Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prodromos Skenderidis | + 3149 word(s) | 3149 | 2022-03-22 02:19:11 | | | |
| 2 | Dean Liu | Meta information modification | 3149 | 2022-03-30 03:08:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Skenderidis, P. Goji Berry. Encyclopedia. Available online: https://encyclopedia.pub/entry/21116 (accessed on 08 February 2026).
Skenderidis P. Goji Berry. Encyclopedia. Available at: https://encyclopedia.pub/entry/21116. Accessed February 08, 2026.
Skenderidis, Prodromos. "Goji Berry" Encyclopedia, https://encyclopedia.pub/entry/21116 (accessed February 08, 2026).
Skenderidis, P. (2022, March 28). Goji Berry. In Encyclopedia. https://encyclopedia.pub/entry/21116
Skenderidis, Prodromos. "Goji Berry." Encyclopedia. Web. 28 March, 2022.
Copy Citation
Goji berry fruits demonstrated anti-oxidative properties that are associated with age-related diseases such as diabetes, atherosclerosis and antitumor and immunoregulatory activities. Bioactive secondary metabolites contained in fruit lead to positive effects for human vision, while other biochemicals contained in the root bark have shown hepatoprotective and inhibitory actions on the rennin/angiotensin system.
goji berry
functional foods
antioxidant
health
antibacterial
1. Introduction
Modern life-styles and dietary habits are the main cause of several human modern diseases such as diabetes, hepatitis and cardiovascular issues [1][2][3]. About 1/5 of the known plant species [4] that have participated in pharmaceutical studies cover a wide range of beneficial effects on human health, animal welfare and crop protection by enhancing human health against free radical damage [5][6][7][8][9][10][11][12][13]. The high concentrations of phytochemicals found in plants are accumulated mainly in their fruits and vegetables. Among beneficial phytochemicals, antioxidant compounds including phenolics, anthocyanins, carotenoids, and tocopherols may be used as a supplement for the human body by acting as natural antioxidants [14]. Thus, the consumption of fruit and vegetables has been linked with several health benefits, as a result of medicinal properties and high nutritional value [15] and is recommended by many scientists throughout the world [16]. In more detail, many studies mention the antioxidant and pharmacological activities of different plant extracts [17][18][19][20].
