Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aref Zayed | + 1993 word(s) | 1993 | 2022-03-17 08:34:21 | | | |
| 2 | Conner Chen | -16 word(s) | 1977 | 2022-03-28 09:59:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zayed, A. High Performance Liquid Chromatography with Fluorescence Detection Methods. Encyclopedia. Available online: https://encyclopedia.pub/entry/21072 (accessed on 04 March 2026).
Zayed A. High Performance Liquid Chromatography with Fluorescence Detection Methods. Encyclopedia. Available at: https://encyclopedia.pub/entry/21072. Accessed March 04, 2026.
Zayed, Aref. "High Performance Liquid Chromatography with Fluorescence Detection Methods" Encyclopedia, https://encyclopedia.pub/entry/21072 (accessed March 04, 2026).
Zayed, A. (2022, March 25). High Performance Liquid Chromatography with Fluorescence Detection Methods. In Encyclopedia. https://encyclopedia.pub/entry/21072
Zayed, Aref. "High Performance Liquid Chromatography with Fluorescence Detection Methods." Encyclopedia. Web. 25 March, 2022.
Copy Citation
Steroids are compounds widely available in nature and synthesized for therapeutic and medical purposes. Although several analytical techniques are available for the quantification of steroids, their analysis is challenging due to their low levels and complex matrices of the samples. The efficiency and quick separation of the high performance liquid chromatography (HPLC) combined with the sensitivity, selectivity, simplicity, and cost-efficiency of fluorescence, make HPLC coupled to fluorescence detection (HPLC-FLD) an ideal tool for routine measurement and detection of steroids.
steroids
HPLC
Fluorescence
Quantification
Clinical
Environmental
Food
Derivitisation
Sample preparation
1. Introduction
Steroids are biologically active molecules that are available in many natural sources such as, animals, plants and fungi, in addition to being manufactured as therapeutics. Steroids share a common four-fused ring core consisting of 17 carbon atoms and vary according to functional groups attached to the core or side chains. Naturally secreted steroids are classified into steroid hormones (sex hormones, mineralo- and glucocorticoids, and anabolic steroids) and cholesterol. These compounds travel the bloodstream bound specifically or non-specifically to plasma proteins, such as albumin and binding globulins [1]. Steroids are key players in many biological, physiological, and pathological events, which are mediated after binding to their cognate tissue receptors. Most prominently, natural steroids play major roles in metabolism, signaling, immunity, reproduction, and salt-retaining activity. On the other hand, many synthetic steroids have been manufactured to mimic natural steroidal activity and have been approved to treat a wide variety of diseases including anti-inflammatory; arthritis, autoimmune disease, asthma and chronic obstructive pulmonary diseases [2][3].
Because steroids can produce biological action at very low concentrations, analytical methods must be very sensitive and precise particularly when quantifying steroid levels in biosamples. Due to the complex nature matrices of most steroid samples, the methods should also be selective to reliably quantify target steroids and resolve them from other similar endogenous compounds and interferences, and to allow for simpler extraction and pre-treatment prior to analysis. This is especially important since sample pre-treatment may lead to the loss of analyte. Steroids possess a naturally lipophilic cyclopentanoperhydrophenanthrene core (Figure 1), although their hydrophilicity is increased by reduction reactions during metabolism. Owing to this hydrophobicity, organic solvents can be used in sample preparation to extract steroids from aqueous media [4].
