| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Víctor M. Víctor | + 2407 word(s) | 2407 | 2020-09-21 15:21:02 | | | |
| 2 | Felix Wu | -799 word(s) | 1608 | 2020-10-21 10:16:59 | | |
Video Upload Options
The rising prevalence of obesity and type 2 diabetes (T2D) is a growing concern worldwide. New discoveries in the field of metagenomics and clinical research have revealed that the gut microbiota plays a key role in these metabolic disorders. The mechanisms regulating microbiota composition are multifactorial and include resistance to stress, presence of pathogens, diet, cultural habits and general health conditions. Recent evidence has shed light on the influence of microbiota quality and diversity on mitochondrial functions. Of note, the gut microbiota has been shown to regulate crucial transcription factors, coactivators, as well as enzymes implicated in mitochondrial biogenesis and metabolism. Moreover, microbiota metabolites seem to interfere with mitochondrial oxidative/nitrosative stress and autophagosome formation, thus regulating the activation of the inflammasome and the production of inflammatory cytokines, key players in chronic metabolic disorders. This review focuses on the association between intestinal microbiota and mitochondrial function and examines the mechanisms that may be the key to their use as potential therapeutic strategies in obesity and T2D management.
1. Introduction
Obesity is a multifactorial disease involving a complex network of physical, systemic and physiological disorders. This condition develops as a consequence of an imbalance due to excessive intake and low expenditure of energy, leading to an abnormal accumulation of lipids in metabolic tissues, particularly adipose tissue and the liver [1][2]. Adipose tissue is mostly implicated in systemic metabolic homeostasis, acting as an autocrine and endocrine organ by releasing active mediators named adipokines. The dysregulation of these secretory factors, caused by excess adiposity and adipocyte dysfunction, can promote macrophage infiltration in the inflamed adipose tissue [3][4][5]. Accordingly, the accumulation of macrophages results in the secretion of different proinflammatory mediators, such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α, which can potentially contribute to the initiation and progression of obesity-induced metabolic complications [2]. If the magnitude of cytokines secretion is large enough, these can leak out of the tissue, raising circulating levels and producing endocrine effects in distant organ systems (such as muscle and the liver), thus exacerbating the systemic insulin resistance and beta (β)-cell dysfunction. These pathogenic states influence each other, leading to persistent hyperglycemia and further initiation of and progression to T2D [5][6][7].
Recently, studies conducted both in human and animal models suggest that obesity and T2D are associated with alterations in the gut microbiota (known as dysbiosis) and related biological pathways [8]. Gut microbes seem to exert a crucial role in the development of metabolic diseases. Indeed, they can affect the host’s metabolic balance by modulating appetite, energy absorption, hepatic fatty storage, gut motility and lipid and glucose metabolisms [8][9]. Moreover, changes in gut microbiota homeostasis can increase intestinal permeability, thus promoting the translocation of bacterial endotoxins into the systemic circulation and further facilitating the metabolic endotoxemia and low-grade inflammation status that characterize these conditions [10]. Unfortunately, the specific mechanisms by which gut microbiota contribute to the pathogenesis of these metabolic disorders are not clearly understood.
2. Mitochondrial Dysfunction in Obesity and T2D
Accumulating evidence has shown that supraphysiological concentrations of Reactive oxygen species (ROS) are responsible for metabolic and redox damage in cell homeostasis. In this sense, a substantial body of the literature is in agreement about the chronic and disruptive oxidative stress damage generated in obesity and T2D [11][12]. Firstly, in obese patients, the excess of macronutrients in adipose tissues stimulates them to release inflammatory mediators that, in turn, propagate the systemic inflammation associated with the development of hyperinsulinemia, insulin resistance and other comorbidities. Interestingly, the altered adipokine profile, which involves the upregulated expression and secretion of proinflammatory cytokines, contributes to the formation of toxic ROS and the subsequent generation of oxidative stress [13] through different processes. For example, in adipose tissue, obesity can induce oxidative stress mainly via catalytic activity of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzyme (NOX). Data reveal that, in the early and intermediate stages of obesity, NADPH oxidase-derived ROS are responsible for the recruitment of macrophages to the inflamed tissue, while in the later stages, mitochondrial-derived ROS maintain a proinflammatory environment and contribute to the development of insulin resistance [12][14]. Moreover, elevated lipid levels, which are abundant in obesity, seem to impair mitochondrial ETC and generate ROS, which can modify the chemical composition of lipids and makes them more reactive, thus maintaining a low level of inflammation [15]. It is also widely established that imbalanced levels of ROS are associated with adipogenesis dysfunction and adipocyte hypertrophy in adipose tissue and harmful systemic effects on vascular dysfunction, such as endothelial inflammation and hypercontractility [16][17].