Among important plant species with significant biomedical issues is the Goji berry where fruit juice, roots and leaves contain ingredients that have a variety of bioactive properties [21][22][23][24].
2. Bioactivities
2.1. Antioxidant Activity
The most studied molecular mechanism of the oxidation of cellular components is that of lipid peroxidation. This is a circular feedback chain process, which, if started and not suspended in time, can oxidize all the biological material. DNA can tolerate a large number of different oxidative lesions, depending on the factors that cause them. Unlike oxidized proteins, which are usually fragmented and their amino acids reused, the oxidized DNA can be repaired in situ. Insufficient DNA repair can result in mutation and, ultimately, cell death by either necrosis or apoptosis.
The amino acids cysteine, methionine, tyrosine, phenylalanine, tryptophan and histidine are more susceptible to oxidative modifications. Relatively recent research results in this field commented on the fact that oxidative modifications of amino acid residues in proteins, besides the negative effects, may also play a positive role by participating in the redox signaling process.
Goji berries have been shown to possess antioxidant properties, neutralizing the oxidative action of free radicals and activating antioxidant mechanisms (Figure 1), such as an increase in superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GPx), catalase (CAT), and erythroid-derived 2-like 2 (Nrf2) expression of several antioxidant and cytoprotective enzymes [25]. Thus, L. barbarum extracts exhibited the binding of peroxide anion radicals and the subsequent reduction of their activity [26].
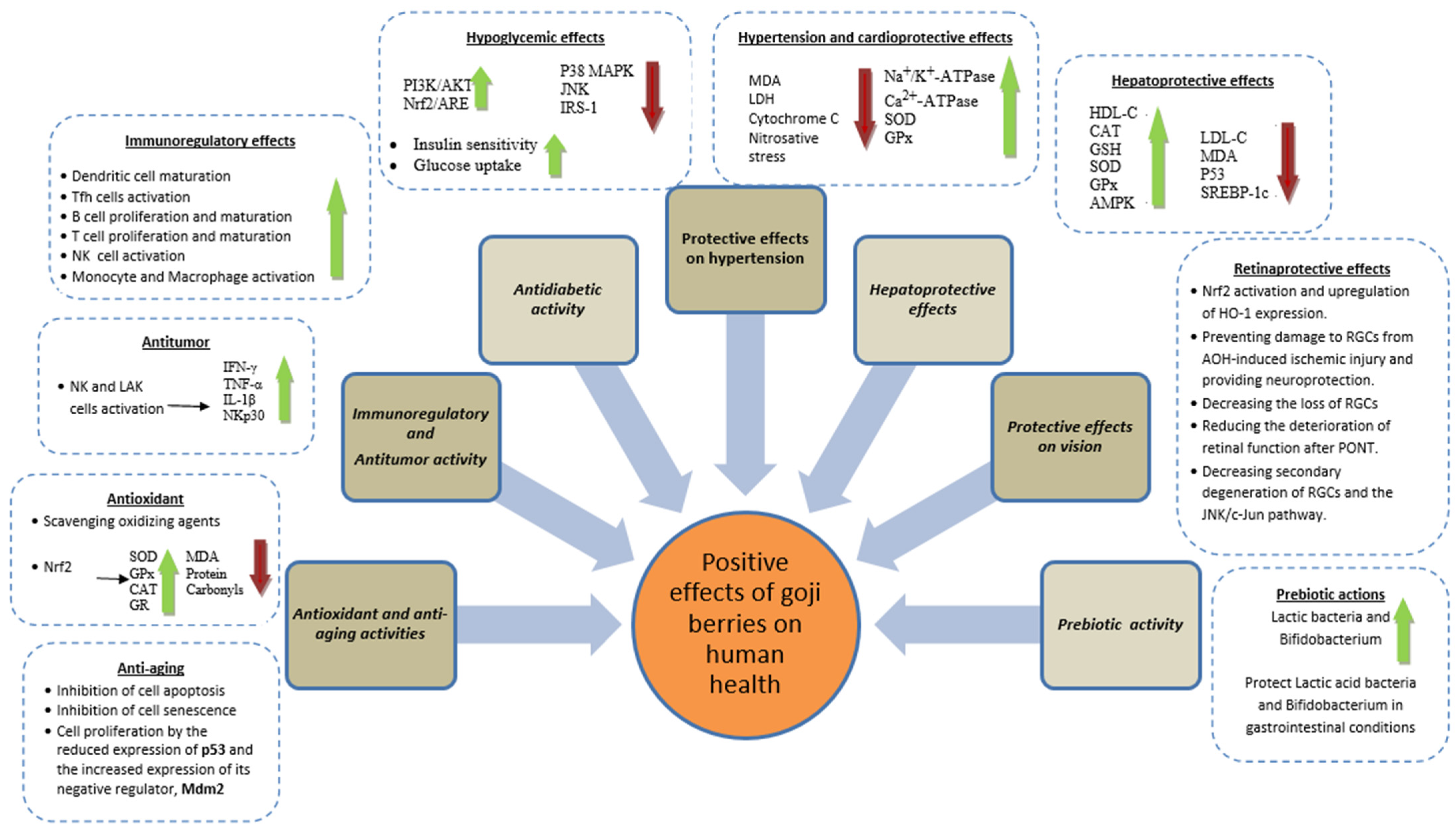
Figure 1. Health-promoting properties of goji berry fruits and extracts.
The protective effect on the inhibition of lipid peroxidation by goji berry extracts is probably due to the polyphenols of goji berry fruit (Figure 2) [27]. Caffeic acid, which is the main hydroxycinnamic acid in goji berries, not only has potent antioxidant effects but also has anti-inflammatory and anti-cancer effects, while recent studies have shown that caffeic acid in its free form or conjugated to other groups, such as quinic acid and sugars, has a protective effect against Alzheimer’s disease [28].
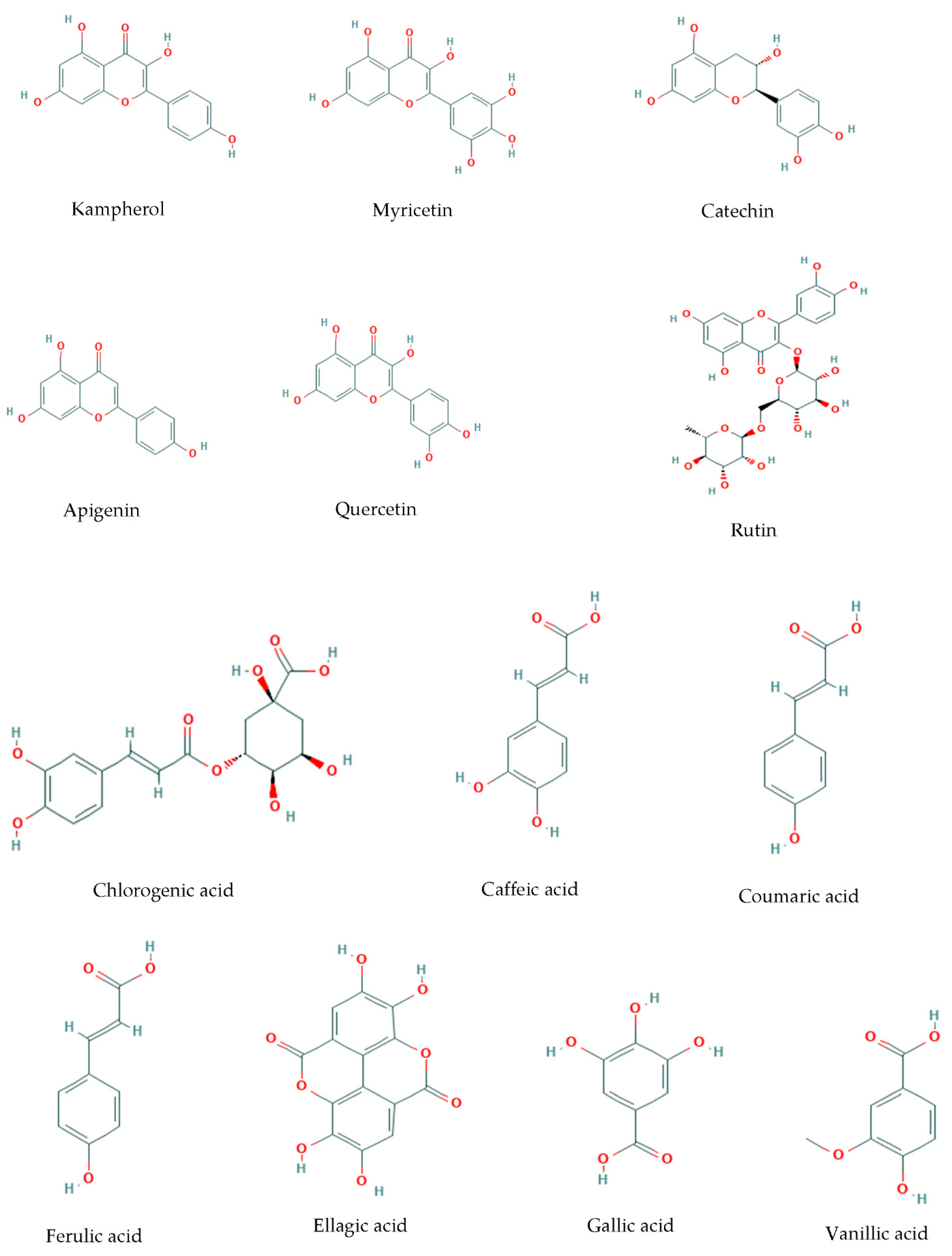
Figure 2. The chemical structure of the polyphenols contained in “Goji berries”.
