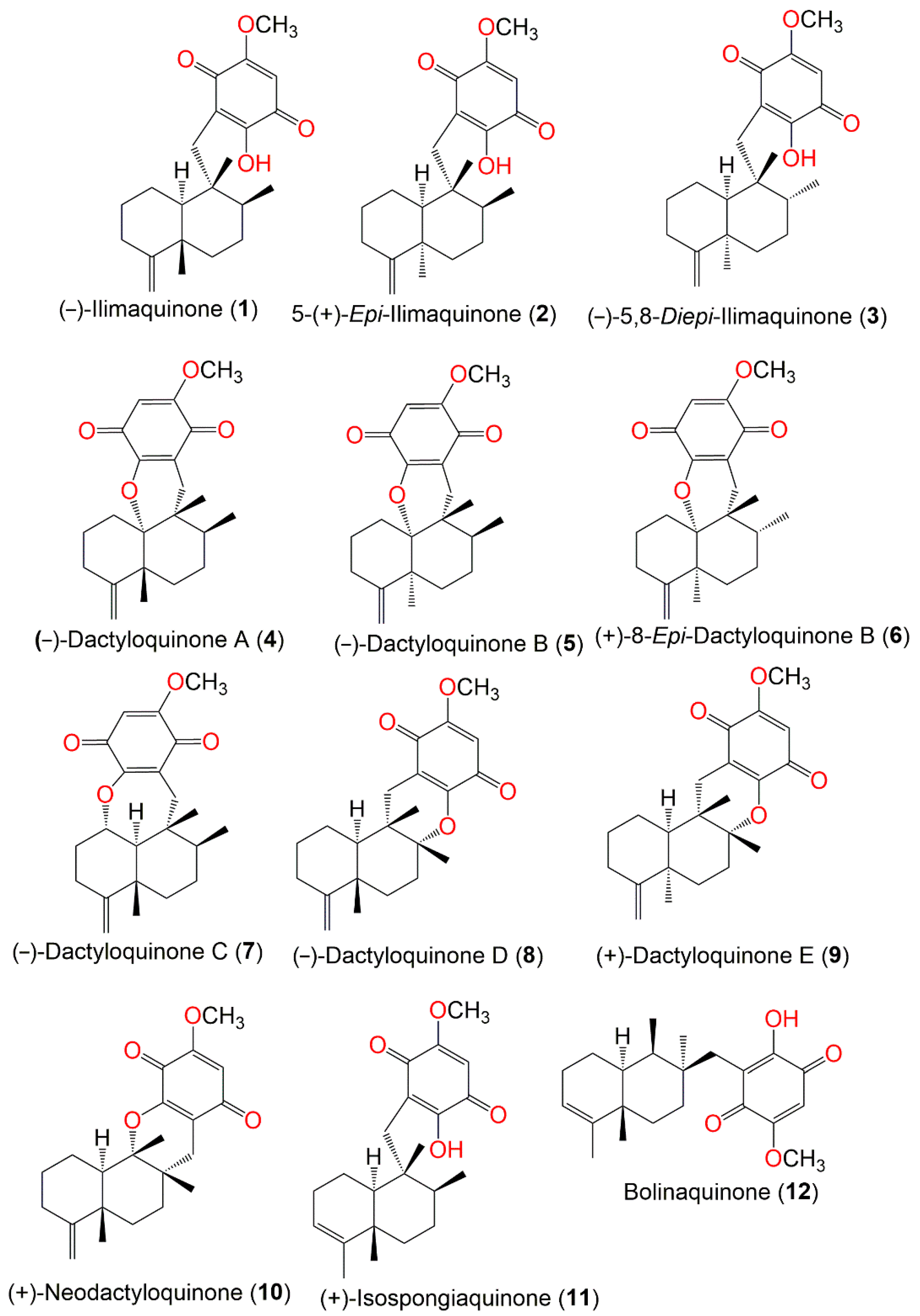
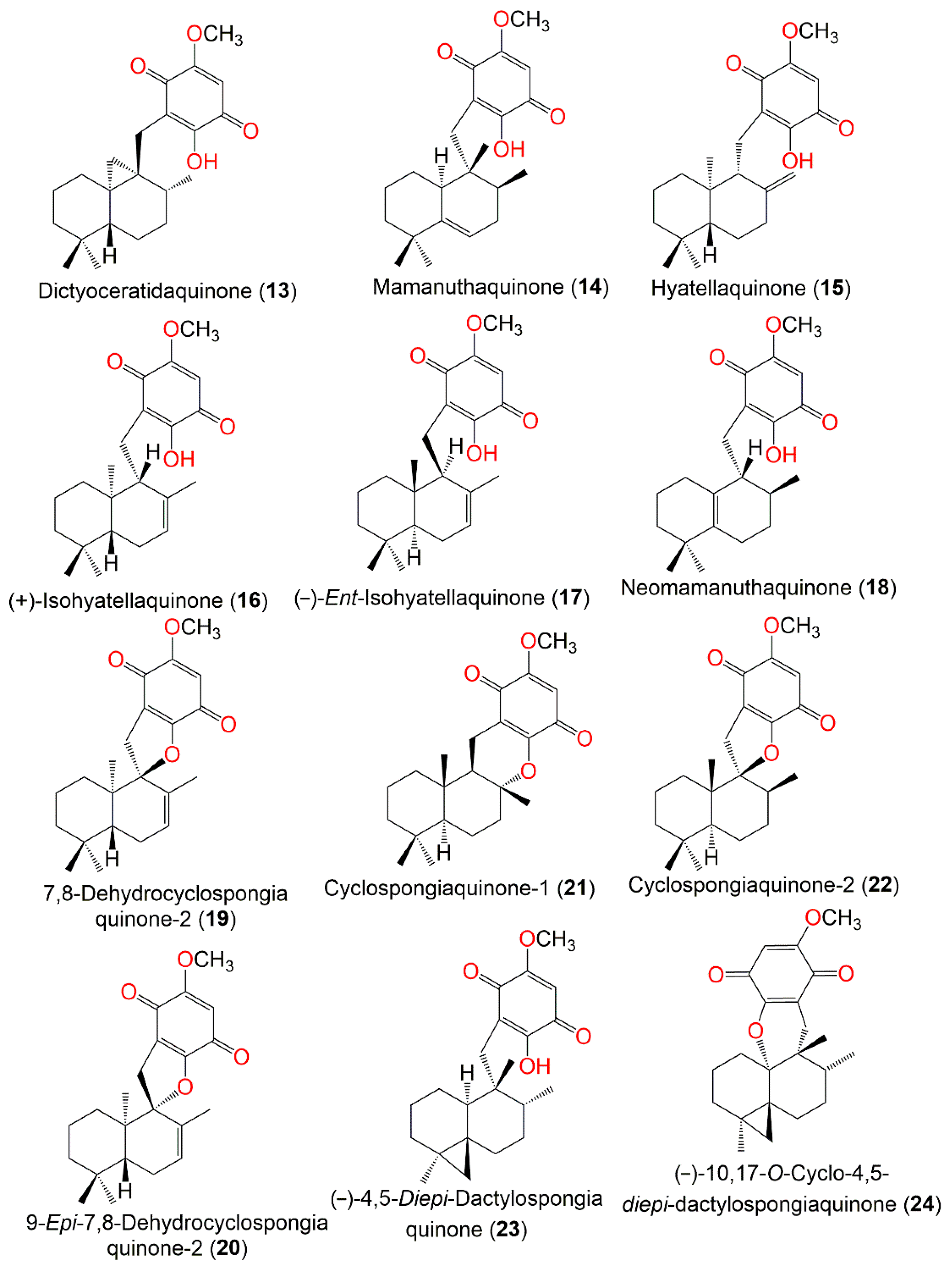
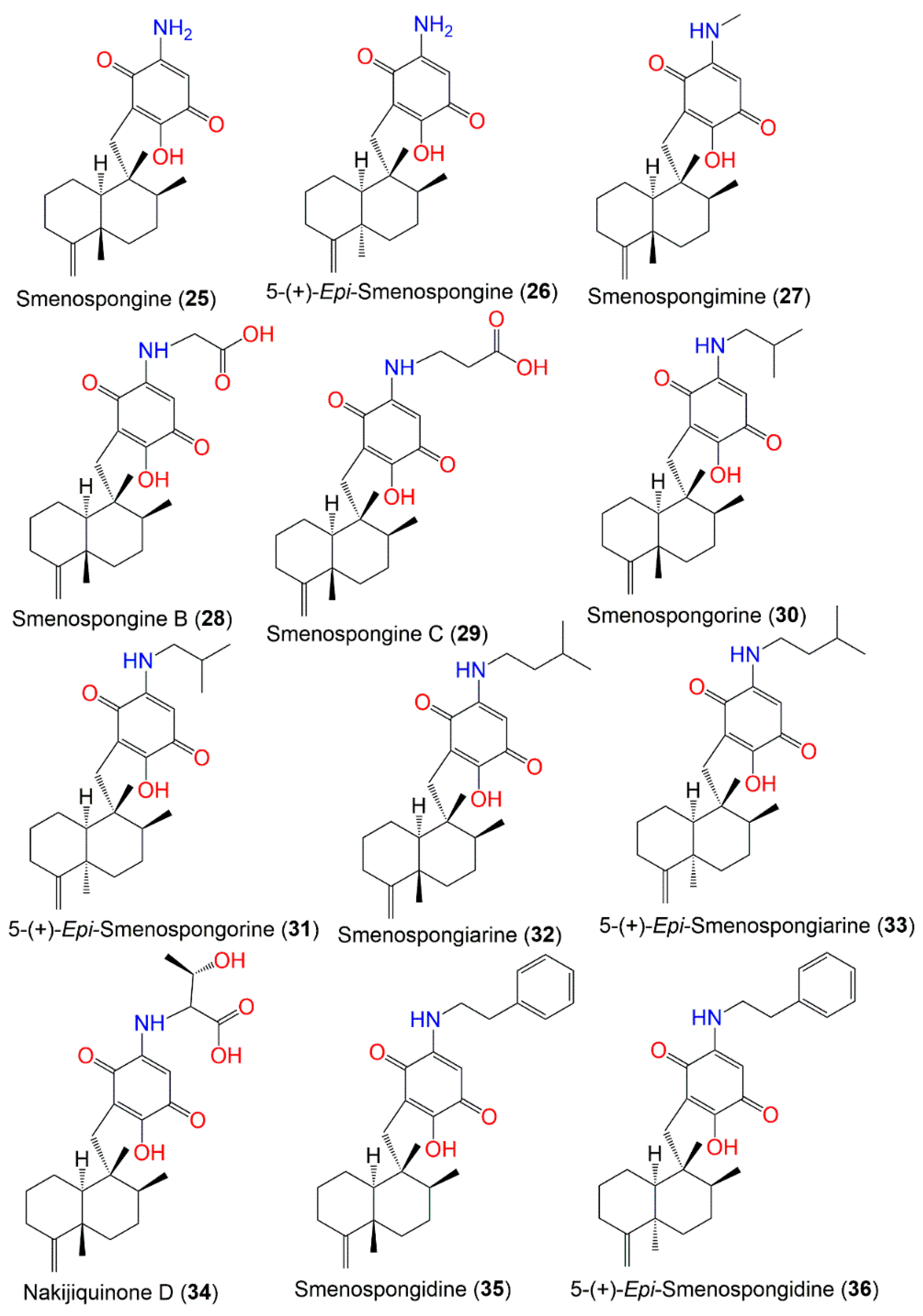
