Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paolo Baldo | + 1147 word(s) | 1147 | 2022-03-15 09:44:04 | | | |
| 2 | Nora Tang | Meta information modification | 1147 | 2022-03-29 09:56:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Baldo, P.; Orzetti, S.; Tommasi, F.; Bortolin, G. Genetic Therapy in Oncology. Encyclopedia. Available online: https://encyclopedia.pub/entry/21123 (accessed on 07 February 2026).
Baldo P, Orzetti S, Tommasi F, Bortolin G. Genetic Therapy in Oncology. Encyclopedia. Available at: https://encyclopedia.pub/entry/21123. Accessed February 07, 2026.
Baldo, Paolo, Sabrina Orzetti, Federica Tommasi, Giorgia Bortolin. "Genetic Therapy in Oncology" Encyclopedia, https://encyclopedia.pub/entry/21123 (accessed February 07, 2026).
Baldo, P., Orzetti, S., Tommasi, F., & Bortolin, G. (2022, March 28). Genetic Therapy in Oncology. In Encyclopedia. https://encyclopedia.pub/entry/21123
Baldo, Paolo, et al. "Genetic Therapy in Oncology." Encyclopedia. Web. 28 March, 2022.
Copy Citation
The impressive advances in the knowledge of biomarkers and molecular targets has enabled significant progress in drug therapy for crucial diseases such as cancer. Specific areas of pharmacology have contributed to these therapeutic outcomes—mainly targeted therapy, immunomodulatory therapy, and gene therapy.
genetic therapy
pharmacovigilance
oncology
1. Introduction
Genetic therapy is a promising and articulated research track in the oncology field. Although it is not currently commonly used in all hospitals/clinics, the scientific and technological concepts underlying it are highly refined and innovative (Figure 1a). Suffice it to say that one of the technologies (mRNA) that made vaccines against the SARS-CoV-2 virus -responsible for the current global pandemic- possible, also derived significantly from cancer research carried out in recent decades [1][2]. The pharmacotherapeutic classes concerning the field of oncology that can be included in the definition of gene therapy are oligonucleotides, oncolytic virus therapy, cell and tissue therapy, and specific vaccines for cancer [3][4]. However, it is not possible to categorically draw boundaries between the various definitions; for example, CAR-T therapy also acts on the immune system, and aptamers exert their therapeutic action as a function of their affinity with biocellular targets [5]. Similarly, immunotherapeutic agents exert their action by interacting with specific cellular target antigens. The utility in the classification of therapeutic agents is often functional, toward greater comprehensibility and schematic representation [6].