Moreover, ethanol extract (70% w/v) of L. chinense protects hepatic cells against oxidative stress-induced cell damage by removing intracellular ROS, SOD recovery, CAT and glutathione action, reducing lipid oxidation, DNA destruction and protein carbonyl values [29]. Further, Changbo et al. [30] have shown that the administration of LBP in mice can reduce the oxidative stress caused after exercise in swimming, increasing the antioxidant enzymes SOD, CAT and GPx.
2.2. Antiaging Activity
Aging is defined as the accumulation of various deleterious changes in cells and tissues [31], especially for elderly people [32].
Several studies have shown that genetic and environmental factors regulate specific pathways involved in hormone signaling, nutritional signaling and the detection of mitochondrial and ROS signaling and genomic survival. It is a common belief that the accumulation of the effects of oxidative stress contributes to the aging process [33][34][35]. For this reason, many of the experimental aging models use the pouring of D-galactose into mouse or rat tissues for a period of 6–8 weeks as a toxin in order to produce free radicals [36]. According to Deng et al. [37], the addition on a daily basis of 100 mg LBP/kg to the diet of mice reduced serum advanced glycation end products (AGE), retrieving the memory pointer back to experimental animals, increasing superoxide dismutase levels in erythrocytes and finally helping them to restore kinetic activity.
The life cycle of Drosophila melanogaster (fruit-fly) has been used as an alternative model for aging studies. Based on this model, the addition of 16 mg LBP/kg shows a statistically significant increase of the average life span of male insects [38].
Furthermore, studies conducted on elderly mice have shown that the consumption of 200–500 mg/kg of LBP promotes oxidative stress reduction, as it reduces the oxidative stress markers associated with the aging process [39]. It has also been reported that LBP activates the antioxidative pathways Nrf2/ARE and Nrf2/HO-1 by activating antioxidants and detoxifying enzymes. One of these enzymes is heme oxygenase-1 (HO-1), which is regulated by the factor associated with the nuclear factor erythroid 2–related factor 2 (Nrf2) [40].
In vivo studies on Factor Nrf2 have shown that it plays an important role in the endogenous antioxidant system by regulating the expression of important antioxidant enzymes, such as oxygenase-1 (HO-1), SOD and CAT. In particular, in oxidative stress or exogenous (pharmacological) activation, Nrf2 moves into the cell nucleus and induces the expression of antioxidant enzymes by blocking the antioxidant response (ARE) [41]. It has also been commented that activation of PI3K/AKT/Nrf2 not only prevents the development of oxidative stress but also prevents metabolic glucose abnormalities such as the occurrence of insulin resistance. Activation of Nrf2 by LBP offers a new alternative therapeutic approach to the prevention of insulin resistance caused by a long-term high-fat diet [42].
The effect of ultraviolet radiation (UVB) causes skin damage by inducing oxidative and inflammatory lesions and thus causes aging and carcinogenicity of the skin. The protective effect of LBPs through the induction of Nrf2 is likely to exert a protective effect against the negative effect of ultraviolet radiation on the skin by binding to the active radicals and reducing DNA damage, resulting in the suppression of the ultraviolet-induced P38 MAP pathway. Based on the previous beneficial effects, LBP could potentially be used as an ingredient in products intended to protect the skin against oxidative damage from environmental conditions [43].
2.3. Antitumor and Immunoregulatory Activity
The defensive mechanisms of vertebrates are also known as the immune system. The immune system recognizes and destroys foreign invaders and toxic substances by a process known as an immune response. The molecule that causes the immune response is called an antigen. In addition, these mechanisms are involved in the body’s effort to remove aged or damaged cells, as well as destroying cancer cells, while sometimes they cause damage against the tissues of the organism itself.
The two main groups of cells in the immune system are the cells of the medullary line and the lymphocytes. Lymphocytes include B-lymphocytes and T-lymphocytes as well as a large granular cell, NK (or natural killer cells). The medullary cells consist of monocytes/macrophages, dendritic cells, neutrophils, eosinophils and basophils. B lymphocytes have an antibody molecule in their membrane, whereas T lymphocytes have an antigen-binding receptor in their membrane. When a B cell encounters an antigen, it quickly divides and differentiates into a B-cell memory and a B-cell effector or plasmid cell. Plasmocytes produce a large number of antibodies (antibody, Ab) or immunoglobulin (immunoglobulin, Ig) that act on the antigen and destroy it. T-lymphocytes, when they meet an antigen or tumor, secrete cytokines (growth factors), directly killing the infected target cell (CD8 killer T cells) and also activating B-cells to make antibody responses and macrophages to destroy microorganisms that either invaded the macrophage or were ingested by it (CD4 helper T cell). There are two types of immunity—humoral and cellular. Humoral immunity is mediated by antibodies produced by B cells and is the main defensive mechanism against extracellular microbes and their toxins, with secreted antibodies binding to them by inducing their elimination. Cellular immunity is mediated by T cells, with dendritic cells playing an important role against antigens.