In addition, oxidative stress has been closely related to β-cell dysfunction, one of the key players in the pathophysiology of T2D [15][16]. Pathological levels of ROS can activate NOX2 and the stress-sensitive serine/threonine kinase (JNK) in β cells and attenuate the insulin cascade, thus increasing insulin resistance [15][16]. Notably, β cells present very low levels of antioxidant enzymes, which make them more susceptible to oxidative stress [17]. This makes β cells highly sensitive to ROS-related signaling and susceptible to oxidative damage. Results by Sasaki et al. [18] reveal a key mechanism by which ROS impairs β-cell functions in diabetic rats: the activation of hypoxia-inducible factor 1α (Hif1α) results in remodeling towards increased aerobic glycolysis and suppressed cytokine-induced cell death. As a result, glucose oxidation and insulin secretion are impaired [18].
3. Gut Microbiota: A Novel Key Player in Obesity and T2D
The human gastrointestinal tract is colonized by large numbers of symbiotic, commensal and pathogenic microorganisms, including archaea, protozoa, fungi, viruses and bacteria, collectively identified as the “gut microbiota”. It consists of up to 100 trillion microbes, more than 1000 different bacteria species and five phyla (Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria and Verrucomicrobia), of which two are dominant: Bacteroidetes (Bacteroides, Xylanibacter and Prevotella) and Firmicutes (Clostridium, Ruminococcus, Eubacterium, Lactobacillus, Roseburia and Faecalibacterium) [19]. The gut microbiota serves several essential functions: preventing colonization by pathogens; producing vitamins such as vitamin K, folate and biotin; participating in energy regulation; fermenting dietary fibers in short-chain fatty acids (SCFAs); metabolizing xenobiotics; modulating brain activity by interacting with the enteric nervous system and assisting in the development of a mature immune system [19]. The bacterial quantity and diversity progressively increase from the stomach to the colon [20], with the colon being home of the densest and metabolically most active community.
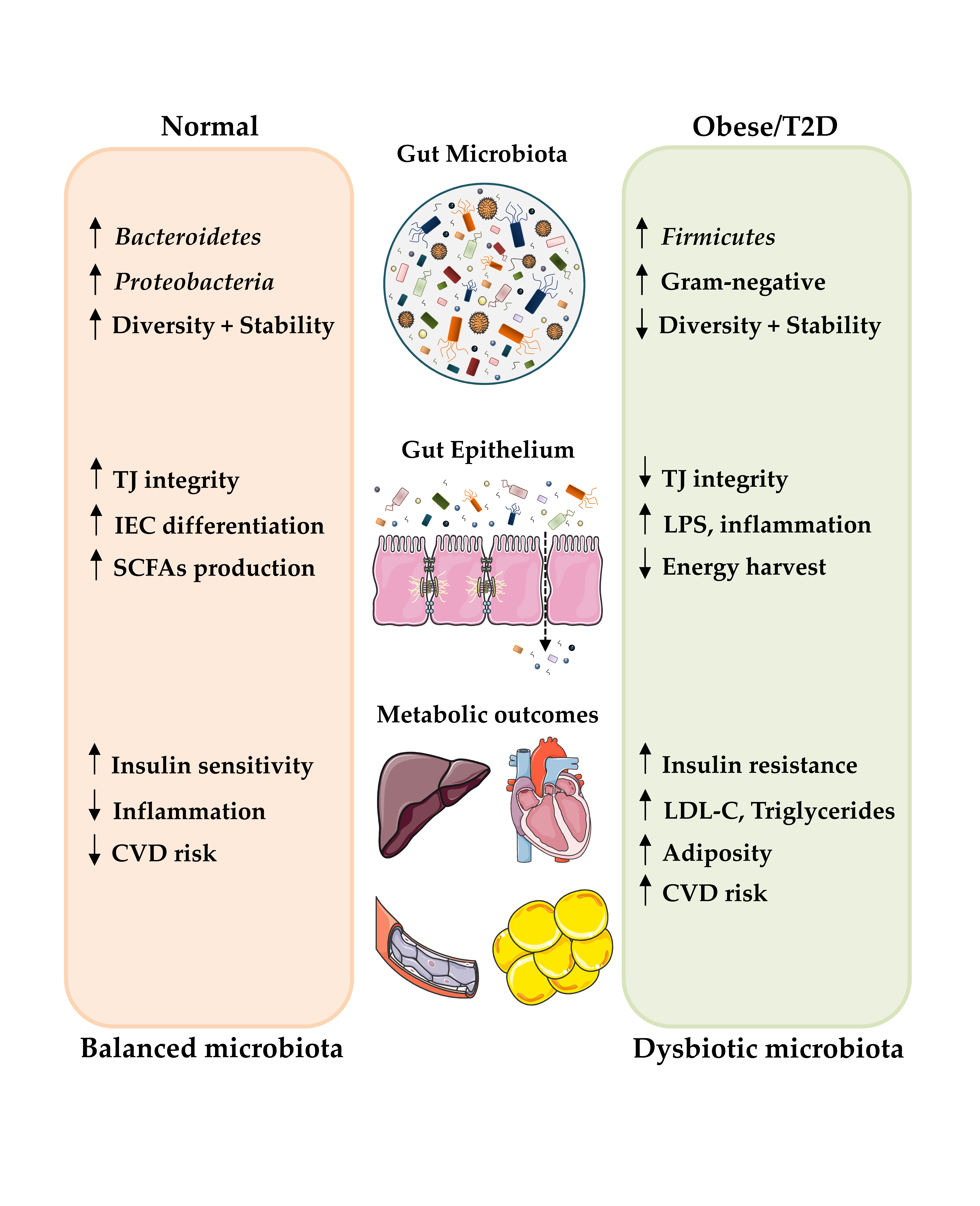