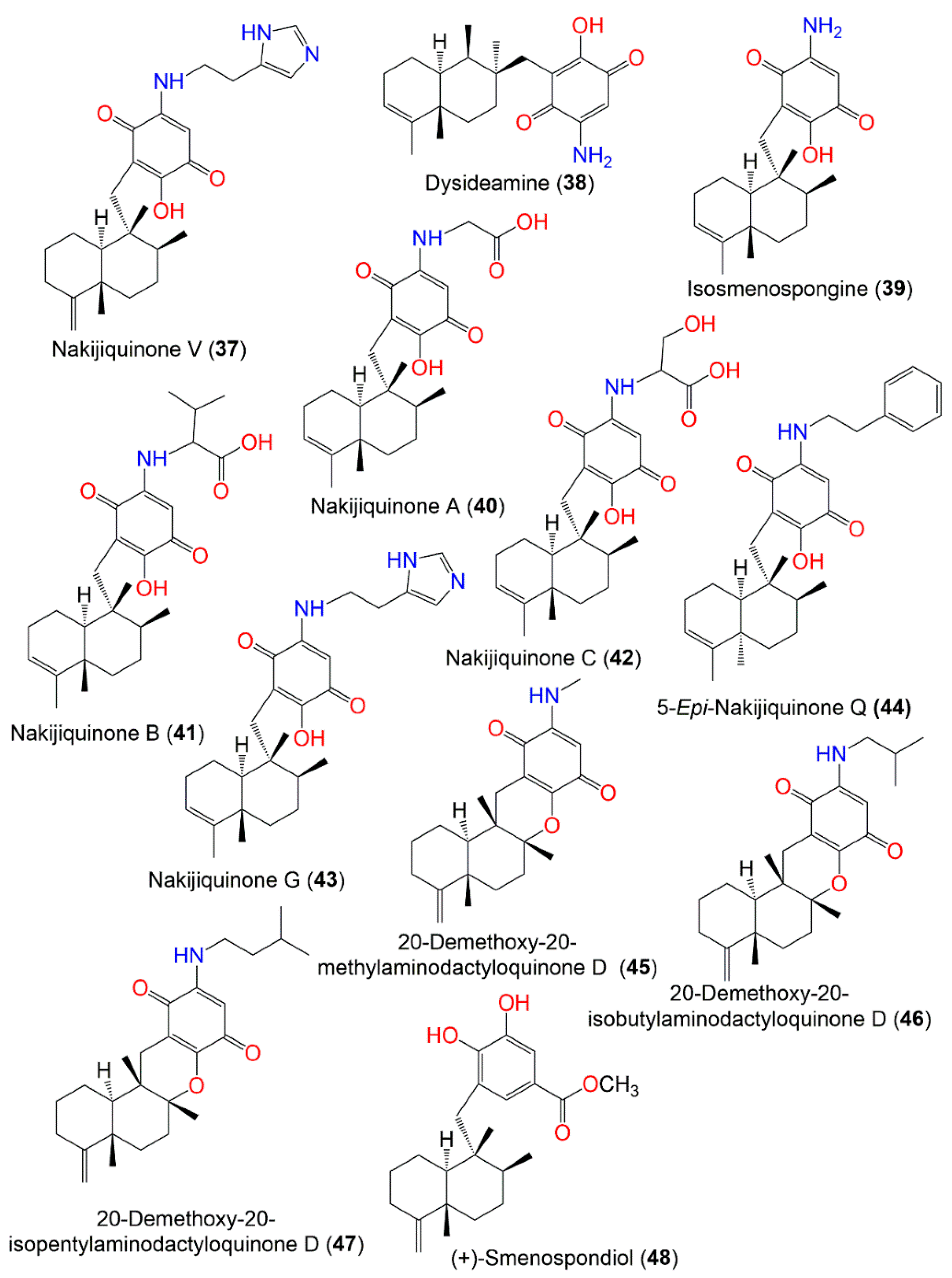
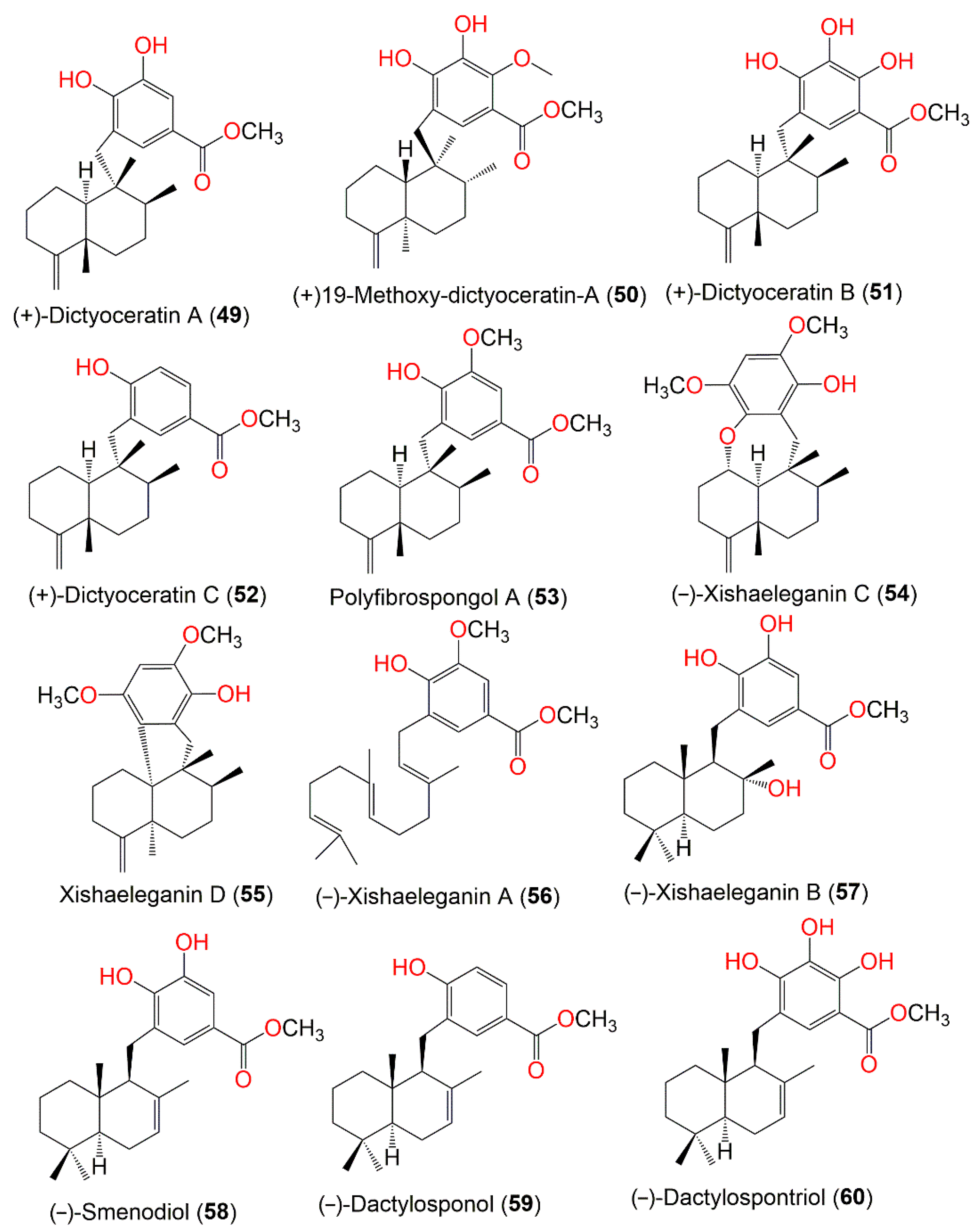
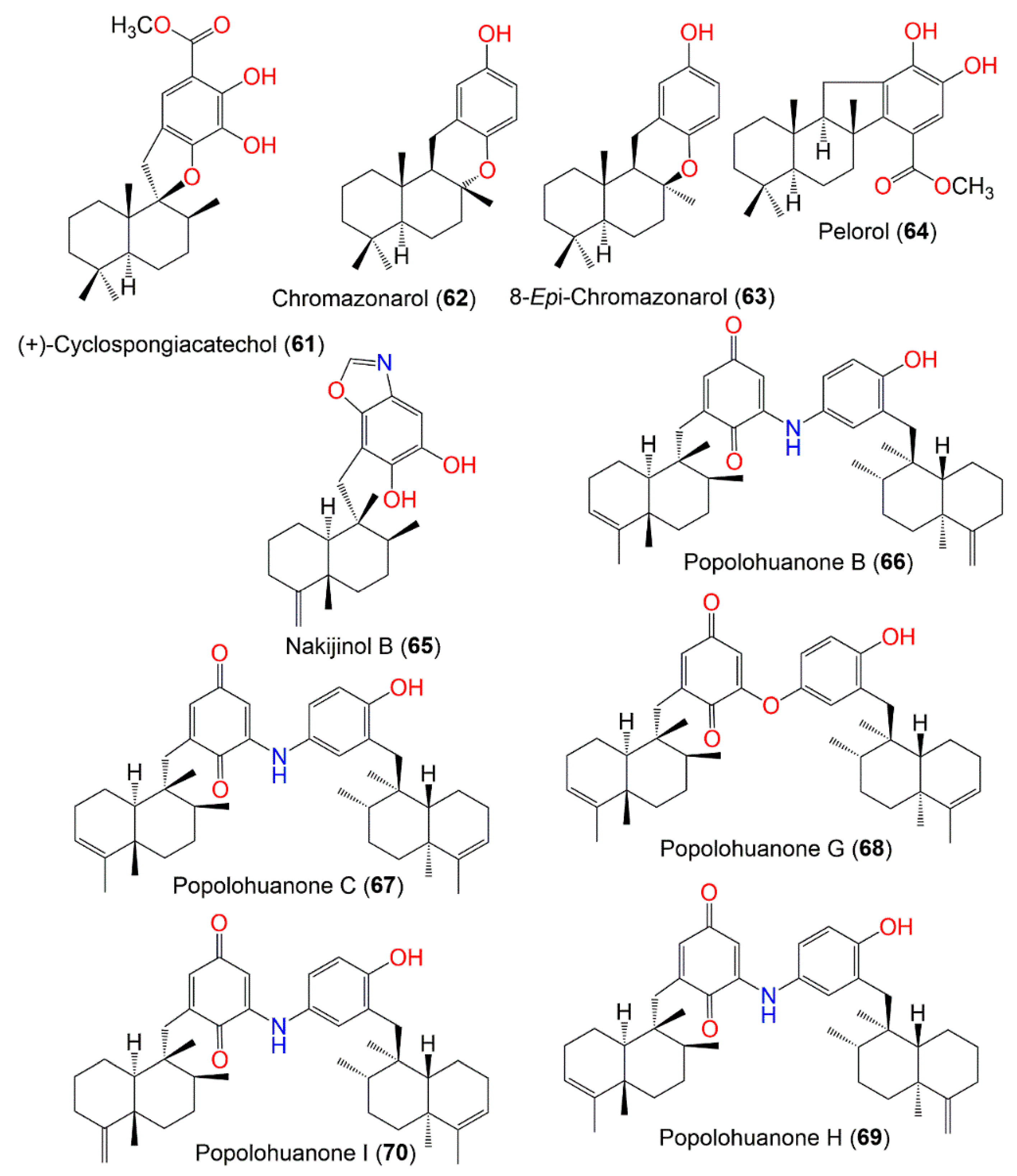
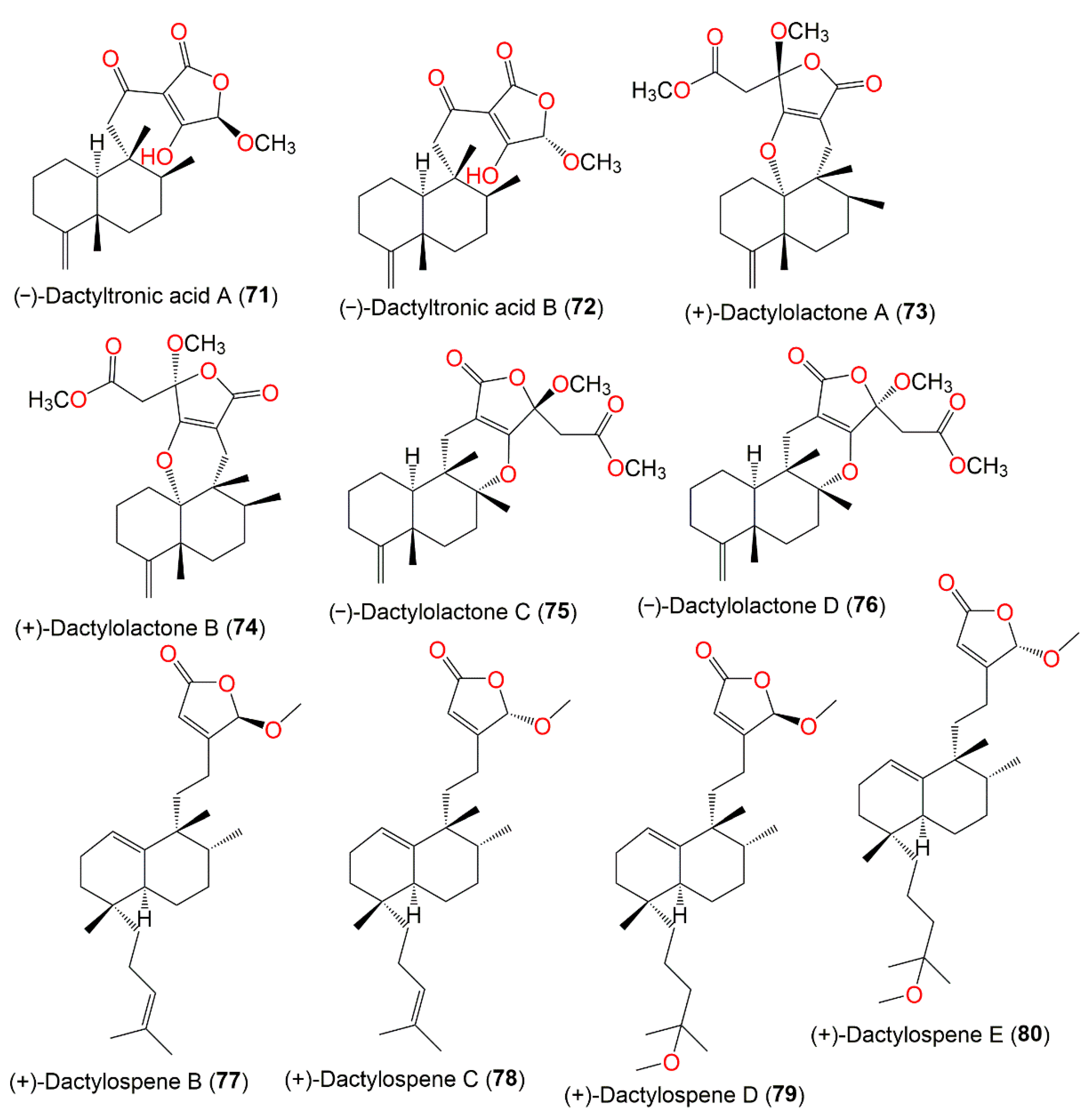