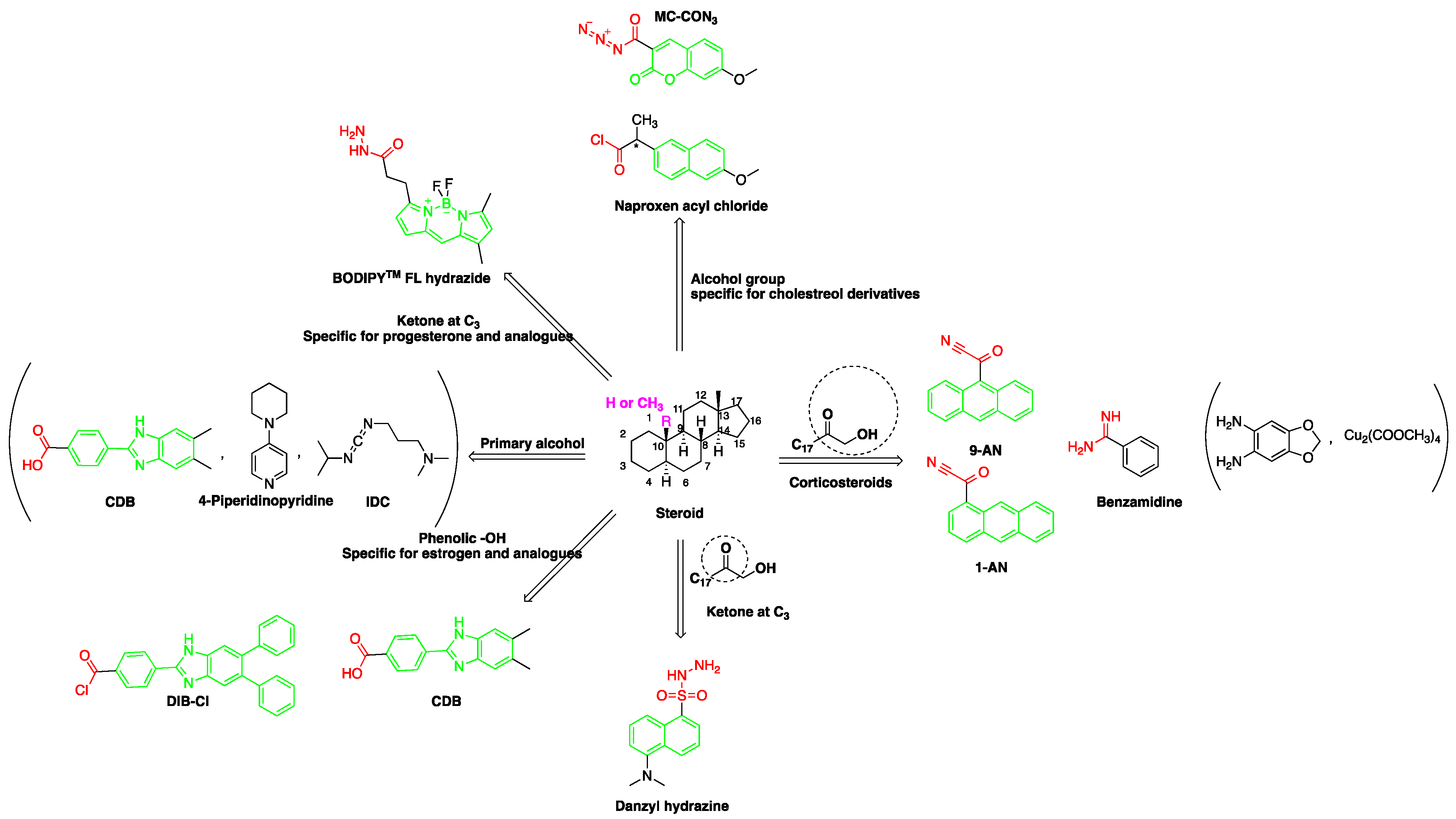
Figure 1. Representation of fluorescent derivatizing agents used in steroid analysis by the high performance liquid chromatography coupled to fluorescence detection (HPLC-FLD).
Commonly used methods in the analysis of steroids include immuno-assays [5][6], gas chromatography-mass spectrometry (GC-MS) [7], high-performance liquid chromatography mass spectrometry (HPLC-MS or MS/MS) [4][8], capillary electrophoresis (CE) [9], and HPLC coupled with UV [10][11][12][13] or fluorescence detection (FLD) [14]. Mass spectrometry (MS) and FLD are relevant to steroid analysis due to their inherited high sensitivity detection and low detection limit. Although both usually require incorporating a derivatization step due to weak ionization of steroids in MS and lack of fluorophores, FLD is considered a simpler and low capital cost technique.
2. HPLC-FLD Methods
HPLC is commonly employed in the analysis of steroids as it provides an excellent separation and quantification tool. Regardless of the compound class, separation using the reversed phase mode is the method of choice in HPLC. Octadecyl silica (ODS or C18) columns are commonly used as the stationary phase in reversed phase-HPLC. Other materials, such as C8, C2, phenyl, amino, and cyano phases can also be used to provide different degrees of selectivity. Selectivity depends also on the composition of the mobile phase. Methanol or acetonitrile are commonly used in combination with various percentages of water to prepare the mobile phase which can be employed in the isocratic or gradient mode. Column temperature, pH of the mobile phase and its modifiers are other parameters that can be used to optimize steroids separation. Using narrow and short columns can successfully decrease the amount of solvent needed and analysis time [4].
Prior to injecting steroid-containing samples on the HPLC, a sample preparation method is commonly employed to produce the required selectivity and sensitivity. Sample pretreatment is carried out through a process of sequential steps depending on the extracted steroid and sample type/matrix. For example, biological samples are often prepared by protein precipitation (PP) and enzyme hydrolysis in order to decrease the interferences from undesired compounds such as plasma proteins and to remove the polar groups added to steroids during metabolism. In addition to PP [9], solid phase extraction (SPE) [15][16] and liquid–liquid extraction (LLE) [17][18] are commonly used extraction techniques for the analysis of steroids. Due to the continuous need for faster and simpler sample preparation procedures, other methods such as solid phase microextraction (SPME) [19], liquid-phase microextraction (LPME) [18], turbulent flow chromatography (TFC) [20], dispersive liquid–liquid microextraction (DLLME) [18], molecularly imprinted solid-phase extraction (MISPE) [21][22], restricted access material (RAM) [4][9] are also used.
HPLC-FLD detection can be carried out through direct or indirect methods, depending on the analyte’s need for extraction or/and derivatization (Figure 2). Researchers seek to develop simple methods that do not require extraction or derivatization. For example, estrogens that are native fluorescents, and certain synthetic steroids, such as the anabolic steroid trenbolone, can fluoresce and, therefore, can be quantified without derivatization. Non-native steroids and non-aromatic compounds, on the other hand, show no fluorescence characteristics and must therefore be derivatized (labeled with fluorescent moieties) prior to analysis (Figure 2).