2.4. Antidiabetic Activity
The number of patients with diabetes worldwide has quadrupled over the last 30 years, and it is the ninth leading cause of death. One in 11 adults today has type II diabetes, accounting for 90% of diabetes cases, and the prediction is that by 2050, one in three will suffer from diabetes. The majority of people suffering from diabetes are aged between 45 and 64.
Sugar, a common constituent of diet, is also a major factor often responsible for elevating the glucose level in diabetic patients [44]. Diabetes mellitus is a metabolic disease characterized by an increase in blood sugar (hyperglycemia) and a metabolic disorder of glucose (C6H12O6), either as a result of decreased insulin secretion or due to a decrease in the sensitivity of cells to insulin. Insulin is a hormone produced in the pancreas that forces the liver and muscle cells to absorb blood glucose and store it as glycogen for future body energy needs. In case the insulin concentration in the body is low or zero and glucose absorption cannot take place, the body begins to use fat as a source of energy by transporting lipids from adipose tissue to the liver [45]. Nowadays, there are known various types of diabetes. The main diabetes types are type I and type II. In general, diabetes is associated with the risk of serious health complications, including myocardial infarction, stroke, kidney failure, vision loss and premature death. So required care for diabetics is likely to be needed for many years [46].
The effect of the antidiabetic action of goji berry extracts has been investigated in various studies. Goji berries may have a positive effect on blood glucose control, as documented in relevant studies described in a study published by Silva et al. [47]. Furthermore, in a study completed by Wu et al. [48] feeding of type 2 diabetic mice with goji berry extract for 4 weeks showed a decrease in blood glucose levels by 35%. Moreover, Zhang et al. [49] suggested that the fraction LBPF4-OL of the LBP promotes lymphocyte proliferation secreting TNF-α and IL-1β. Luo et al. [50] also showed that L. barbarum extracts have hypoglycemic and hypolipidemic effects as well as strong antioxidant activity in rabbits with diabetes and hyperlipidemia from aloxane.
2.5. Hypertension and Heart Protective Effects
Hypertension is today one of the major public health problems due to its high incidence, its importance in cardiovascular disease and its correlation with a large number of health problems leading to death. Nearly one in two adults (about 103 million people) suffer from hypertension in the United States only [51]. Hypertension is influenced by factors such as genetics, lack of exercise and dietary intake of sodium, which is one of the most common causes of hypertension. It has been reported that the dietary sodium intake has been correlated with blood pressure, confirming the sensitivity of blood pressure to salt [52].
Regarding LBP’s protective positive effects on myocardial I/R damage Shao Ping and Pin-Ting [53] used in their study of Wistar adult male rats. In their study, it was presented that LBPs protected rat hearts from I/R injury via upregulation of heart Na+/K+-ATPase and inhibition of cardiomyocyte apoptosis concluding the cardioprotective effect of LBP stems caused by their antioxidant, anti-inflammatory and anti-apoptotic activities.
Prophylactic activity of LBP against cardiotoxic side effects of doxorubicin (DOX), which is a potent antitumor agent, has been also demonstrated in acute DOX-induced cardiotoxicity in rats [54][55] and beagle dogs [56]. Data of previous studies indicated that L. barbarum fruits and extracts may exert a potent protective effect on DOX-induced cardiomyocyte damage, mainly via antioxidative and free radical-scavenging pathways. Zhang et al. [57] in their study, related the anti-hypertensive effect of L. barbarum to down regulated expression of renal endothelial lncRNAs ONE in a rat model of salt-sensitive hypertension. In conclusion, their study commented that L. barbarum treatment can restore blood pressure to normal levels. At the same time, the expression of long noncoding RNA (lncRNA) was found to be reduced by the suppression of the antisense mRNA (sONE). Moreover, the improvement of endothelial nitric oxide synthase (eNOS) levels in the hypertensive model rats treated with L. barbarum compared with that receiving a high-salt diet was also observed. In addition, Guo et al. [58], using a meta-analysis of randomized controlled trials, presented that L. barbarum treatment significantly reduced fasting glucose concentrations while marginally reducing concentrations of total cholesterol and yielded no benefit in terms of bodyweight and blood pressure.
2.6. Hepatoprotective Activity
Alcohol use is the third leading risk factor contributing to the global burden of disease, after high blood pressure and tobacco smoking. According to a WHO report published in September 2018, alcohol causes 3 million annual deaths globally and accounts for 5.3% of all deaths. Despite the three above mentioned factors affecting liver Demori and Voci [59] commented that modern eating habits involving high-calorie diets that lead to obesity also can cause liver diseases such as hepatic steatosis. Chronic alcohol overdrinking (CAO) typically progresses through the stages of fatty liver or simple steatosis, alcoholic hepatitis and chronic hepatitis with hepatic fibrosis or cirrhosis [60].
The use of L. barbarum was originally proposed in traditional Chinese medicine for the treatment of liver diseases. Nowadays, studies done by Xiao et al. [61] have proved that feeding alcohol-induced liver injury rats with 300 mg/kg LBP for 30 days showed positive reverse effects, reducing liver injury, preventing the progression of alcohol-induced fatty liver and improving antioxidant function, in contrast with the ethanol group.