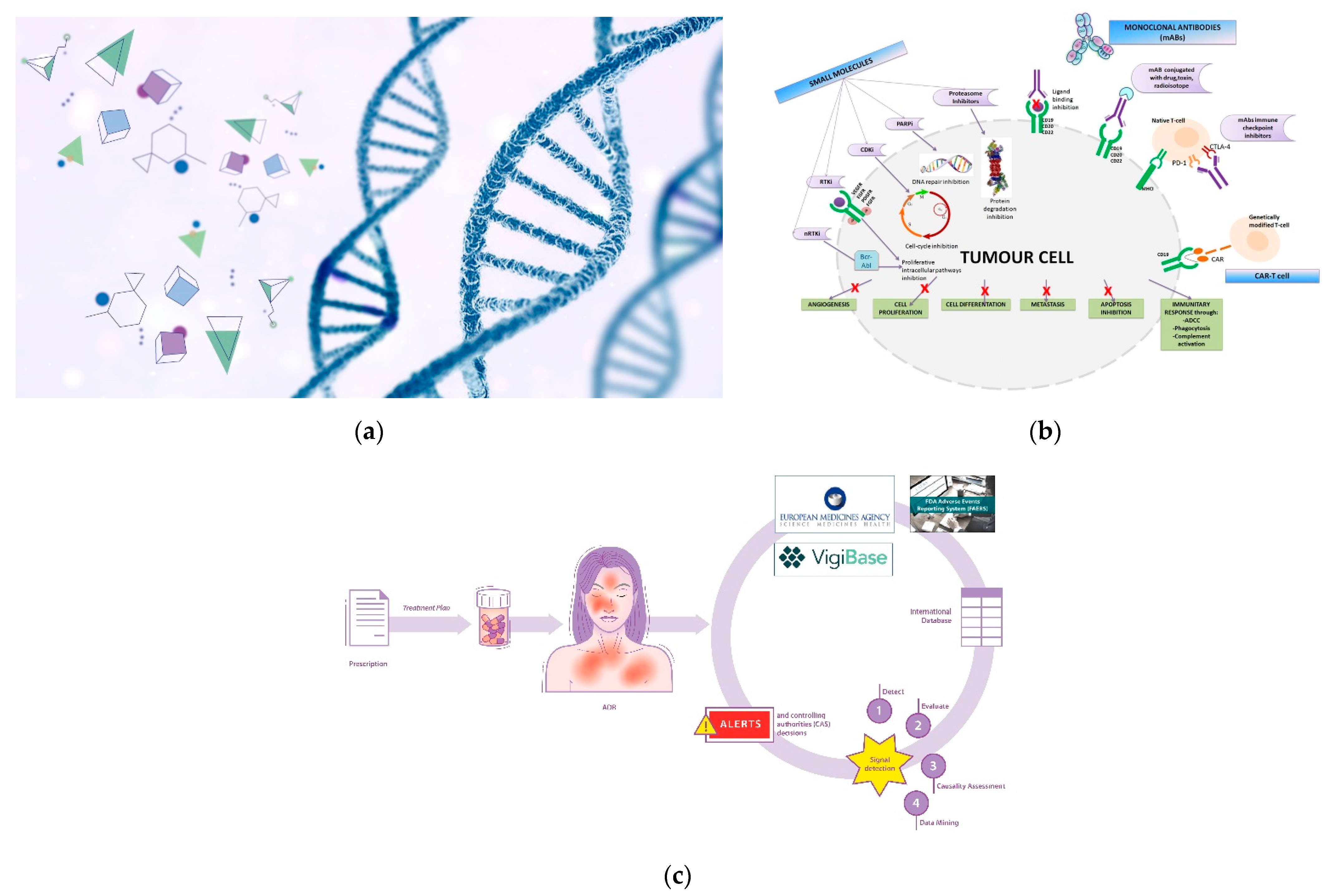
Figure 1. (a) Genetic therapy involves the interaction between pharmacological molecules and the genetic material of the cell; (b) Targeted therapy involve specific sites to interact with molecular targets in the cell. BCR-ABL: Breakpoint Cluster Region-Abelson gene; nRTKi: Non-Receptor Tyrosine Kinase inhibitors; RTKi: Receptor Tyrosine Kinase inhibitors; VEGFR: Vascular Endothelial Growth Factor Receptor; EGFR: Epidermal Growth Factor Receptor; PDGFR: Platelet-Derived Growth Factor Receptor; FGFR: Fibroblast Growth Factor Receptor; CDKi: Cyclin-Dependent Kinase inhibitors; PARPi: Poly Adenosine diphosphate-Ribose Polymerase inhibitors; MHCI: Major Histocompatibility Complex; PD-1: Programmed cell Death Protein 1; CTLA-4: Cytotoxic T-Lymphocyte Antigen 4; CAR: Chimeric Antigen Receptor; ADCC: Antibody-Dependent Cellular Cytotoxicity. (c) Pictorial rappresentation of the workflow of the International sistem of pharmacovigilance. Spontaneous reports of ADRs (adverse drug reactions) are collected from international databases (Vigibase, FAERS system and Eudravigilance) in order to generate alerts and implement post-marketing drug surveillance.
2. Oncolytic Viruses (OVs)
Viruses interact biologically with human cells in vivo, expressing selectivity for cancer cells and killing them. This is why they are then referred to as “oncolytic”. Although this type of approach is now included in the context of gene therapy against cancer, research in oncolytic viruses (OVs) has its origin in the early 1950s [7]. Although they belong to different families (Adenoviridae, Herpesviridae, Paramyxoviridae, Parvoviridae, Picornaviridae, Poxviridae, Reoviridae, Rhabdoviridae) [8], there are essentially three viral agents currently registered for therapeutic application, the first of which was Rigvir® in 2004 [9], while many agents are under investigation for use in diagnostic and therapeutic techniques in different types of cancer [10]. OVs are engineered to infect cancer cells, replicate, and cause cell lysis while sparing healthy cells. In addition to this mechanism of action, OVs contribute to the global response of the organism by expressing substances and antigens in the tumor microenvironment (MEV). They also contribute to an organic/biological reactivity that can be exploited for more accurate diagnosis by aiding in the use of imaging technologies (e.g., fluorescence, luminescence) [11][12][13].