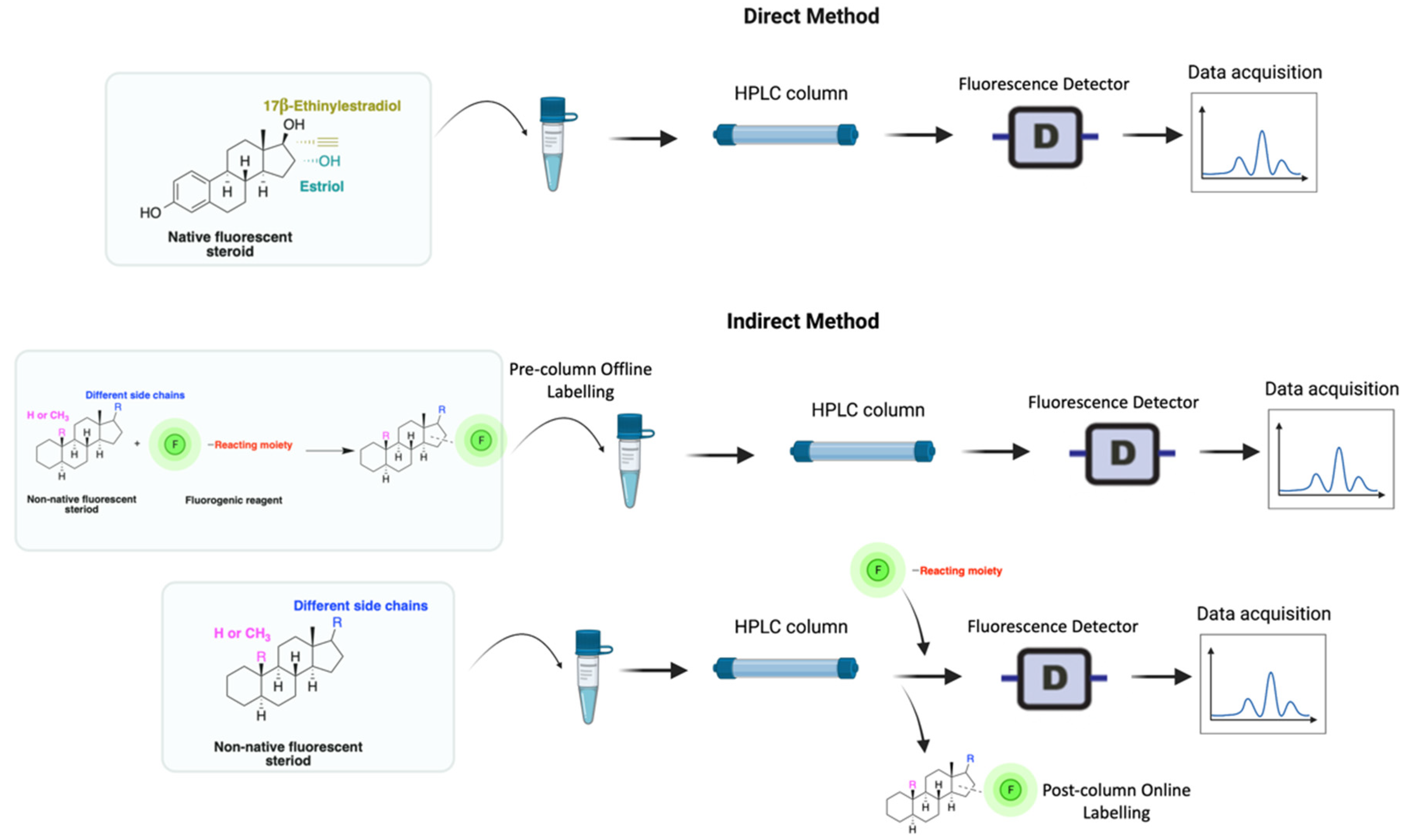
Figure 2. Derivatization using fluorophore-containing reagents for steroids analysis by HPLC-FLD.
A good derivatization procedure should be simple with high yield and minimum side products and is performed under mild conditions to avoid decomposition of the target steroid [4]. Derivatization enhances the detection of steroids and can be done by either pre-column offline labeling or post-column online labeling (Figure 2), which ideally must be rapid. These procedures can, however, be accelerated by modifying the reaction’s temperature and pH or incorporating a catalyst. Figure 1 is a representation for the most common fluorescent derivatizing pathways and their targets in steroid analysis. There are several classes of fluorescent derivatizing agents including anthracenes, coumarins and phenanthrenes. The derivatization process depends on functionalization of specific groups that are targeted in steroids structure as well as the type of derivatization agent used. For example, steroids with primary alcohol moiety, e.g., cortisone, can be targeted with a fluorescent agent in a selective reaction thus differentiating them from steroids containing secondary and tertiary alcohol groups such as prednisolone. Similarly, other steroids, such as estrogens, have specific phenolic groups, which can also be targeted by other agents. The various derivatization procedures reported in the literature used for steroid detection by HPLC-FLD including the reagents employed and conditions used in each procedure are summarized in Table 1.