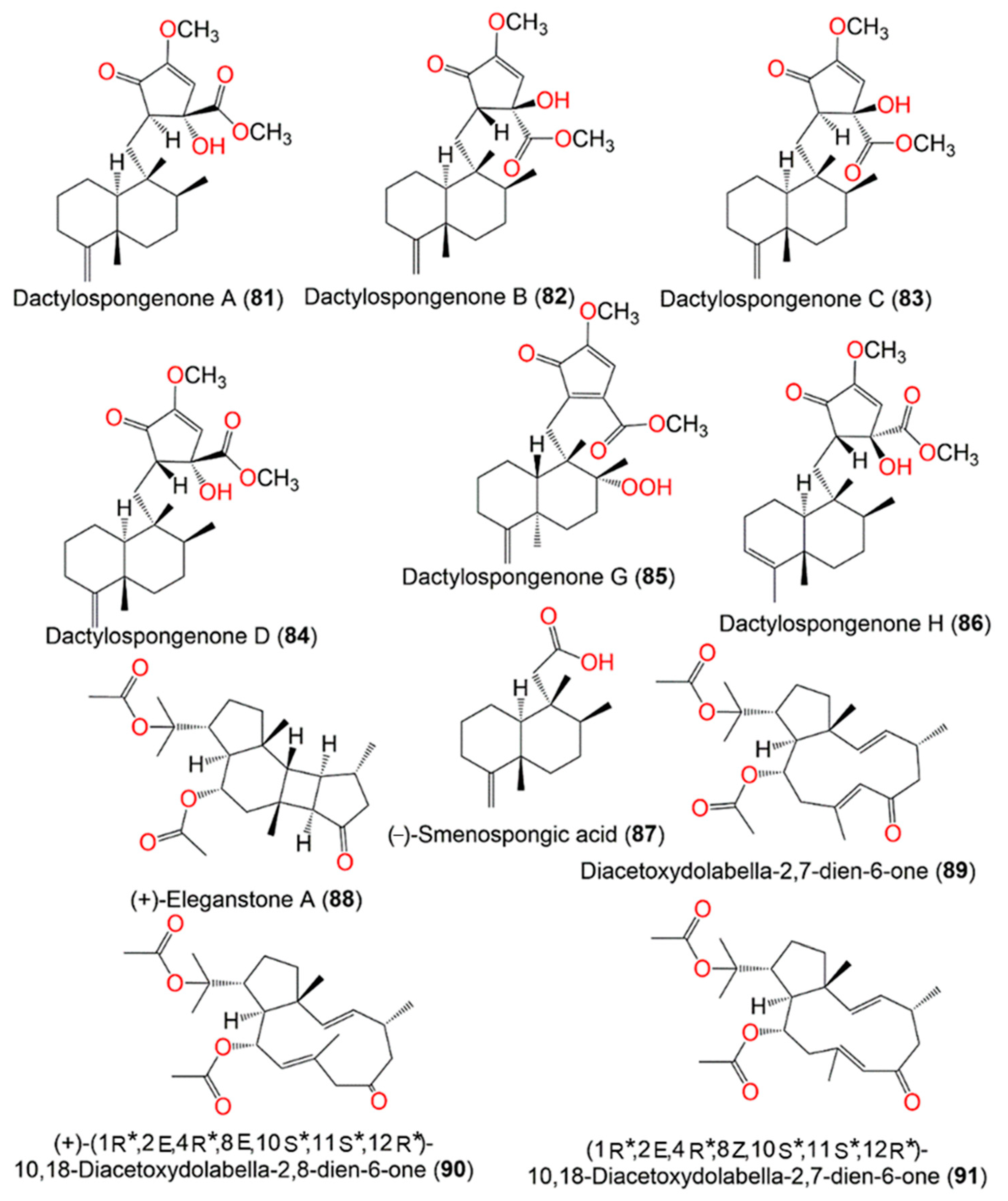
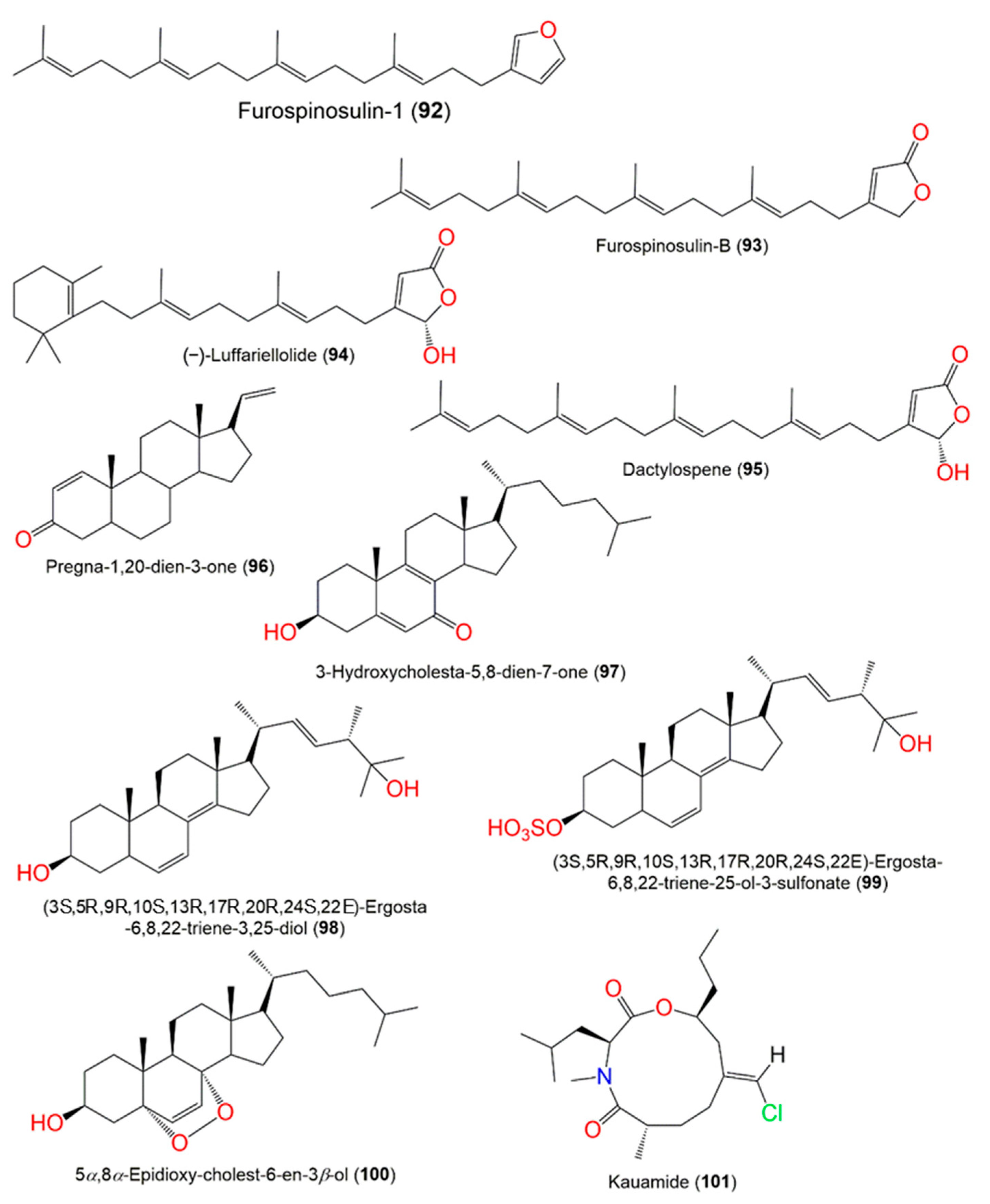
3. Biosynthetic Pathways of D. elegans Metabolites