3. Cell- and Tissue-Based Therapy for Cancer
Although this product category can be considered borderline in terms of immunotherapy (a sort of cell immunotherapy), it best represents innovation in the field of biotechnology and advanced therapy. Chimeric antigen receptor (CAR-T) cells and T cell redirecting bispecific T cell engager (BiTE) are approved for use in several forms of hematologic malignancies [14]. The main concept underlying the mechanism of action of this class of drugs is the redirection of T cell reactivity against specific tumor antigens. CAR-T cells are genetically engineered T cells with a chimeric antigen receptor [15][16]. The CAR is composed of an extracellular single-chain variable fragment (scFv), a domain that recognizes tumor-specific antigens and intracellular signaling targets. BiTEs are recombinant proteins consisting of two scFv fragments of separate antibodies, one to target a tumor-specific antigen and one to intercept and recruit active T cells. Recruited T cells are then redirected to kill cancer cells.
4. Cancer-Specific Vaccines
Although the therapeutic potential of vaccines in the treatment of various forms of cancer has yet to be attained, the technologies used for the rapid development of vaccines against the SARS-CoV-2 virus, particularly viral-vector and DNA/RNA-based technologies, derive from decades of scientific and laboratory research in the fight against cancer [17]. The success of prophylactic strategies against pathogens such as polio and smallpox viruses, or viral-driven cancers such as hepatitis B virus (HBV), which causes hepatocarcinoma, and human papilloma virus (HPV), which causes cervical cancer [18][19], suggests potential new perspectives for the development of “preventive” (or prophylactic) anticancer vaccines. Still, so far, research has not yielded satisfactory results for other forms of cancer.
In parallel, the class of anticancer vaccines, defined as therapeutic, includes agents that belong to several categories: cell-, peptide-, DNA- or RNA-, viral-vector, or bacterial-vector based vaccines [20][21]. To quickly understand the potential benefits expected of gene therapy through this category of agents, here must bear in mind that the goal of any therapeutic cancer vaccine is to increase and reactivate the body’s latent immune response, specifically that of non-active T cells in the tumor microenvironment, by stimulating dendritic cells (DCs), thus conferring the T cells with the property of being tumor-specific antigens (TAAs). Moreover, efficient delivery of vaccines is required through nanocarriers or specific adjuvant molecules or ligands that favor an effective interaction with the tumor microenvironment and, therefore, the release of therapeutic or cytotoxic agents to specific cellular targets.
5. Combination Therapies and Therapeutic Oligonucleotides
Several studies are currently evaluating combination therapy strategies involving various agents, immunomodulatory therapy, bi-specific T cell engagement, cell-tissue therapy with CAR-T, and the use of specific vaccines targeted to various forms of cancer. Recent reviews by Shi et al. [22] and Chaurasiya et al. [23] have presented comprehensive summaries of ongoing studies on oligonucleotides/aptamers. Since 1990, technologies involving antisense oligonucleotides (ASOs), aptamers, microRNA (miRNAs), small interfering RNAs (siRNAs), and catalytic DNA with enzymatic properties (DNAzymes) have been investigated to uncover new therapeutic possibilities and overcome some of the limitations in the curative potential of monoclonal antibodies (mAbs) and targeted therapy. These are considered promising approaches to the treatment of resistant types of cancer [24]. Therapeutic oligonucleotides/aptamers interact with target cells, causing RNA alterations/modifications by several different mechanisms (mRNA degradation, pre-mRNA splicing, or mRNA translation) [25]. Besides being a potential strategy for cancer therapy, the use of oligonucleotides holds promise for treating also many forms of illness due to genetic aberrations (for example, neurological and ocular diseases). They also deserve to be used clinically in diagnostic procedures, such as liquid biopsy [26][27][28]. Safety may be a major concern for this type of molecule, along with a lack of efficacy, potentially due to difficulty in delivering the active components to the site of action.
References
- Amanpour, S. The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism. Arch. Razi. Inst. 2021, 76, 1–6.
- Zhang, W.W.; Li, L.; Li, D.; Liu, J.; Li, X.; Li, W.; Xu, X.; Zhang, M.J.; Chandler, L.A.; Lin, H.; et al. The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic. Hum. Gene Ther. 2018, 29, 160–179.
- Liu, J.; Pandya, P.; Afshar, S. Therapeutic Advances in Oncology. Int. J. Mol. Sci. 2021, 22, 2008.
- Fu, Z.; Xiang, J. Aptamers, the Nucleic Acid Antibodies, in Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 2793.
- Crowther, M.D.; Svane, I.M.; Met, Ö. T-Cell Gene Therapy in Cancer Immunotherapy: Why It Is No Longer Just CARs on The Road. Cells 2020, 9, 1588.
- Bhatia, K.; Bhumika; Das, A. Combinatorial drug therapy in cancer-New insights. Life Sci. 2020, 258, 118134.
- Moore, A.E. Viruses with oncolytic properties and their adaptation to tumors. Ann. N. Y. Acad. Sci. 1952, 54, 945–952.