Table 1. Derivatization reagents and conditions used in steroid quantification by HPLC-FLD.
| Reagent | Target Steroid | Sample Preparation and Derivatization Conditions | Reference |
|---|---|---|---|
p-Nitrobenzoyl chloride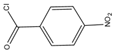 |
Estrogens | Sample was extracted by SPE C18. Residue reacted with p-nitrobenzoyl chloride at 25 °C, 30 min |
Mao et al. 2004 [23] |
1-Anthroyl nitrile (1-AN)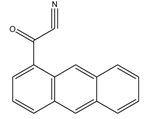 |
18-Oxygenated corticosteroids, 18-hydroxycortisol, 18-hydroxycortisone and 18-oxocortisol Pregnenolone and C21 steroids; especially corticoids | Sample was extracted by SPE or LLE with a mixture of diethyl ether and dichloromethane. Extract reacted with 1-anthroyl nitrile in acetonitrile at room temperature for 10 min. |
Kurosawa et al. 1995 [24] Shimada et al. 1996 [25] Shimada et al. 1991 [26] |
9-Anthroyl nitrile (9-AN)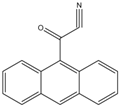 |
Glucocorticoids; F and E, Hydroxysteroids Corticosteroids |
Sample was extracted by LLE or SPE. Extracts reacted with 9-AN in a mixture of quinuclidine and triethylamine for 30 min at RT. |
Glowka et al. 2009 [27] Goto et al. 1983 [28] Shibata et al. 1997 [29] Haegele et al. 1991 [30] Kosicka et al. 2018 [31] Neufeld et al. 1998 [32] Shimada et al. 1991 [26] |
2-(11H-Benzo[a]carbazole-11-yl) ethyl carbonochloridate (BCEC-Cl)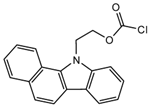 |
E1, E2, E3, BPA, NP, OP | Sample was extracted by DLLME. Extracts were added to BCEC-Cl in a NaHCO3 buffer at pH 10; it was shaken for 10 s, and then allowed to stand for 14 min at 43 °C. |
Wu et al. 2015 [33] |
| 9-Phenanthrene boronic acid 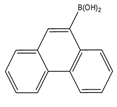 |
Brassinosteroids | Sample was extracted by LLE. Extracts reacted with 9-phenanthrene-boronic acid in a mixture of pyridine and acetonitrile for 10 min at 70 °C. |
Gamoh and Takatsuto. 1989 [34] |
| 10-Ethyl-acridone- 2-sulfonyl chloride (EASC) 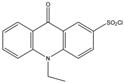 |
Estrogens and Biogenic Amines (aliphatic amines) |
Sample was extracted by LLE or SPE. Extracts reacted with EASC in anhydrous acetonitrile and NaHCO3 buffer pH (10.2), for 5 min at 60 °C. |
Zhang et al. 2012 [35] |
Sulfuric acid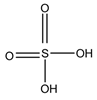 |
F and Corticosterone |
Sample was extracted by SPE or LLE. Extracts reacted with sulfuric acid in ethanol; it was cooled in crushed ice for 15 min in the dark. |
Nozaki et al. 1991 [36] Nozaki et al. 1992 [37] Sudo et al. 1990 [38]Gao et al. 2010 [39] |
7-Methoxycoumarin-3-carbonyl azide (MC-CON3)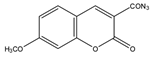 |
7α-hydroxycholesterol | Sample was extracted by LLE and purified by normal-phase SPE. Extracts reacted with MC-CON3 in ethyl acetate (AcOEt) for 40 min at 140 °C. |
Saisho et al. 1998 [40] |
2-(4-Carboxyphenyl)-5,6-dimethylbenzimidazole(CDB)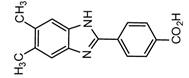 |
Corticosteroids, BPA and alkylphenols | Sample was extracted by LLE. 0.02% w/v CDB solution, 2.0% w/v 1-isopropyl-3-(3-dimethylaminopropyl) carbodiimide perchlorate (IDC) solution, and 0.01% w/v (10 mg/mL) 4-piperidinopyridine, for 60 min at 40 °C. |
Katayama et al. 1991 [41] Katayama et al. 1992 [41] Katayama et al. 2001 [42] |
Benzamidine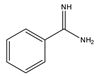 |
17-hydroxycorticosteroids | Sample was extracted by LLE. Extracts reacted with benzamidine in a basic medium of sodium hydroxide solution, with a mixture of propanol and water for 5 min at 95 °C. |
Seki et al. 1984 [43] |
| 4-(4,5-Diphenyl-1H- imidazol-2-yl) benzoyl chloride (DIB-Cl) 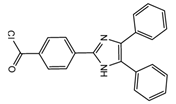 |
BPA | Sample was extracted by LLE or SPE. Extracts reacted with DIB-Cl in a mixture of acetonitrile and triethylamine for 20 min at RT. |
Sun et al. 2004 [44] Kuroda et al. 2003 [45] Sun et al. 2002 [46] |
4-(4,5-Diphenyl-1H-imidazol-2-yl) iodobenzene (DIB-I)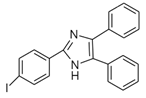 |
EE | Sample was extracted by the SPE disc method (C18 SPE disk). A total of 50 μL of the extracts reacted with 50 μL of DIB-I (3.0 mM), in a mixture of 50 μL of solution containing 0.2 mM of PdCl2 and 0.3 mM of CuI, and 50 μL of DIPEA (3.0 mM). The vial contents were deoxygenated by N2 purge for 10 sec, heated at 100 °C for 40 min, cooled, then filtered through a 0.45-μm membrane filter before injection into the HPLC-FLD system. |