Pretreatment with 50 µg/mL LBP of rat normal hepatocyte line BRL-3A cells has shown a significant reduction of 24-hour ethanol-induced over expression of thioredoxin-interacting protein (TXNIP) increasing cellular apoptosis. Xiao et al. [62] also observed an activation of NOD-like receptor 3 (NLRP3) inflammasome and reduction of the antioxidant enzyme expression and ROS. Non-alcoholic fatty liver disease is an important factor in causing hepatocarcinoma and is associated with obesity, insulin resistance and metabolic syndrome. As mentioned previously, obesity leads to a decrease in insulin sensitivity (IR), a decrease in the antioxidant enzymes SOD, CAT and GSH-Px but also an increase in ROS, leading to liver dysfunction, hepatic steatosis and depletion of the hepatocyte population [59][63][64].
2.7. Eye and Vision Activity
Zeaxanthin and lutein are two common carotenoids found in plants and are constituents of the yellow macular pigment in human retina [65]. Biological functions of these macular pigments include the absorption of spectra. The function of these pigments is to absorb the blue light that can cause harm to the retina, but this chronic process of absorption may affect these macular pigments [66].
Glaucoma is the second most common cause of blindness and is a degenerative disease of retinal ganglion cells (RGCs) and the optic nerve and is expected to affect about 111.8 million people between 40 and 80 years by 2040 [67]. The most common types of glaucoma are primary open angle glaucoma (POAG) and primary angle closure glaucoma (PACG) [68]. The appearance of glaucoma caused mainly by the progressive disruption of RGC axonal transport or with retinal ischemia. Pathologically, glaucoma is characterized by the death of RGCs and increased intraocular pressure (IOP) [69]. Increased IOP is an important contributor to POAG. Elevation of IOP could cause many changes that are involved in the pathogenesis of glaucoma, such as oxidative stress, glutamate toxicity and ischemia [68].
The positive effects of goji berries on eye diseases such as glaucoma, cataract and rhinitis pigmentosa (RP) have been proposed by Chinese herbalists due to their high concentration of zeaxanthin and their esters, which are ready absorbed into serum, resulting in protection of the retina against free radicals and blue light damage. Leung et al. [70] reported that the levels of these two carotenoids in the serum and tissues of rhesus monkeys after feeding with L. barbarum fruits were significantly higher against the control group. Furthermore, clinical studies focused on L. barbarum as a therapy for retinal diseases in humans exist in the scientific literature. Chan et al. [71], in a study involving retinitis pigmentosa patients, showed that L. barbarum treatment can provide a neuroprotective effect for the retina and could help delay or minimize cone degeneration in RP. The positive effect of goji berries on glaucoma is due to the activation of the microglia at a moderate level resulting RGCs protection against IOP regulating important intracellular pathways that stimulate the body’s defense under stress situations.
2.8. Pre-Biotic Activity
The term probiotic originates from the Greek words pre + bios and has been used with many different meanings in recent decades. Initially, the term “probiotic” was used to describe compounds produced by a protozoan that stimulated the growth of another [72]. Finally, experts from the Food and Agriculture Organization/World Health Organization have identified probiotics as “living microorganisms, which when consumed in sufficient quantities as part of the feed contribute to the beneficial effect of the host” [73].
The use of probiotics extends back to a time before the discovery of microbes. Fermented dairy products were depicted in Egyptian hieroglyphics, and buffalo milk fermentation was traditionally used by Mongolian nomads to preserve their milk during their long journeys [74]. So far, many microorganisms such as fungi, yeasts, bacteria or their mixed combination have been considered or used as probiotics. The two main bacterial genera mainly referred to as probiotics are those of Lactobacillus and Bifidobacterium [75].
Historically, during the 1800s, the positive effect on human health of the consumption of fermented dairy products was observed by scientists. Although Louis Pasteur identified bacteria and yeasts that were responsible for the fermentation process did not associate these microbes with any apparent health effects. In 1905, Elie Metchnikoff, who had worked with Pasteur in the 1860s, observed that Bulgarian shepherd’s longevity was mainly due to the lactobacilli used for yogurt fermentation and the presence of these lactobacilli in the sheep intestine in Bulgaria not with the yogurt they consumed but with [76]. In particular, Metchnikoff, in his study, “The Prologue of Life” in 1908, assumed that lactic acid bacteria detected in Bulgarian yogurts, the so-called Balkan Bulgarian, later known as Lactobacillus bulgaricus (now called L. delbrueckii subsp. bulgaricus) and Streptococcus thermophilus, are responsible for enhancing the intestinal system by inhibiting microbial fermentation, resulting in a reduction in unwanted by-products, such as amines and ammonia. Thus, for the first time, Metchnikoff highlighted the importance of specific micro-organisms and their contribution to human health and longevity.
Prebiotics are components of non-digestible foods that effectively affect the host by favoring growth and/or bacterial activity in the large intestine [77][78].
2.9. Other Bioactivities
Additional bioactive effects of goji berries, such as skin protection and its synergistic potential within fertility treatment by inducing spermatogenesis, have been reported [25][79][80][81][82]. Studies carried out with phenolic compounds isolated from the fruits of L. barbarum showed that the extracts had a bactericidal effect against Gram positive and Gram-negative bacteria [10]. On the other hand, L. chinense leaf extracts were found to be more potent as antimicrobial agents than the fruit extracts, with the best microbiocidal activity exerted on Bacillus subtilis [83].
References
- Knight, K.; Badamgarav, E.; Henning, J.M.; Hasselblad, V.; Anacleto, P.; Gano, P.; Ofman, J.J.; Weingarten, S.R. A Systematic review of diabetes disease management programs. Am. J. Manag. Care 2005, 11, 242–250.