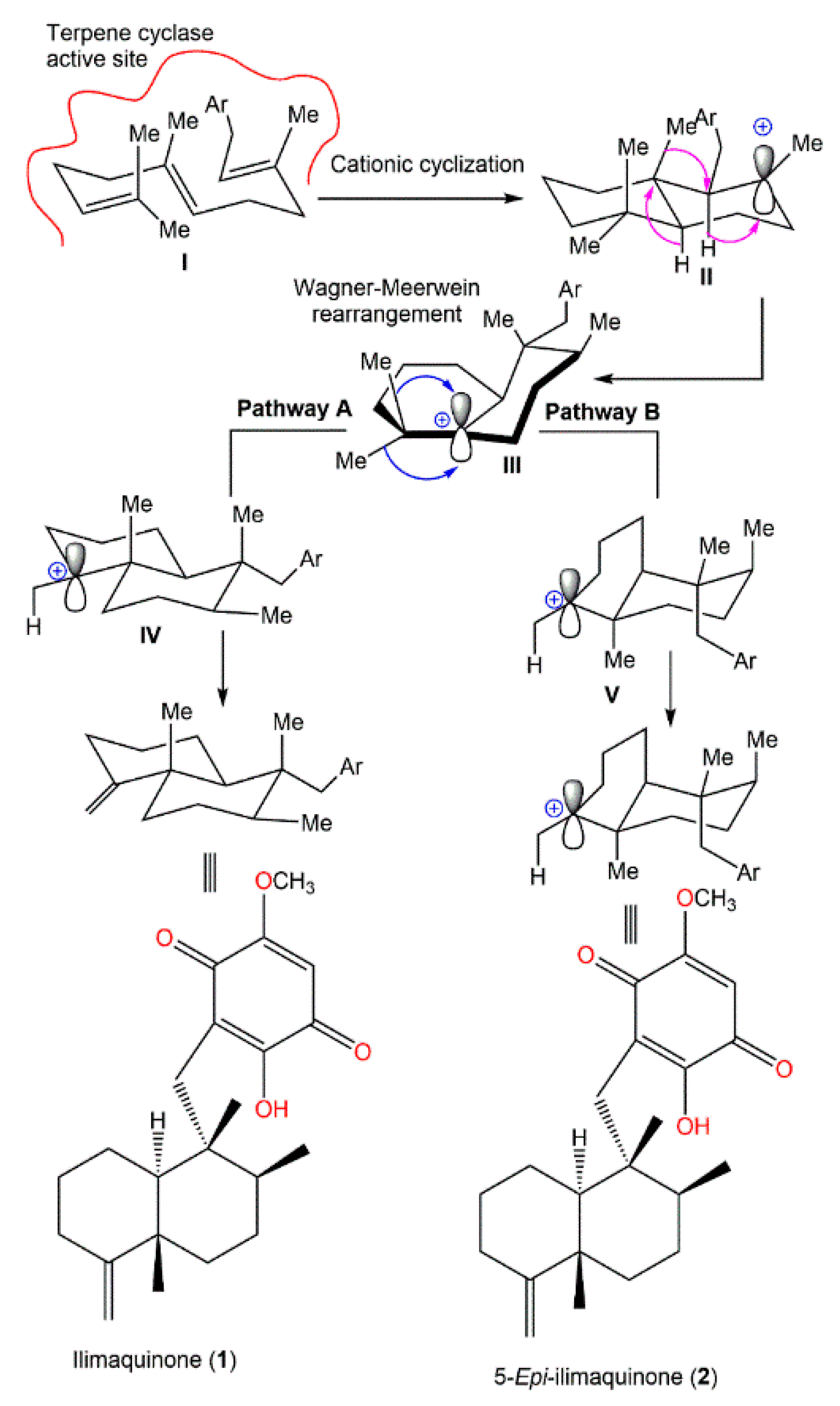
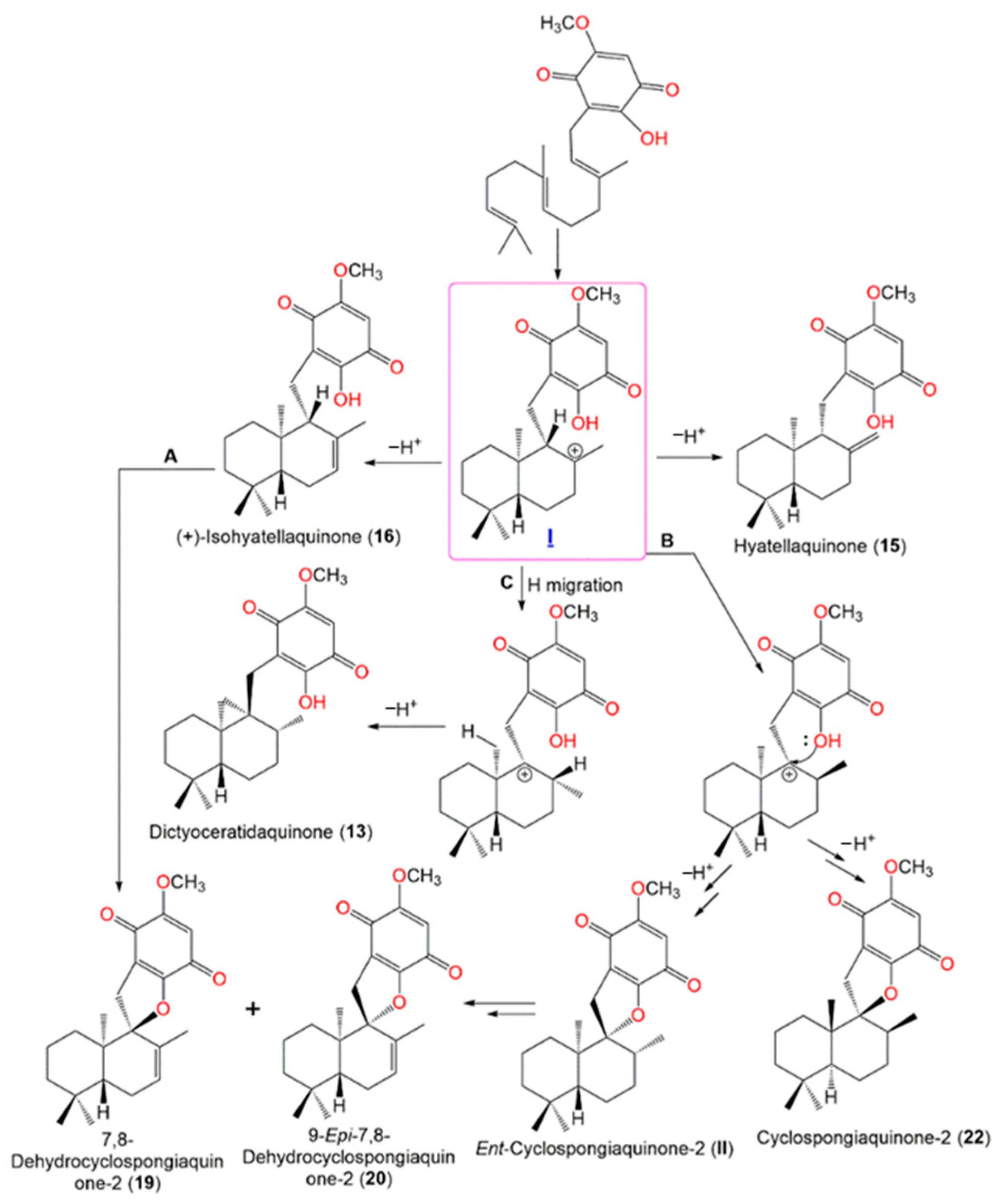
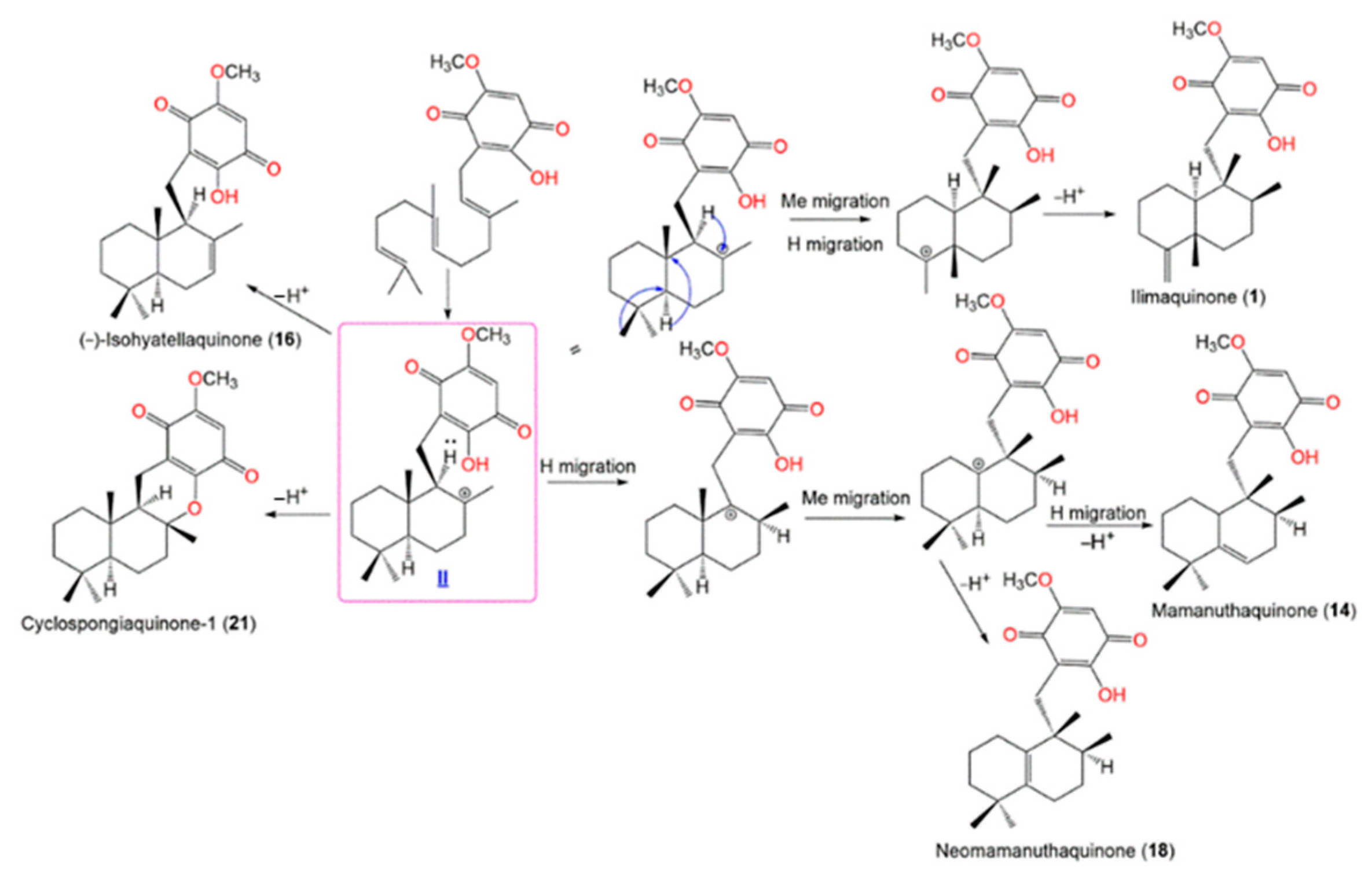
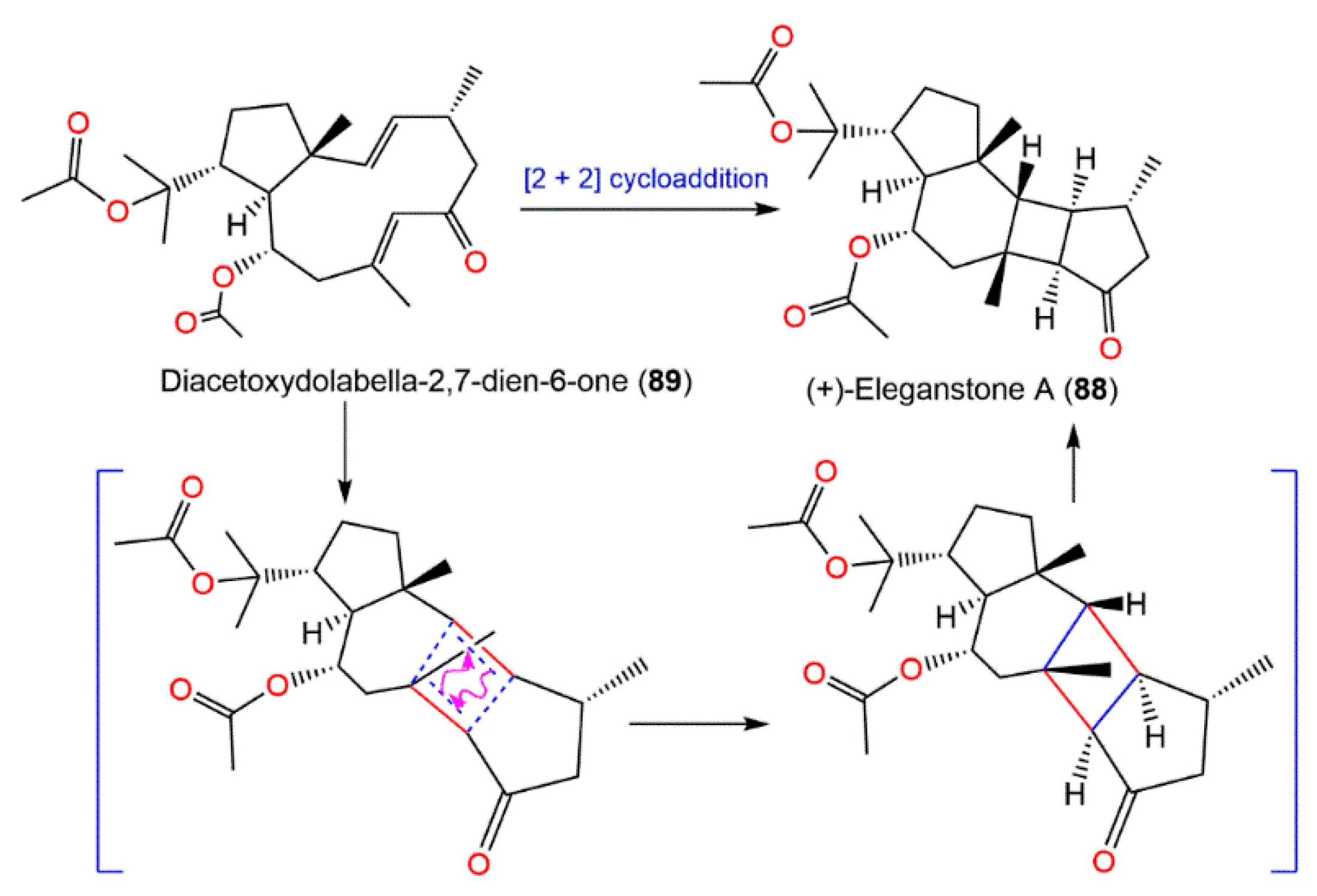