Ali et al. 2020 [47] |
1,2-Diamino-4,5-methylenedioxybenzene (DMB)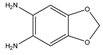 |
α-Dicarbonyl compounds; prednisolone, PN and 21-hydroxycorticosteroids | Sample was extracted by LLE. Extracts reacted with DMB for 40 min at 60 °C. |
Yamaguchi et al. 1991 [48] Yoshitake et al. 1989 [49] Yamaguchi et al. 1989 [50] |
2-(4-Carboxyphenyl)-5,6-dimethylbenzimidazole (BODIPY FL hydrazide)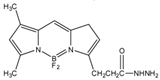 |
Aldehydes and ketones; progesterone, 17-hydroxyprogesterone, and other 3-keto steroids. |
Sample was extracted by LLE. Extracts reacted with BODIPY FL hydrazide in ethanol for 15 h at RT. |
Katayama et al. 1998 [51] |
Dansyl hydrazine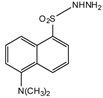 |
F, Butane acid-(5-androsten-17-one-3beta-ol)-diester (A1998)[52], alfaxalone and pregnanolone [53] | Sample was extracted by LLE. Extracts reacted with dansyl hydrazine in organic solvent for 30 min at RT in acidic medium. |
Kawasaki et al. 1979 [54] Visser et al. 2000 [52] Peng et al. 2007 [53] |
Naproxen acyl chloride in toluene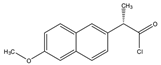 |
Cholesterol and sitosterol | Sample was extracted by LLE. Extracts reacted with naproxen acyl chloride in toluene, while shaking for 1.5 h at 90 °C. Diethylamine in toluene was then added to inactivate the excess naproxen acyl chloride, while shaking for 5 min at 30 °C. |
Lin et al. 2007 [19] |
Dansylaminophenylboronic acid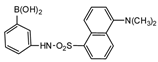 |
Brassinolide and castasterone | Sample was extracted by LLE. Extracts reacted with dansylaminophenylboronic acid in a mixture of pyridine and acetonitrile for 20 min at 70 °C. |
Motegi et al. 1994 [55] |
9-Fluorenylmethyl chloroformate (Fmoc-Cl)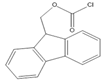 |
E1, E2, E3, BPA, NP, OP | Samples were extracted by MSPE. Extracts were reacted with Fmoc-Cl in NaHCO3 (pH = 10.5) at 60 °C for 10 min, and then added to a mixture of aqueous acetic acid and acetonitrile. The mixture was cooled to RT. |
Qianyu Li et al. 2018 [56] |
| 2-(11H-Benzo[a]carbazole-11-yl)- ethyl-4-methylbenzenesulfonate (BCETS) 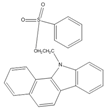 |
FFA | The samples were extracted by supercritical CO2 and organic solvent extraction. The extracted samples were derivatized in BCETS in a solution of K2CO3 at 84 °C; then the mixture was cooled down to RT and diluted with DMF. | Li et al. 2011 [57] |
Benzimidazo[2, 1-b]quinazoline-12(6H)-one-5-ethylimidazole ester (BQEIC)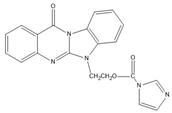 |
OP, NP, TBP, BPA, E1, E2, E3 | The samples were extracted by LLE. BQEIC in a solvent of DMAP at 80 °C for 60 min. Finally, the mixture was cooled to RT and diluted with acetonitrile. |
Liu et al. 2018 [58] |
Abbreviation: solid-phase extraction (SPE), liquid–liquid extraction (LLE), 4-octylphenol (OP), 4-tert-octylphenol (4-t-OP), 4-nonylphenol (NP), 4-tert-butylphenol (TBP), bisphenol A (BPA), estrone (E1), 17β-estradiol (17β-E2), 17α-estradiol (17α-E2), estriol (E3), free fatty acids(FFA), 17α-ethinylestradiol (EE2), ethinylestradiol (EED), cortisol (F), cortisone (E), 4,40-(1,2-diethylethylene)diphenol (HEX), prednisolone (PL), prednisone (PN), 6β-hydroxycortisol (6β-OHF), 6β-hydroxyprednisolone (6β-OHP) and 6β-hydroxycortisone (6β-OHE), room temperature (RT).
References
- Benc, D.; Icin, T.; Pejakovic, S.; Bajkin, I.; Prodanovic, J.; Vukovic, B.; Novakovic-Paro, J.; Tomic-Naglic, D.; Zvezdin, B.; Mitrovic, M. Glucocorticoid therapy and adrenal suppression. Med. Pregl. 2017, 70, 465–471.
- Rudolph, L.M.; Cornil, C.A.; Mittelman-Smith, M.A.; Rainville, J.R.; Remage-Healey, L.; Sinchak, K.; Micevych, P.E. Actions of steroids: New neurotransmitters. J. Neurosci. 2016, 36, 11449–11458.
- Cole, T.J.; Short, K.L.; Hooper, S.B. The science of steroids. Semin. Fetal Neonatal Med. 2019, 24, 170–175.
- Makin, H.L.J.; Honour, J.W.; Shackleton, C.H.L.; Griffiths, W.J. General Methods for the Extraction, Purification, and Measurement of Steroids by Chromatography and Mass Spectrometry. Steroid Anal. 2010, 163–282.
- Snyder, S.A.; Keith, T.L.; Verbrugge, D.A.; Snyder, E.M.; Gross, T.S.; Kannan, K.; Giesy, J.P. Analytical methods for detection of selected estrogenic compounds in aqueous mixtures. Environ. Sci. Technol. 1999, 33, 2814–2820.