Numerous studies in animal models and humans have demonstrated that gut microbiota dysbiosis can be considered a major player in the development of obesity and T2D [21][22][23]. Specific bacterial phyla, class, order or species and/or bacterial activity seems to be detrimental or beneficial to the onset of such syndromes. Indeed, although the composition of the intestinal microbiota fluctuates markedly in healthy individuals, those exhibiting insulin resistance, overall adiposity and dyslipidemia are characterized by low bacterial variety [24] (Figure 1).
Figure 1. Role of gut microbiota in the development of obesity and T2D, including some of the mechanisms thought to contribute to alterations of the host metabolic state. Up and down arrows indicate an increase and decrease, respectively. Abbreviations: IEC, intestinal epithelial cells; LPS, lipopolysaccharide; LDL-C, low-density lipoproteins-cholesterol; SCFAs, short-chain fatty acids; CVD, cardiovascular disease and TJ, tight junction.
Obese and T2D individuals report consistent changes in the intestinal microbiota composition in comparison to lean individuals. Of note, the prevalence of Bacteroidetes is lower in obese and diabetic subjects and increases with weight loss [25][26]. Clostridium and Lactobacillus species are correlated with insulin resistance; Clostridium is negatively associated with fasting glucose and glycated hemoglobin levels, while Lactobacillus is positively correlated with such parameters. In addition, two large metagenome-wide association studies showed that T2D individuals present a lower proportion of butyrate-producing Clostridiales (Faecalibacterium prausnitzii and Roseburia) and higher proportions of Clostridiales that do not produce butyrate, indicating a protective role of butyrate-producing bacteria against T2D [21][27]. In addition, several reports show that obese people present a low amount of Akkermansia muciniphila, a mucin-degrading bacterium responsible for the unsealing of the intestinal barrier [28]. A lower abundance of this microorganism leads to an increased gut permeability and a further translocation of bacterial endotoxins into the systemic circulation, thus contributing to the chronic inflammation related to both obesity and insulin resistance [29].