- Kannel, W.B. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979, 241, 2035–2038.
- Sowers, J.R.; Epstein, M.; Frohlich, E.D. Diabetes, hypertension, and cardiovascular disease. Hypertension 2001, 37, 1053–1059.
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542.
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42.
- Leontopoulos, S.; Skenderidis, P.; Kalorizou, H.; Petrotos, K. Bioactivity potential of polyphenolic compounds in human health and their effectiveness against various food borne and plant pathogens. A review. J. Food Biosyst. Eng. 2017, 7, 1–19.
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and extraction methods of polyphenolic compounds derived from pomegranate (Punica granatum) peels. A mini review. Processes 2021, 9, 236.
- Leontopoulos, S.; Skenderidis, P.; Vagelas, I.K. Potential use of polyphenolic compounds obtained from olive mill waste waters on plant pathogens and plant parasitic nematodes. In Plant Defence: Biological Control; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 137–177. ISBN 978-3-030-51034-3.
- Greathead, H. Plants and plant extracts for improving animal productivity. Proc. Nutr. Soc. 2003, 62, 279–290.
- Skenderidis, P.; Mitsagga, C.; Giavasis, I.; Petrotos, K.; Lampakis, D.; Leontopoulos, S.; Hadjichristodoulou, C.; Tsakalof, A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019, 13, 2017–2031.
- Leontopoulos, S.; Skenderidis, P.; Petrotos, K.; Giavasis, I. Corn silage supplemented with pomegranate (Punica granatum) and avocado (Persea americana) pulp and seed wastes for improvement of meat characteristics in poultry production. Molecules 2021, 26, 5901.
- Zengin, Z.B.; Meza, L.; Pal, S.K.; Grivas, P. Chemoimmunotherapy in urothelial cancer: Concurrent or sequential? Lancet Oncol. 2021, 22, 894–896.
- Negi, G.; Kumar, A.; Joshi, R.P.; Sharma, S.S. Oxidative stress and Nrf2 in the pathophysiology of diabetic neuropathy: Old perspective with a new angle. Biochem. Biophys. Res. Commun. 2011, 408, 1–5.
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337.
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40.
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023.
- Rocchetti, G.; Senizza, B.; Putnik, P.; Kovačević, D.B.; Barba, F.J.; Trevisan, M.; Lucini, L. Untargeted screening of the bound/free phenolic composition in tomato cultivars for industrial transformation. J. Sci. Food Agric. 2019, 99, 6173–6181.
- Rocchetti, G.; Lucini, L.; Corrado, G.; Colla, G.; Cardarelli, M.; De Pascale, S.; Rouphael, Y. Phytochemical profile, mineral content, and bioactive compounds in leaves of seed-propagated artichoke hybrid cultivars. Molecules 2020, 25, 3795.
- Skenderidis, P.; Mitsagga, C.; Lampakis, D.; Petrotos, K.; Giavasis, I. The effect of encapsulated powder of goji berry (Lycium barbarum) on growth and survival of probiotic bacteria. Microorganisms 2020, 8, 57.
- Skenderidis, P.; Leontopoulos, S.; Petrotos, K.; Giavasis, I. Vacuum microwave-assisted aqueous extraction of polyphenolic compounds from avocado (Persea Americana) solid waste. Sustainability 2021, 13, 2166.
- Amagase, H.; Sun, B.; Borek, C. Lycium barbarum (goji) juice improves in vivo antioxidant biomarkers in serum of healthy adults. Nutr. Res. 2009, 29, 19–25.
- Hsu, C.-H.; Nance, D.M.; Amagase, H. A meta-analysis of clinical improvements of general well-being by a standardized Lycium barbarum. J. Med. Food 2012, 15, 1006–1014.
- Yao, R.; Heinrich, M.; Zou, Y.; Reich, E.; Zhang, X.; Chen, Y.; Weckerle, C. Quality variation of goji (Fruits of Lycium spp.) in China: A comparative morphological and metabolomic analysis. Front. Pharmacol. 2018, 9, 151.
- Skenderidis, P.; Lampakis, D.; Giavasis, I.; Leontopoulos, S.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Chemical properties, fatty-acid composition, and antioxidant activity of goji berry (Lycium barbarum L. and Lycium chinense Mill.) Fruits. Antioxidants 2019, 8, 60.
- Cao, S.; Du, J.; Hei, Q. Lycium barbarum polysaccharide protects against neurotoxicity via the Nrf2-HO-1 pathway. Exp. Ther. Med. 2017, 14, 4919–4927.
- Ahmed, N.; Wang, M.; Shu, S. Effect of commercial Bacillus thuringiensis toxins on Tyrophagus putrescentiae (Schrank) fed on wolfberry (Lycium barbarum L.). Int. J. Acarol. 2016, 42, 1–6.
- Cui, B.; Liu, S.; Lin, X.; Wang, J.; Li, S.; Wang, Q.; Li, S. Effects of Lycium barbarum aqueous and ethanol extracts on high-fat-diet induced oxidative stress in rat liver tissue. Molecules 2011, 16, 9116–9128.
- Habtemariam, S. Protective effects of caffeic acid and the Alzheimer’s brain: An update. Mini-Rev. Med. Chem. 2017, 17, 667–674.
- Zhang, R.; Kang, K.A.; Piao, M.J.; Kim, K.C.; Kim, A.D.; Chae, S.; Park, J.S.; Youn, U.J.; Hyun, J.W. Cytoprotective effect of the fruits of Lycium chinense Miller against oxidative stress-induced hepatotoxicity. J. Ethnopharmacol. 2010, 130, 299–306.