- Varriale, A.; Pennacchio, A.; Pinto, G.; Oliviero, G.; D’Errico, S.; Majoli, A.; Scala, A.; Capo, A.; Pennacchio, A.; Di Giovanni, S.; et al. A Fluorescence Polarization Assay to Detect Steroid Hormone Traces in Milk. J. Agric. Food Chem. 2015, 63, 9159–9164.
- Xiao, X.Y.; McCalley, D.V.; McEvoy, J. Analysis of estrogens in river water and effluents using solid-phase extraction and gas chromatography–negative chemical ionisation mass spectrometry of the pentafluorobenzoyl derivatives. J. Chromatogr. A 2001, 923, 195–204.
- Shackleton, C. Clinical steroid mass spectrometry: A 45-year history culminating in HPLC-MS/MS becoming an essential tool for patient diagnosis. J. Steroid Biochem. Mol. Biol. 2010, 121, 481–490.
- Appelblad, P.; Irgum, K. Separation and detection of neuroactive steroids from biological matrices. J. Chromatogr. A 2002, 955, 151–182.
- Patel, D.; Namdev, K.K.; Verma, K.; Gururani, R.; Tiwari, A.; Kumar, P.; Dewangan, R.P.; Wabaidur, S.M.; Sharma, S.; Dwivedi, J. HPLC-UV and spectrofluorimetric methods for simultaneous estimation of fluticasone furoate and vilanterol in rabbit plasma: A pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1132, 121842.
- Yilmaz, B.; Kadioglu, Y. Determination of 17 β-estradiol in pharmaceutical preparation by UV spectrophotometry and high performance liquid chromatography methods. Arab. J. Chem. 2017, 10, S1422–S1428.
- Arsova-Sarafinovska, Z.; Ugrinova, L.; Starkoska, K.; Djordjev, D.; Dimitrovska, A. Determination of ethinylestradiol and levonorgestrel in oral contraceptives with HPLC methods with UV detection and UV/fluorescence detection. Maced. Pharm. Bull. 2006, 52, 9–16.
- Arsova-sarafinovska, Z.; Polozhani, A.; Dimitrovska, A. Determination of ethinylestradiol and drospirenone in oral contraceptives with HPLC method with UV and fluorescence detection Determination of ethinylestradiol and drospirenone in oral contraceptives with HPLC method with UV and fluorescence detection. Arch. Public Health 2009, 1, 67.
- Zhang, G.; Yang, Y.; Lu, Y.; Chen, Y.; Li, W.; Wang, S. Effect of heavy metal ions on steroid estrogen removal and transport in SAT using DLLME as a detection method of steroid estrogen. Water (Switzerland) 2020, 12, 589.
- Liu, N.; Shi, Y.E.; Li, M.; Zhang, T.D.; Gao, S. Simultaneous determination of four trace estrogens in feces, leachate, tap and groundwater using solid-liquid extraction/auto solid-phase extraction and high-performance liquid chromatography with fluorescence detection. J. Sep. Sci. 2015, 38, 3494–3501.
- Vallejo-Rodríguez, R.; Lopez-Lopez, A.; Saldarriaga-Noreña, H.; Murillo-Tovar, M.; Hernández-Mena, L. Optimization of Analytical Conditions to Determine Steroids and Pharmaceuticals Drugs in Water Samples Using Solid Phase-Extraction and HPLC. Am. J. Anal. Chem. 2011, 02, 863–870.
- Socas-Rodríguez, B.; Asensio-Ramos, M.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á. Analysis of oestrogenic compounds in dairy products by hollow-fibre liquid-phase microextraction coupled to liquid chromatography. Food Chem. 2014, 149, 319–325.
- Lima, D.L.D.; Silva, C.P.; Otero, M.; Esteves, V.I. Low cost methodology for estrogens monitoring in water samples using dispersive liquid-liquid microextraction and HPLC with fluorescence detection. Talanta 2013, 115, 980–985.
- Xiong, L.; Yan, P.; Chu, M.; Gao, Y.Q.; Li, W.H.; Yang, X.L. A rapid and simple HPLC–FLD screening method with QuEChERS as the sample treatment for the simultaneous monitoring of nine bisphenols in milk. Food Chem. 2018, 244, 371–377.
- Sánchez-Guijo, A.; Hartmann, M.F.; Shi, L.; Remer, T.; Wudy, S.A. Determination of free cortisol and free cortisone in human urine by on-line turbulent flow chromatography coupled to fused-core chromatography-tandem mass spectrometry (TFC-HPLC-MS/MS). Anal. Bioanal. Chem. 2014, 406, 793–801.
- Jiang, T.; Zhao, L.; Chu, B.; Feng, Q.; Yan, W.; Lin, J.M. Molecularly imprinted solid-phase extraction for the selective determination of 17β-estradiol in fishery samples with high performance liquid chromatography. Talanta 2009, 78, 442–447.
- Guedes-Alonso, R.; Santana-Viera, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Molecularly imprinted solid-phase extraction coupled with ultra high performance liquid chromatography and fluorescence detection for the determination of estrogens and their metabolites in wastewater. J. Sep. Sci. 2015, 38, 3961–3968.