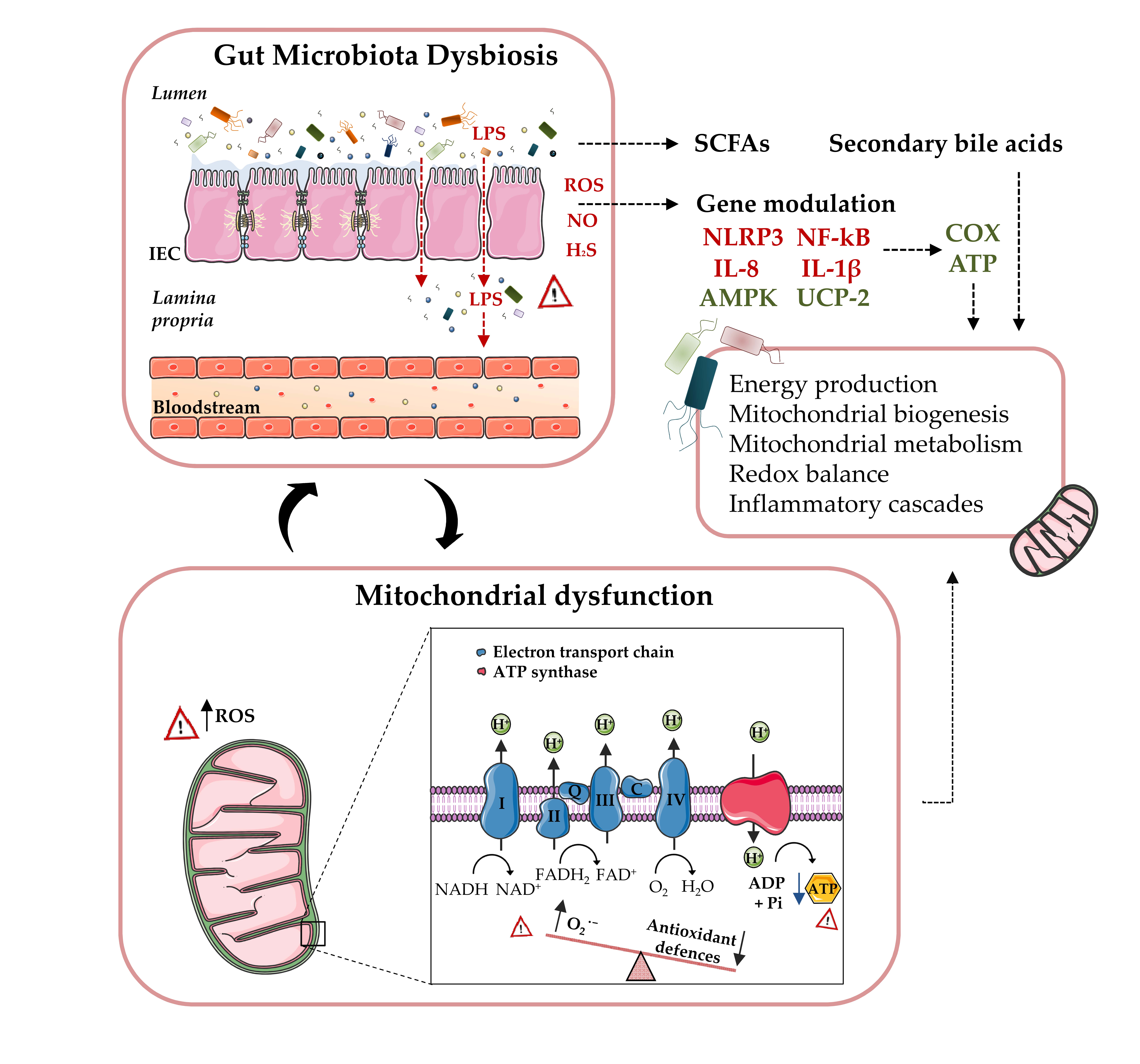
Unfortunately, the mechanisms by which gut microbiota contribute to the pathogenesis of these metabolic disorders are not clearly understood. In this sense, recent data have shed light on interactions between microbiota quality and diversity and mitochondrial function [30]. Of note, the intestinal microbiota seems to play a crucial role in the regulation of several transcription factors, transcriptional coactivators and enzymes associated with the mitochondrial biogenesis and metabolism [30][31][32][33][34]. Furthermore, microbiota metabolites seem to interfere with mitochondrial oxidative/nitrosative stress and autophagosome formation, thus regulating the activation of the NLRP3 inflammasome and the release of different inflammatory cytokines [30][32], all key players in chronic metabolic disorders (Figure 2).