- Changbo, D. Supplementation of Lycium barbarum polysaccharides protection of skeletal muscle from exercise-induced oxidant stress in mice. Afr. J. Pharm. Pharmacol. 2012, 6, 643–647.
- Bucheli, P.; Gao, Q.; Redgwell, R.; Karine, V.; Wang, J.; Zhang, W.; Nong, S.; Cao, B. Chapter 14 Wolfberry biomolecular and clinical aspects of Chinese. In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2013; pp. 1–17.
- Kaur, D.; Rasane, P.; Singh, J.; Kaur, S.; Kumar, V.; Mahato, D.K.; Dey, A.; Dhawan, K.; Kumar, S. Nutritional interventions for elderly and considerations for the development of geriatric Foods. Curr. Aging Sci. 2019, 12, 15–27.
- Li, X.; Zhou, A. Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polym. J. 2007, 43, 488–497.
- Yi, R.; Liu, X.-M.; Dong, Q. A study of Lycium barbarum polysaccharides (LBP) extraction technology and its anti-aging effect. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 171–174.
- Xia, G.; Xin, N.; Liu, W.; Yao, H.; Hou, Y.; Qi, J. Inhibitory effect of Lycium barbarum polysaccharides on cell apoptosis and senescence is potentially mediated by the p53 signaling pathway. Mol. Med. Rep. 2014, 9, 1237–1241.
- Ho, S.-C.; Liu, J.-H.; Wu, R.-Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18.
- Deng, H.-B.; Cui, D.-P.; Jiang, J.-M.; Feng, Y.-C.; Cai, N.-S.; Li, D.-D. Inhibiting effects of Achyranthes bidentata polysaccharide and Lycium barbarum polysaccharide on non-enzyme glycation in D-galactose induced mouse aging model. Biomed. Environ. Sci. 2003, 16, 267–275.
- Wang, Y.; Zhao, H.; Sheng, X.; Gambino, P.E.; Costello, B.; Bojanowski, K. Protective effect of Fructus lycii polysaccharides against time and hyperthermia-induced damage in cultured seminiferous epithelium. J. Ethnopharmacol. 2002, 82, 169–175.
- Ji, L.L. Antioxidant signaling in skeletal muscle: A brief review. Exp. Gerontol. 2007, 42, 582–593.
- Ma, Q. Role of Nrf2 in Oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426.
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE Pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J. Diabetes Res. 2017, 2017, 4826724.
- Yang, P.; Li, D.; Jin, S.; Ding, J.; Guo, J.; Shi, W.; Wang, C. Stimuli-responsive biodegradable poly (methacrylic acid) based nano-capsules for ultrasound traced and triggered drug delivery system. Biomaterials 2014, 35, 2079–2088.
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic. Res. 2017, 51, 200–210.
- Singh, J.; Rasane, P.; Kaur, S.; Kumar, V.; Dhawan, K.; Mahato, D.K.; Malhotra, S.; Sarma, C.; Kaur, D.; Bhattacharya, J. Nutritional interventions and considerations for the development of low calorie or sugar free foods. Curr. Diabetes Rev. 2020, 16, 301–312.
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98.
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the Year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053.
- Silva, C.; Alves, B.; Azzalis, L.; Junqueira, V.; Fonseca, R.; Fonseca, A.; Fonseca, F. Goji Berry (Lycium Barbarum) in the treatment of diabetes melitus: A systematic review. Food Res. 2017, 1, 221–224.
- Wu, H.; Guo, H.; Zhao, R. Effect of Lycium barbarum Polysaccharide on the improvement of antioxidant ability and DNA damage in NIDDM Rats. Yakugaku Zasshi 2006, 126, 365–371.
- Zhang, M.; Tang, X.; Wang, F.; Zhang, Q.; Zhang, Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int. J. Biol. Macromol. 2013, 61, 270–275.
- Luo, Q.; Cai, Y.; Yan, J.; Sun, M.; Corke, H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004, 76, 137–149.
- Finegold, J.A.; Asaria, P.; Francis, D.P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013, 168, 934–945.
- Levy, D.; Ehret, G.B.; Rice, K.; Verwoert, G.C.; Launer, L.J.; Dehghan, A.; Glazer, N.L.; Morrison, A.C.; Johnson, A.D.; Aspelund, T.; et al. Genome-wide association study of blood pressure and hypertension. Nat. Genet. 2009, 41, 677–687.
- Lu, S.-P.; Zhao, P.-T. Chemical characterization of Lycium barbarum polysaccharides and their reducing myocardial injury in ischemia/reperfusion of rat heart. Int. J. Biol. Macromol. 2010, 47, 681–684.
- Xin, Y.-F.; Zhou, G.-L.; Deng, Z.-Y.; Chen, Y.-X.; Wu, Y.-G.; Xu, P.-S.; Xuan, Y.-X. Protective effect of Lycium barbarum on doxorubicin-induced cardiotoxicity. Phytother. Res. 2007, 21, 1020–1024.
- Xin, Y.-F.; Wan, L.-L.; Peng, J.-L.; Guo, C. Alleviation of the acute doxorubicin-induced cardiotoxicity by Lycium barbarum polysaccharides through the suppression of oxidative stress. Food Chem. Toxicol. 2011, 49, 259–264.