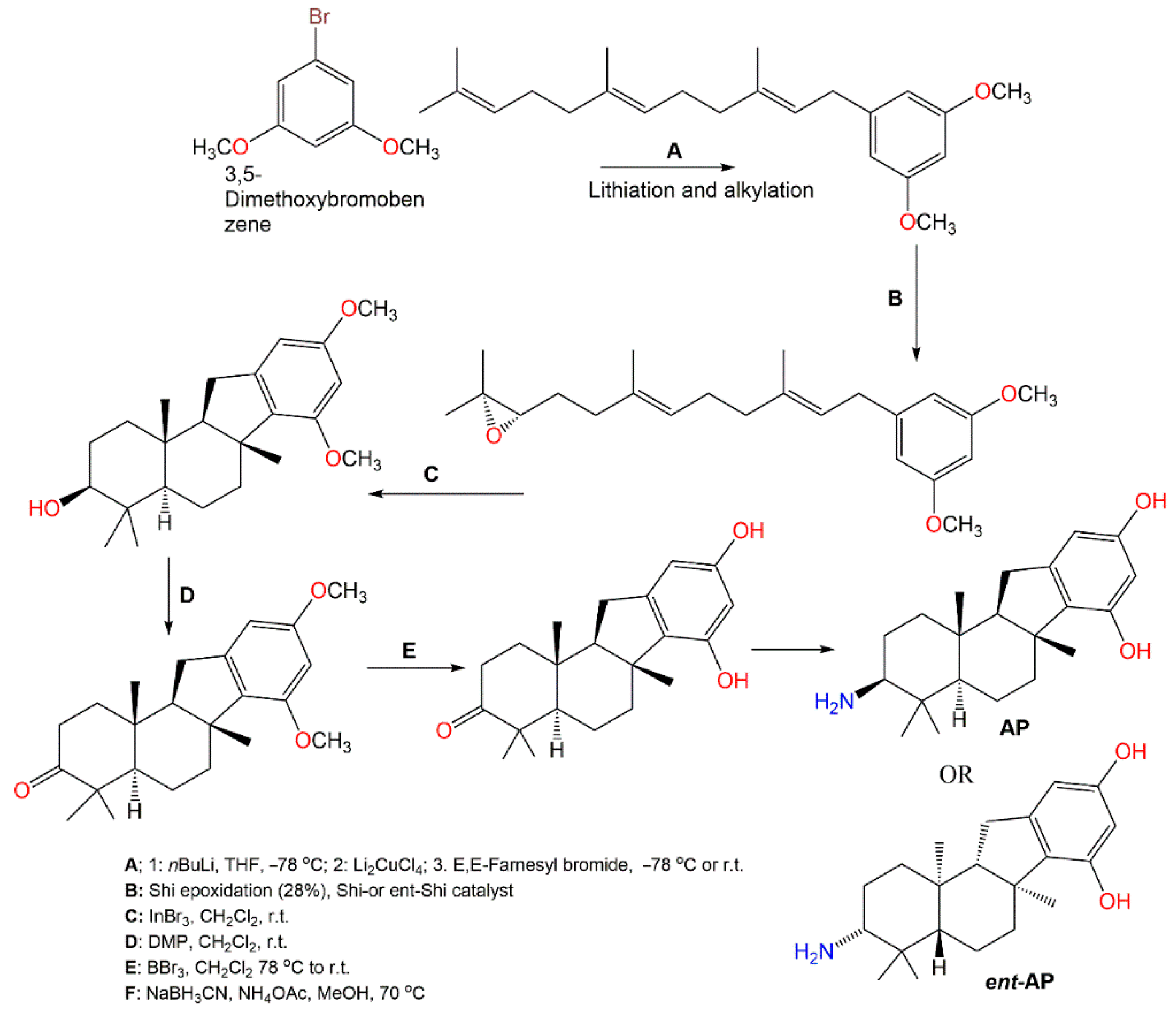
4. Synthesis of D. elegans Metabolites
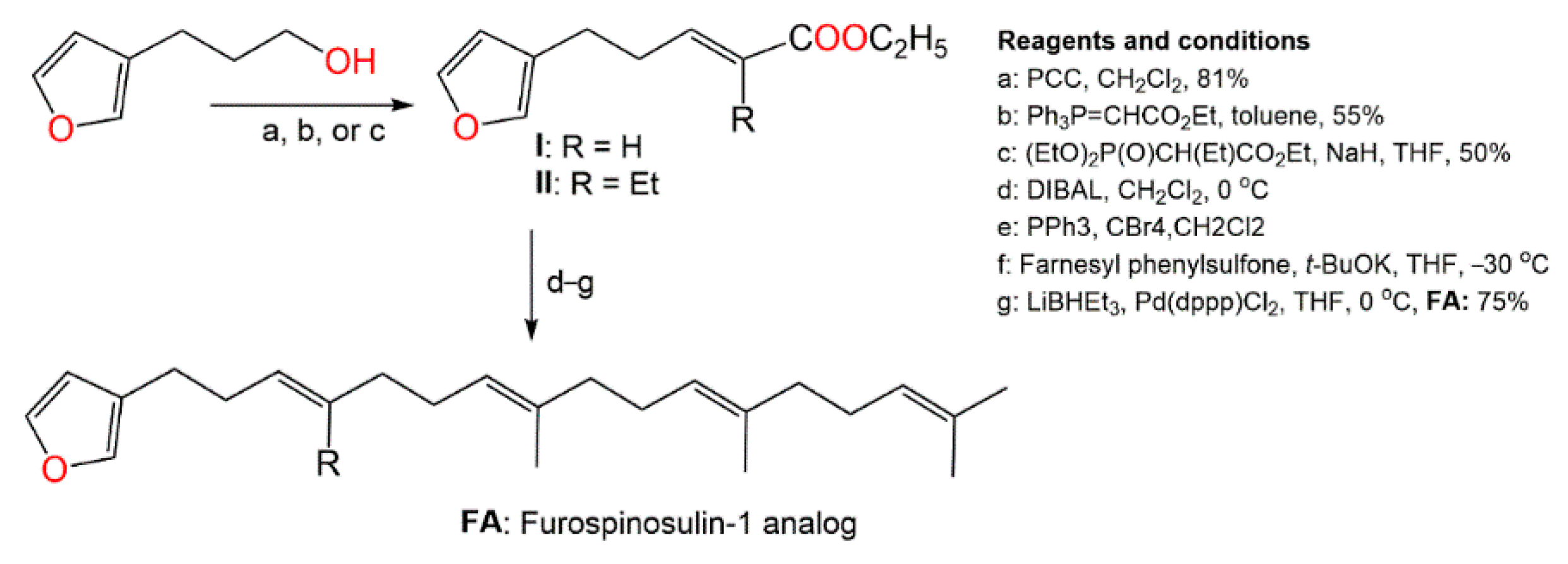