- Mao, L.; Sun, C.; Zhang, H.; Li, Y.; Wu, D. Determination of environmental estrogens in human urine by high performance liquid chromatography after fluorescent derivatization with p-nitrobenzoyl chloride. Anal. Chim. Acta 2004, 522, 241–246.
- Kurosawa, S.; Koike, T.; Yoshimura, T.; Kurosawa, T.; Tohma, M.; Chiba, H.; Kobayashi, K. Simultaneous determination of 18-oxygenated corticosteroids by high-performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. 1995, 18, 2383–2396.
- Shimada, K.; Nakagi, T. Studies on neurosteroids. IV. Quantitative determination of pregnenolone in rat brains using high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 2593–2602.
- Shimada, K.; Nonaka, M. Utility of cyclodextrin in mobile phase for high-performance liquid chromatographic separation of C21 steroids. J. Liq. Chromatogr. 1991, 14, 2109–2117.
- Główka, F.K.; Kosicka, K.; Karaźniewicz-Łada, M. HPLC method for determination of fluorescence derivatives of cortisol, cortisone and their tetrahydro- and allo-tetrahydro-metabolites in biological fluids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 283–289.
- Goto, J.; Goto, N.; Shamsa, F.; Saito, M.; Komatsu, S.; Suzaki, K.; Nambara, T. New sensitive derivatization of hydroxysteroids for high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 1983, 147, 397–400.
- Shibata, N.; Hayakawa, T.; Takada, K.; Hoshino, N.; Minouchi, T.; Yamaji, A. Simultaneous determination of glucocorticoids in plasma or urine by high-performance liquid chromatography with precolumn fluorimetric derivatization by 9-anthroyl nitrile. J. Chromatogr. B Biomed. Appl. 1998, 706, 191–199.
- Haegele, A.D.; Wade, S.E. Ultrasensitive differential measurement of cortisol and cortisone in biological samples using fluorescent ester derivatives in normal phase hplc. J. Liq. Chromatogr. 1991, 14, 1133–1148.
- Kosicka, K.; Siemiątkowska, A.; Szpera-Goździewicz, A.; Krzyścin, M.; Bręborowicz, G.; Główka, F. High-performance liquid chromatography methods for the analysis of endogenous cortisol and cortisone in human urine: Comparison of mass spectrometry and fluorescence detection. Ann. Clin. Biochem. 2019, 56, 82–89.
- Neufeld, E.; Chayen, R.; Stern, N. Fluorescence derivatisation of urinary corticosteroids for high-performance liquid chromatographic analysis. J. Chromatogr. B Biomed. Appl. 1998, 718, 273–277.
- Wu, H.; Li, G.; Liu, S.; Hu, N.; Geng, D.; Chen, G.; Sun, Z.; Zhao, X.; Xia, L.; You, J. Monitoring the contents of six steroidal and phenolic endocrine disrupting chemicals in chicken, fish and aquaculture pond water samples using pre-column derivatization and dispersive liquid-liquid microextraction with the aid of experimental design metho. Food Chem. 2016, 192, 98–106.
- Gamoh, K.; Omote, K.; Okamoto, N.; Takatsuto, S. High-performance liquid chromatography of brassinosteroids in plants with derivatization using 9-phenanthreneboronic acid. J. Chromatogr. A 1989, 469, 424–428.
- Zhang, S.; You, J.; Sun, Z.; Song, C.; Ning, S.; Zhao, C.; Suo, Y. A sensitive method for extraction and determination of endocrine-disrupting compounds from wastewater using 10-ethyl-acridone-2-sulfonyl chloride as pre-column labeling reagent by high-performance liquid chromatography with fluorescence detection. Microchem. J. 2012, 103, 90–96.
- Nozaki, O.; Ohata, T.; Ohba, Y.; Moriyama, H.; Kato, Y. Determination of serum cortisol by reversed-phase liquid chromatography using precolumn sulphuric acid-ethanol fluorescence derivatization and column switching. J. Chromatogr. B Biomed. Sci. Appl. 1991, 570, 1–11.
- Nozaki, O.; Ohata, T.; Ohba, Y.; Moriyama, H.; Kato, Y. Determination of urinary free cortisol by high performance liquid chromatography with sulphuric acid–ethanol derivatization and column switching. Biomed. Chromatogr. 1992, 6, 109–114.
- Sudo, A. Analysis of corticosterone in rat urine by high-performance liquid chromatography and fluorimetry using post-column reaction with sulphuric acid. J. Chromatogr. B Biomed. Sci. Appl. 1990, 528, 453–458.
- Gao, W.; Xie, Q.; Jin, J.; Qiao, T.; Wang, H.; Chen, L.; Deng Huihua, H.; Lu, Z. HPLC-FLU detection of cortisol distribution in human hair. Clin. Biochem. 2010, 43, 677–682.
- Saisho, Y.; Shimada, C.; Umeda, T. Determination of 7α-hydroxycholesterol in dog plasma by high- performance liquid chromatography with fluorescence detection. Anal. Biochem. 1998, 265, 361–367.
- Katayama, M.; Masuda, Y.; Taniguchi, H. Determination of corticosteroids in plasma by high-performance liquid chromatography after pre-column derivatization with 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole. J. Chromatogr. B Biomed. Sci. Appl. 1993, 612, 33–39.