Figure 2. Microbiota-mitochondria inter-talk: Increased gut permeability in obese and T2D patients can induce LPS translocation to the bloodstream, along with a further inflammatory cascade, ROS and H2S production, as well as inflammasome NLRP3 activation, thus resulting in a mitochondria-mediated inflammatory response. The inhibition of AMPK activation and decreased expression of UCP-2 lead to a reduced glucose metabolism and to the partial uncoupling of mitochondrial oxidative phosphorylation, as well as an increased ROS production. Moreover, microbiota-released metabolites can also undermine or promote mitochondrial biogenesis and energy metabolism. Concurrently, mitochondrial ROS production can regulate gut microbiota activity and composition by modulating mucosal immune responses and intestinal barrier functions. Abbreviations: AMPK, AMP-activated protein kinase; ATP, adenosine triphosphate; COX, cyclooxygenase; H2S, hydrogen sulphide; IEC, intestinal epithelial cells; LPS, lipopolysaccharide; NLRP3, inflammasome NOD-like receptor family and pyrin domain containing 3; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitrogen oxide; ROS, reactive oxygen species; SCFAs, short-chain fatty acids and UCP-2, uncoupling protein 2.
References
- Undurti N. Das; Obesity: Genes, brain, gut, and environment. Nutrition 2010, 26, 459-473, 10.1016/j.nut.2009.09.020.
- Nathalie Esser; Sylvie Legrand-Poels; Jacques Piette; A.J. Scheen; Nicolas Paquot; Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Research and Clinical Practice 2014, 105, 141-150, 10.1016/j.diabres.2014.04.006.
- Erin E. Kershaw; Jeffrey S. Flier; Adipose Tissue as an Endocrine Organ. The Journal of Clinical Endocrinology & Metabolism 2004, 89, 2548-2556, 10.1210/jc.2004-0395.
- Katja Rabe; Michael Lehrke; Klaus G. Parhofer; Uli C. Broedl; Adipokines and Insulin Resistance. Molecular Medicine 2008, 14, 741-751, 10.2119/2008-00058.rabe.
- Alba Fernández-Sánchez; Eduardo Madrigal-Santillán; Mirandeli Bautista; Jaime Esquivel-Soto; Ángel Morales-González; Cesar Esquivel-Chirino; Irene Durante-Montiel; Graciela Sánchez-Rivera; Carmen Valadez-Vega; José A. Morales-González; et al. Inflammation, Oxidative Stress, and Obesity. International Journal of Molecular Sciences 2011, 12, 3117-3132, 10.3390/ijms12053117.
- Olivia Osborn; Jerrold M Olefsky; The cellular and signaling networks linking the immune system and metabolism in disease. Nature Medicine 2012, 18, 363-374, 10.1038/nm.2627.
- Niv Zmora; Stavros Bashiardes; Maayan Levy; Eran Elinav; The Role of the Immune System in Metabolic Health and Disease. Cell Metabolism 2017, 25, 506-521, 10.1016/j.cmet.2017.02.006.
- D. Festi; Ramona Schiumerini; Leonardo Henry Eusebi; Giovanni Marasco; Martina Taddia; Antonio Colecchia; Gut microbiota and metabolic syndrome. World Journal of Gastroenterology 2014, 20, 16079-94, 10.3748/wjg.v20.i43.16079.
- Muhammad Tanweer Khan; Max Nieuwdorp; Fredrik Bäckhed; Microbial Modulation of Insulin Sensitivity. Cell Metabolism 2014, 20, 753-760, 10.1016/j.cmet.2014.07.006.