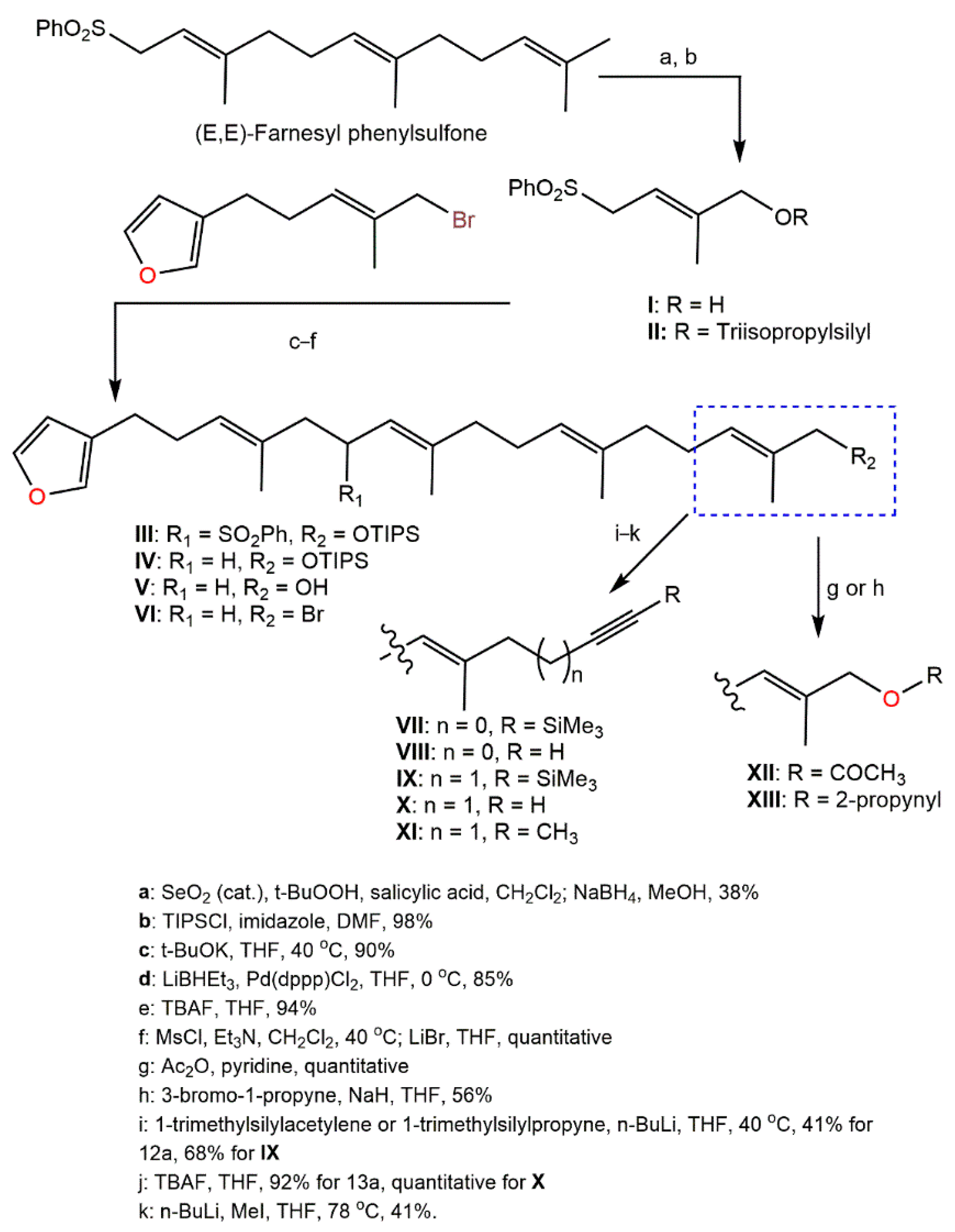
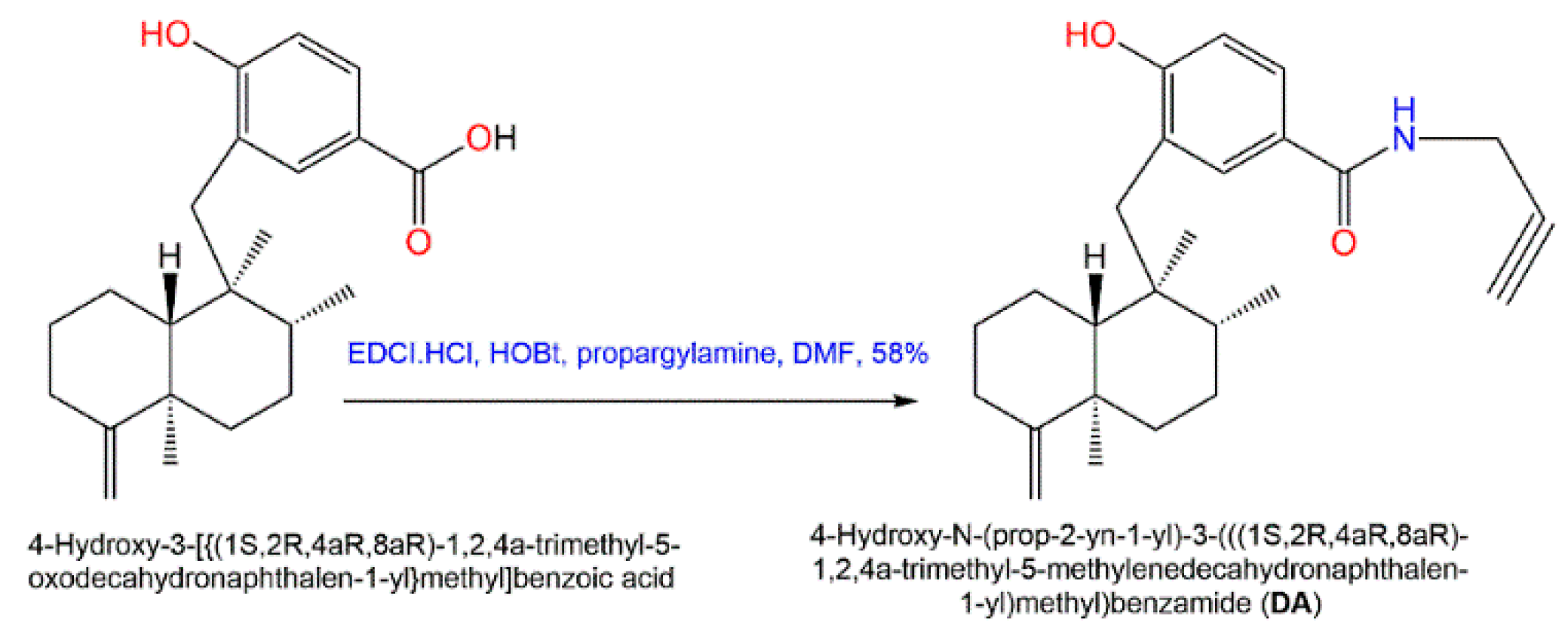
5. Activities of D. elegans Extracts and Fractions
References
- Kobayashi, J. Search for new bioactive marine natural products and application to drug development. Chem. Pharm. Bull. 2016, 64, 1079–1083.