- Katayama, M.; Sasaki, T.; Matsuda, Y.; Kaneko, S.; Iwamoto, T.; Tanaka, M. Sensitive determination of bisphenol A and alkylphenols by high performance liquid chromatography with pre-column derivatization with 2-(4-carboxyphenyl)-5,6-dimethylbenzimidazole. Biomed. Chromatogr. 2001, 15, 403–407.
- Seki, T.; Yamaguchi, Y. New fluorimetric determination of 17-hydroxycorticosteroids after high-performance liquid chromatography using post-column derivatization with benzamidine. J. Chromatogr. B Biomed. Sci. Appl. 1984, 305, 188–193.
- Sun, Y.; Irie, M.; Kishikawa, N.; Wada, M.; Kuroda, N.; Nakashima, K. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomed. Chromatogr. 2004, 18, 501–507.
- Kuroda, N.; Kinoshita, Y.; Sun, Y.; Wada, M.; Kishikawa, N.; Nakashima, K.; Makino, T.; Nakazawa, H. Measurement of bisphenol A levels in human blood serum and ascitic fluid by HPLC using a fluorescent labeling reagent. J. Pharm. Biomed. Anal. 2003, 30, 1743–1749.
- Sun, Y.; Nakashima, M.N.; Takahashi, M.; Kuroda, N.; Nakashima, K. Determination of bisphenol A in rat brain by microdialysis and column switching high-performance liquid chromatrography with fluorescence detection. Biomed. Chromatogr. 2002, 16, 319–326.
- Ali, M.F.B.; Uejo, Y.; Kishikawa, N.; Ohyama, K.; Kuroda, N. A selective and highly sensitive high performance liquid chromatography with fluorescence derivatization approach based on Sonogashira coupling reaction for determination of ethinyl estradiol in river water samples. J. Chromatogr. A 2020, 1628, 461440.
- Yamaguchi, M.; Ishida, J.; Yoshitake, T.; Nakamura, M. Determination of prednisolone and prednisone in plasma by liquid chromatography with fluorescence detection. Anal. Chim. Acta 1991, 242, 113–116.
- Yoshitake, T.; Hara, S.; Yamaguchi, M.; Nakamura, M.; Ohkura, Y.; Görög, S. Measurement of 21-hydroxycorticosteroids in human and rat sera by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B Biomed. Sci. Appl. 1989, 489, 364–370.
- YAMAGUCHI, M.; YOSHITAKE, T.; ISHIDA, J.; NAKAMURA, M. Determination of 21-hydroxycorticosteroids in human urine by high-performance liquid chromatography with fluorescence detection. Chem. Pharm. Bull. 1989, 37, 3022–3025.
- Katayama, M.; Nakane, R.; Matsuda, Y.; Kaneko, S.; Hara, I.; Sato, H. Determination of progesterone and 17-hydroxyprogesterone by high performance liquid chromatography after pre-column derivatization with 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a- diaza-s-indacene-3-propionohydrazide. Analyst 1998, 123, 2339–2342.
- Visser, S.A.G.; Smulders, C.J.G.M.; Gladdines, W.W.F.T.; Irth, H.; Van Der Graaf, P.H.; Danhof, M. High-performance liquid chromatography of the neuroactive steroids alphaxalone and pregnanolone in plasma using dansyl hydrazine as fluorescent label: Application to a pharmacokinetic-pharmacodynamic study in rats. J. Chromatogr. B Biomed. Sci. Appl. 2000, 745, 357–363.
- Peng, X.D.; Xu, D.H.; Jin, J.; Mei, X.T.; Lv, J.Y.; Xu, S.B. Determination of a new active steroid by high performance liquid chromatography with laser-induced fluorescence detection following the pre-column derivatization. Int. J. Pharm. 2007, 337, 25–30.
- Kawasaki, T.; Maeda, M.; Tsuji, A. Determination of plasma and urinary cortisol by high-performance liquid chromatography using fluorescence derivatization with dansyl hydrazine. J. Chromatogr. B Biomed. Sci. Appl. 1979, 163, 143–150.
- Motegi, C.; Takatsuto, S.; Gamoh, K. Identification of brassinolide and castasterone in the pollen of orange (Citrus sinensis Osbeck) by high-performance liquid chromatography. J. Chromatogr. A 1994, 658, 27–30.
- Li, N.; Wu, D.; Liu, J.; Hu, N.; Shi, X.; Dai, C.; Sun, Z.; Suo, Y.; Li, G.; Wu, Y. Magnetic covalent organic frameworks based on magnetic solid phase extraction for determination of six steroidal and phenolic endocrine disrupting chemicals in food samples. Microchem. J. 2018, 143, 350–358.
- Li, G.; You, J.; Suo, Y.; Song, C.; Sun, Z.; Xia, L.; Zhao, X.; Shi, J. A developed pre-column derivatization method for the determination of free fatty acids in edible oils by reversed-phase HPLC with fluorescence detection and its application to Lycium barbarum seed oil. Food Chem. 2011, 125, 1365–1372.
- Liu, J.; You, J.; Zhang, S.; Song, C.; Ji, Z.; Zhuang, J.; Yu, Y. New fluorescent labeling reagent Benzimidazoquinazoline-12(6H) -one-5-ethylimidazole ester and its application in the analysis of endocrine disrupting compounds in milk by high performance liquid chromatography with fluorescence detection. Microchem. J. 2018, 138, 309–315.
More
Information
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.0K
Revisions:
2 times
(View History)
Update Date:
01 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