- Julian R. Marchesi; David Adams; Francesca Fava; Gerben D A Hermes; Gideon M Hirschfield; Georgina Hold; Mohammed Nabil Quraishi; James Kinross; Hauke Smidt; Kieran M. Tuohy; et al.Linda V ThomasE G ZoetendalAilsa Hart The gut microbiota and host health: a new clinical frontier. Gut 2015, 65, 330-339, 10.1136/gutjnl-2015-309990 [doi]..
- Magdalene K Montgomery; Nigel Turner; Mitochondrial dysfunction and insulin resistance: an update. Endocrine Connections 2015, 4, R1-R15, 10.1530/ec-14-0092.
- Gabriella D’Angelo; Sara Manti; Gabriella D’Angelo; Antonio Nicotera; Eleonora Parisi; Gabriella Di Rosa; Eloisa Gitto; Teresa Arrigo; Oxidative Stress in Obesity: A Critical Component in Human Diseases. International Journal of Molecular Sciences 2014, 16, 378-400, 10.3390/ijms16010378.
- Pallavi M.; Suchitra M. M.; Alok Sachan; Lakshmi A. Y.; Srinivasa Rao P. V. L. N.; Role of adipokines, oxidative stress, and endotoxins in the pathogenesis of non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. International Journal of Research in Medical Sciences 2019, 7, 1644-1652, 10.18203/2320-6012.ijrms20191652.
- Chang Yeop Han; Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes & Metabolism Journal 2016, 40, 272-279, 10.4093/dmj.2016.40.4.272.
- Prasenjit Manna; Sushil K. Jain; Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metabolic Syndrome and Related Disorders 2015, 13, 423-444, 10.1089/met.2015.0095.
- Clara Lefranc; Malou Friederich-Persson; Roberto Palacios-Ramirez; Aurelie Nguyen Dinh Cat; Mitochondrial oxidative stress in obesity: role of the mineralocorticoid receptor. Journal of Endocrinology 2018, 238, R143-R159, 10.1530/joe-18-0163.
- Chih‐Hao Wang; Ching‐Chu Wang; Hsin‐Chang Huang; Yau‐Huei Wei; Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS Journal 2013, 280, 1039-1050, 10.1111/febs.12096.
- Mayumi Sasaki; Shimpei Fujimoto; Yuichi Sato; Yuichi Nishi; Eri Mukai; Gen Yamano; Hiroki Sato; Yumiko Tahara; Kasane Ogura; Kazuaki Nagashima; et al.Nobuya Inagaki Reduction of Reactive Oxygen Species Ameliorates Metabolism-Secretion Coupling in Islets of Diabetic GK Rats by Suppressing Lactate Overproduction. Diabetes 2013, 62, 1996-2003, 10.2337/db12-0903.
- Elizabeth Thursby; Nathalie Juge; Introduction to the human gut microbiota. Biochemical Journal 2017, 474, 1823-1836, 10.1042/bcj20160510.
- Gregory P. Donaldson; S. Melanie Lee; Sarkis K. Mazmanian; Gut biogeography of the bacterial microbiota. Nature Reviews Microbiology 2015, 14, 20-32, 10.1038/nrmicro3552.
- Lijuan Sun; Lanjing Ma; Yubo Ma; Faming Zhang; Changhai Zhao; Yongzhan Nie; Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein & Cell 2018, 9, 397-403, 10.1007/s13238-018-0546-3.
- Junjie Qin; Yingrui Li; Zhiming Cai; Shenghui Li; Jianfeng Zhu; Fan Zhang; Suisha Liang; Wenwei Zhang; Yuanlin Guan; Dongqian Shen; et al.Yangqing PengDongya ZhangZhuye JieWenxian WuYouwen QinWenbin XueJunhua LiLingchuan HanDonghui LuPeixian WuYali DaiXiaojuan SunZesong LiAifa TangShilong ZhongXiaoping LiWeineng ChenRan XuMingbang WangQiang FengMeihua GongJing YuYanyan ZhangMing ZhangTorben HansenGaston SanchezJeroen RaesGwen FalonyShujiro OkudaMathieu AlmeidaEmmanuelle Le ChatelierPierre RenaultNicolas PonsJean-Michel BattoZhaoxi ZhangHua ChenRuifu YangWeimou ZhengSonggang LiHuanming YangJian WangS. Dusko EhrlichRasmus NielsenOluf PedersenKarsten KristiansenJun Wang A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55-60, 10.1038/nature11450.