- Omar, A.M.; Mohamed, G.A.; Ibrahim, S. Chaetomugilins and chaetoviridins-promising natural metabolites: Structures, separation, characterization, biosynthesis, bioactivities, molecular docking, and molecular dynamics. J. Fungi 2022, 8, 127.
- Radjasa, O.K.; Vaske, Y.M.; Navarro, G.; Vervoort, H.C.; Tenney, K.; Linington, R.G.; Crews, P. Highlights of marine invertebrate-derived biosynthetic products: Their biomedical potential and possible production by microbial associants. Bioorg. Med. Chem. 2011, 19, 6658–6674.
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98.
- Mohamed, G.A.; Ibrahim, S.R.M. Untapped potential of marine-associated Cladosporium species: An overview on secondary metabolites, biotechnological relevance, and biological activities. Mar. Drugs 2021, 19, 645.
- Gao, Z.M.; Zhou, G.W.; Huang, H.; Wang, Y. The Cyanobacteria-dominated sponge Dactylospongia elegans in the South China Sea: Prokaryotic community and metagenomic insights. Front. Microbiol. 2017, 8, 1387.
- Hentschel, U.; Hopke, J.; Horn, M.; Friedrich, A.B.; Wagner, M.; Hacker, J.; Moore, B.S. Molecular evidence for a uniform microbial community in sponges from different oceans. Appl. Environ. Microbiol. 2002, 68, 4431–4440.
- Esposito, R.; Ruocco, N.; Viel, T.; Federico, S.; Zupo, V.; Costantini, M. Sponges and their symbionts as a source of valuable compounds in cosmeceutical field. Mar. Drugs 2021, 19, 444.
- Bell, J.J. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 2008, 79, 341–353.
- Lee, Y.K.; Lee, J.H.; Lee, H.K. Microbial symbiosis in marine sponges. J. Microbiol. 2001, 39, 254–264.
- Thomas, T.R.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association-a review. Mar. Drugs 2010, 8, 1417–1468.
- Galitz, A.; Nakao, Y.; Schupp, P.J.; Wörheide, G.; Erpenbeck, D. A soft spot for chemistry–current taxonomic and evolutionary implications of sponge secondary metabolite distribution. Mar. Drugs 2021, 19, 448.
- Sladić, D.; Gasić, M.J. Reactivity and biological activity of the marine sesquiterpene hydroquinone avarol and related compounds from sponges of the order Dictyoceratida. Molecules 2006, 11, 1–33.
- Sagar, S.; Kaur, M.; Minneman, K.P. Antiviral lead compounds from marine sponges. Mar. Drugs 2010, 8, 2619–2638.
- Wada, Y.; Fujioka, H.; Kita, Y. Synthesis of the marine pyrroloiminoquinone alkaloids, discorhabdins. Mar. Drugs 2010, 8, 1394–1416.
- Butler, M.S.; Capon, R.J. Beyond polygodial: New drimane sesquiterpene from a Southern marine sponge, Dysidea sp. Aust. J. Chem. 1993, 46, 1255–1267.
- Yong, K.W.L.; Jankam, A.; Hooper, J.N.A.; Suksamrarn, A.; Garson, M.J. Stereochemical evaluation of sesquiterpene quinones from two sponges of the genus Dactylospongia and the implication for enantioselective processes in marine terpene biosynthesis. Tetrahedron 2008, 64, 6341–6348.
- Poigny, S.; Huor, T.; Guyot, M.; Samadi, M. Synthesis of (−)-hyatellaquinone and revision of absolute configuration of naturally occurring (+)-hyatellaquinone. J. Org. Chem. 1999, 64, 9318–9320.
- Boufridi, A.; Lachkar, D.; Erpenbeck, D.; Beniddir, M.A.; Evanno, L.; Petek, S.; Debitus, C.; Poupon, E. Ilimaquinone and 5-epi-ilimaquinone: Beyond a simple diastereomeric ratio, biosynthetic considerations from NMR-based analysis. Aust. J. Chem. 2017, 70, 743–750.
- Yu, H.-B.; Gu, B.-B.; Wang, S.-P.; Cheng, C.-W.; Yang, F.; Li, H.-W. New diterpenoids from the marine sponge Dactylospongia elegans. Tetrahedron 2017, 73, 6657–6661.
- Liang, L.-F.; Kurtán, T.; Mándi, A.; Yao, L.-G.; Li, J.; Zhang, W.; Guo, Y.-W. Unprecedented diterpenoids as a PTP1B inhibitor from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller. Org. Lett. 2012, 15, 274–277.
- Kotoku, N.; Fujioka, S.; Nakata, C.; Yamada, M.; Sumii, Y.; Kawachi, T.; Arai, M.; Kobayashi, M. Concise synthesis and structure activity relationship of furospinosulin-1, a hypoxia-selective growth inhibitor from marine sponge. Tetrahedron 2011, 67, 6673–6678.
- Kotoku, N.; Nakata, C.; Kawachi, T.; Sato, T.; Guo, X.H.; Ito, A.; Sumii, Y.; Arai, M.; Kobayashi, M. Synthesis and evaluation of effective photoaffinity probe molecule of furospinosulin-1, a hypoxia-selective growth inhibitor. Bioorg. Med. Chem. 2014, 22, 2102–2112.
- Sumii, Y.; Kotoku, N.; Fukuda, A.; Kawachi, T.; Sumii, Y.; Arai, M.; Kobayashi, M. Enantioselective synthesis of dictyoceratin-A (smenospondiol) and -C, hypoxia-selective growth inhibitors from marine sponge. Bioorg. Med. Chem. 2015, 23, 966–975.
- Yang, L.; Williams, D.E.; Mui, A.; Ong, C.; Krystal, G.; van Soest, R.; Andersen, R.J. Synthesis of pelorol and analogues: Activators of the inositol 5-phosphatase SHIP. Org. Lett. 2005, 7, 1073–1076.
- Meimetis, L.G.; Nodwell, M.; Yang, L.; Wang, X.; Wu, J.; Harwig, C.; Stenton, G.R.; Mackenzie, L.F.; MacRury, T.; Patrick, B.O.; et al. Synthesis of SHIP1-activating analogs of the sponge meroterpenoid pelorol. Eur. J. Org. Chem. 2012, 2012, 5195–5207.
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781.
- Li, J.; Wu, W.; Yang, F.; Liu, L.; Wang, S.P.; Jiao, W.H.; Xu, S.H.; Lin, H.W. Popolohuanones G–I, dimeric sesquiterpene quinones with IL-6 inhibitory activity from the marine sponge Dactylospongia elegans. Chem. Biodivers. 2018, 15, e1800078.
- Rivera, A.P.; Uy, M.M. In vitro antioxidant and cytotoxic activities of some marine sponges collected off misamis oriental coast, Philippines. E-J. Chem. 2012, 9, 354–358.