- Xin, Y.; Zhang, S.; Gu, L.; Liu, S.; Gao, H.; You, Z.; Zhou, G.; Wen, L.; Yu, J.; Xuan, Y. Electrocardiographic and Biochemical evidence for the cardioprotective effect of antioxidants in acute doxorubicin-induced cardiotoxicity in the beagle dogs. Biol. Pharm. Bull. 2011, 34, 1523–1526.
- Zhang, X.; Yang, X.; Lin, Y.; Suo, M.; Gong, L.; Chen, J.; Hui, R. Anti-hypertensive effect of Lycium barbarum L. with down-regulated expression of renal endothelial lncRNA sONE in a rat model of salt-sensitive hypertension. Int. J. Clin. Exp. Pathol. 2015, 8, 6981–6987.
- Guo, X.F.; Li, Z.H.; Cai, H.; Li, D. The Effects of Lycium Barbarum L. (L. Barbarum) on cardiometabolic risk factors: A me-ta-analysis of randomized controlled trials. Food Funct. 2017, 8, 1741–1748.
- Demori, I.; Voci, A.; Fugassa, E.; Burlando, B. Combined effects of high-fat diet and ethanol induce oxidative stress in rat liver. Alcohol 2006, 40, 185–191.
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. (Aust.) 2013, 28, 77–84.
- Xiao, J.; Wang, J.; Xing, F.; Han, T.; Jiao, R.; Liong, E.C.; Fung, M.-L.; So, K.-F.; Tipoe, G.L. Zeaxanthin Dipalmitate therapeutically improves hepatic functions in an alcoholic fatty liver disease model through modulating MAPK pathway. PLoS ONE 2014, 9, e95214.
- Xiao, J.; Zhu, Y.; Liu, Y.; Tipoe, G.L.; Xing, F.; So, K.-F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 2014, 69, 73–78.
- Assy, N.; Kaita, K.; Mymin, D.; Levy, C.; Rosser, B.; Minuk, G. Fatty infiltration of liver in hyperlipidemic patients. Am. J. Dig. Dis. Sci. 2000, 45, 1929–1934.
- Lee, Y.M.; Choi, J.S.; Kim, M.H.; Jung, M.H.; Lee, Y.S.; Song, J. Effects of dietary genistein on hepatic lipid metabolism and mitochondrial function in mice fed high-fat diets. Nutrition 2006, 22, 956–964.
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of Iutein and Zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171.
- Taylor, H.R.; West, S.; Muñoz, B.; Rosenthal, F.S.; Bressler, S.B.; Bressler, N.M. The Long-term effects of visible light on the eye. Arch. Ophthalmol. 1992, 110, 99–104.
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090.
- Quigley, H.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267.
- Prasanna, G.; Hulet, C.; Desai, D.; Krishnamoorthy, R.R.; Narayan, S.; Brun, A.-M.; Suburo, A.M.; Yorio, T. Effect of elevated intraocular pressure on endothelin-1 in a rat model of glaucoma. Pharmacol. Res. 2005, 51, 41–50.
- Leung, I.; Tso, M.; Li, W.; Lam, T. Absorption and tissue distribution of Zeaxanthin and Iutein in rhesus monkeys after taking Fructus lycii (Gou Qi Zi) extract. Investig. Ophthalmol. Vis. Sci. 2001, 42, 466.
- Chan, H.H.-L.; Lam, C.H.-I.; Choi, K.-Y.; Li, S.Z.-C.; Lakshmanan, Y.; Yu, W.-Y.; Chang, R.C.-C.; Lai, J.S.-M.; So, K.-F. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J. Ethnopharmacol. 2019, 236, 336–344.
- Lilly, D.M.; Stillwell, R.H. Probiotics: Growth-promoting factors produced by microorganisms. Science 1965, 147, 747–748.
- FAO/WHO. Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation; Food and Nutrition Paper; FAO: Rome, Italy, 2001; Volume 85.
- Guo, L.; Li, T.; Tang, Y.; Yang, L.; Huo, G. Probiotic properties of Enterococcus strains isolated from traditional naturally fermented cream in China. Microb. Biotechnol. 2015, 9, 737–745.
- Ohimain, E.I.; Ofongo, R.T.S. The Effect of probiotic and prebiotic feed supplementation on chicken health and gut microflora: A Review. Int. J. Anim. Vet. Adv. 2012, 4, 135–143.
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412.
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G.; et al. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19.
- Trowell, H.; Southgate, D.A.T.; Wolever, T.M.S.; Leeds, A.R.; Gassull, M.A.; Jenkins, D.J.A. Dietary fibre redefined. Lancet 1976, 307, 967.
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Konstantinos, P.; Hadjichristodoulou, C. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257.
- Reeve, V.E.; Allanson, M.; Arun, S.J.; Domanski, D.; Painter, N. Mice drinking goji berry juice (Lycium barbarum) are protected from UV radiation-induced skin damage via antioxidant pathways. Photochem. Photobiol. Sci. 2010, 9, 601–607.
- Shi, G.-J.; Zheng, J.; Wu, J.; Qiao, H.-Q.; Chang, Q.; Niu, Y.; Sun, T.; Li, Y.-X.; Yu, J.-Q. Beneficial effects of Lycium barbarum polysaccharide on spermatogenesis by improving antioxidant activity and inhibiting apoptosis in streptozotocin-induced diabetic male mice. Food Funct. 2017, 8, 1215–1226.
- Vidović, B.B.; Milinčić, D.D.; Marčetić, M.D.; Djuriš, J.D.; Ilić, T.D.; Kostić, A.Ž.; Pešić, M.B. Health benefits and applications of goji berries in functional food products development: A review. Antioxidants 2022, 11, 248.
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.-M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
30 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