- Chianese, A.; Santella, B.; Ambrosino, A.; Stelitano, D.; Rinaldi, L.; Galdiero, M.; Zannella, C.; Franci, G. Oncolytic Viruses in Combination Therapeutic Approaches with Epigenetic Modulators: Past, Present, and Future Perspectives. Cancers 2021, 13, 2761.
- Alberts, P.; Tilgase, A.; Rasa, A.; Bandere, K.; Venskus, D. The advent of oncolytic virotherapy in oncology: The Rigvir® story. Eur. J. Pharmacol. 2018, 837, 117–126.
- Cao, G.D.; He, X.B.; Sun, Q.; Chen, S.; Wan, K.; Xu, X.; Feng, X.; Li, P.P.; Chen, B.; Xiong, M.M. The Oncolytic Virus in Cancer Diagnosis and Treatment. Front. Oncol 2020, 10, 1786.
- Yano, S.; Tazawa, H.; Kishimoto, H.; Kagawa, S.; Fujiwara, T.; Hoffman, R.M. Real-Time Fluorescence Image-Guided Oncolytic Virotherapy for Precise Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 879.
- Rojas, J.J.; Thorne, S.H. Theranostic potential of oncolytic vaccinia virus. Theranostics 2012, 2, 363–373.
- Chulpanova, D.S.; Solovyeva, V.V.; Kitaeva, K.V.; Dunham, S.P.; Khaiboullina, S.F.; Rizvanov, A.A. Recombinant Viruses for Cancer Therapy. Biomedicines 2018, 6, 94.
- Edeline, J.; Houot, R.M.; Marabelle, A.; Alcantara, M. CAR-T cells and BiTEs in solid tumors: Challenges and perspectives. J. Hematol. Oncol. 2021, 14, 65.
- June, C.H.; Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 2018, 379, 64–73.
- Locke, F.L.; Jacobson, C.A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42.
- Yu, W.L.; Hua, Z.C. Chimeric antigen receptor T-cell (CAR T) therapy for hematologic and solid malignancies: Efficacy and safety—a systematic review with meta-analysis. Cancers 2019, 11, 47.
- Cecco, S.; Muraro, E.; Giacomin, E.; Martorelli, D.; Lazzarini, R.; Baldo, P.; Dolcetti, R. Cancer vaccines in phase II/III clinical trials: State of the art and future perspectives. Curr. Cancer Drug Targets 2011, 11, 85–102.
- Donninger, H.; Li, C.; Yaddanapudi, K. Cancer Vaccines: Promising Therapeutics or an Unattainable Dream. Vaccines 2021, 9, 668.
- Hajj Hussein, I.; Chams, N.; Chams, S.; El Sayegh, S.; Badran, R.; Raad, M.; Gerges-Geagea, A.; Leone, A.; Jurjus, A. Vaccines Through Centuries: Major Cornerstones of Global Health. Front. Public Health 2015, 3, 269.
- Filin, I.Y.; Solovyeva, V.V.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A. Current Trends in Cancer Immunotherapy. Biomedicines 2020, 8, 621.
- Shi, T.; Song, X.; Wang, Y.; Liu, F.; Wei, J. Combining Oncolytic Viruses With Cancer Immunotherapy: Establishing a New Generation of Cancer Treatment. Front. Immunol. 2020, 11, 683.
- Chaurasiya, S.; Fong, Y.; Warner, S.G. Oncolytic Virotherapy for Cancer: Clinical Experience. Biomedicines 2021, 9, 419.
- Xiong, H.; Veedu, R.N.; Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021, 22, 3295.
- Adachi, H.; Hengesbach, M.; Yu, Y.T.; Morais, P. From Antisense RNA to RNA Modification: Therapeutic Potential of RNA-Based Technologies. Biomedicines 2021, 9, 550.
- Roy, D.; Pascher, A.; Juratli, M.A.; Sporn, J.C. The Potential of Aptamer-Mediated Liquid Biopsy for Early Detection of Cancer. Int. J. Mol. Sci. 2021, 22, 5601.
- Junjie, F.; Bo, L.; Jiaxu, Y.; Weilun, P.; Chunchen, L.; Tingting, L.; Huixian, L.; Lei, Z. Liquid biopsy: Application in Early Diagnosis and Monitoring of Cancer. Small Struct. 2020, 1, 2000063.
- Zhang, Y.; Lai, B.S.; Juhas, M. Recent Advances in Aptamer Discovery and Applications. Molecules 2019, 24, 941.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
935
Revisions:
2 times
(View History)
Update Date:
03 Nov 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