- Aftab Ahmad; Wanwei Yang; Guofang Chen; Muhammad Shafiq; Sundus Javed; Syed Shujaat Ali Zaidi; Ramla Shahid; Chao Liu; Habib Bokhari; Analysis of gut microbiota of obese individuals with type 2 diabetes and healthy individuals. PLOS ONE 2019, 14, e0226372, 10.1371/journal.pone.0226372.
- Emmanuelle Le Chatelier; MetaHIT Consortium; Trine Nielsen; Junjie Qin; Edi Prifti; Falk Hildebrand; Gwen Falony; Mathieu Almeida; Manimozhiyan Arumugam; Jean-Michel Batto; et al.Sean KennedyPierre LeonardJunhua LiKristoffer BurgdorfNiels GrarupTorben JørgensenIvan BrandslundHenrik Bjørn NielsenAgnieszka S. JunckerMarcelo BertalanFlorence LevenezNicolas PonsSimon RasmussenShinichi SunagawaJulien TapSebastian TimsErwin G. ZoetendalSøren BrunakKarine ClémentJoël DoréMichiel KleerebezemKarsten KristiansenPierre RenaultThomas Sicheritz-PontenWillem M. De VosJean-Daniel ZuckerJeroen RaesTorben HansenPeer BorkJun WangStanislav EhrlichOluf Pedersen Richness of human gut microbiome correlates with metabolic markers. Nature Cell Biology 2013, 500, 541-546, 10.1038/nature12506.
- Ruth E. Ley; Peter J. Turnbaugh; Samuel Klein; Jeffrey I. Gordon; Human gut microbes associated with obesity. Nature 2006, 444, 1022-1023, 10.1038/4441022a.
- G. Blandino; R. Inturri; F. Lazzara; M. Di Rosa; L. Malaguarnera; Impact of gut microbiota on diabetes mellitus. Diabetes & Metabolism 2016, 42, 303-315, 10.1016/j.diabet.2016.04.004.
- Fredrik H. Karlsson; Valentina Tremaroli; Intawat Nookaew; Göran Bergström; Carl Johan Behre; Björn Fagerberg; Jens Bo Nielsen; Fredrik Bäckhed; Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99-103, 10.1038/nature12198.
- Sharon Y. Geerlings; Ioannis Kostopoulos; Willem M. De Vos; Clara Belzer; Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How?. Microorganisms 2018, 6, 75, 10.3390/microorganisms6030075.
- Patrice D. Cani; Jacques Amar; Miguel Angel Iglesias; Marjorie Poggi; Claude Knauf; Delphine Bastelica; Audrey M. Neyrinck; Francesca Fava; Kieran M. Tuohy; Chantal Chabo; et al.Aurélie WagetEvelyne DelméeBéatrice CousinThierry SulpiceBernard ChamontinJean FerrièresJean-François TantiGlenn R. GibsonLouis CasteillaNathalie M. DelzenneMarie Christine AlessiRémy Burcelin Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761-1772, 10.2337/db06-1491.
- Yann Saint-Georges-Chaumet; Marvin Edeas; Microbiota–mitochondria inter-talk: consequence for microbiota–host interaction. Pathogens and Disease 2015, 74, ftv096, 10.1093/femspd/ftv096.
- Franco-Obregon, A.; Gilbert, J.A. TheMicrobiome-Mitochondrion Connection: Common Ancestries, Common Mechanisms, Common Goals. mSystems 2017, 2, e00017–e00018.
- Lucattini, R.; Likic, V.A.; Lithgow, T. Bacterial proteins predisposed for targeting to mitochondria. Mol. Biol. Evol. 2004, 21, 652–658.
- Lobet, E.; Letesson, J.J.; Arnould, T. Mitochondria: A target for bacteria. Biochem. Pharmacol. 2015, 94, 173–185.
- Neish, A.S.; Rheinallt, M.J. Redox signaling mediates symbiosis between the gut microbiota and the intestine. Gut Microbes 2014, 5, 250–253.