Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gabriele Christine Saretzki | + 2629 word(s) | 2629 | 2022-03-14 09:27:12 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Saretzki, G.; Porika, M. CRISPR/Cas in Research of Telomeres. Encyclopedia. Available online: https://encyclopedia.pub/entry/20920 (accessed on 07 February 2026).
Saretzki G, Porika M. CRISPR/Cas in Research of Telomeres. Encyclopedia. Available at: https://encyclopedia.pub/entry/20920. Accessed February 07, 2026.
Saretzki, Gabriele, Mahendar Porika. "CRISPR/Cas in Research of Telomeres" Encyclopedia, https://encyclopedia.pub/entry/20920 (accessed February 07, 2026).
Saretzki, G., & Porika, M. (2022, March 23). CRISPR/Cas in Research of Telomeres. In Encyclopedia. https://encyclopedia.pub/entry/20920
Saretzki, Gabriele and Mahendar Porika. "CRISPR/Cas in Research of Telomeres." Encyclopedia. Web. 23 March, 2022.
Copy Citation
Telomeres are highly specialized nucleoprotein complexes that play a critical role in cell senescence and aging. Each chromosomal end must be “capped” with a critical amount of telomere repeats to prevent DNA repair pathways from being activated. As a result of the clustered regularly interspaced short palindromic repeats (CRISPR)-associated system’s (Cas) method, targeted genetic studies are now underway to change telomerase, the genes that govern it as well as telomeres.
genome editing
CRISPR/Cas
telomeres
aging
therapy
1. Introduction
The previous century has seen an incredible increase in human life expectancy across the world. However, this rise in life span has not been supported by a similar increase in health span [1][2]. Aging is the leading cause for most pathological conditions such as immunosenescence, cardiometabolic disorders, osteoporosis, sarcopenia, arthritis, cataracts, neurological diseases, and most malignancies that shorten life expectancy and increase chronic illnesses in older people [3].
Telomeres are highly specialized nucleoprotein complexes that play a critical role in cell senescence and aging. Each chromosomal end must be “capped” with a critical amount of telomere repeats to prevent DNA repair pathways from being activated [4][5]. Telomeres consist of a six-protein complex called shelterin that binds to telomeric DNA containing tandemly repeated hexanucleotides (TTAGGG in mammals, including humans). Human telomeric DNA is about 5 to 15 kb in length and is composed of double-stranded (ds) telomeric repeats that end in a single-stranded (ss) 3′ G-rich overhang [6][7]. In addition, telomeres and shelterin form a telomeric T-loop and a displacement D- loop [8]. The latter sequesters the ss overhang away and protects it from degradation and emanation of a DNA damage signal. With each DNA replication, telomeric DNA shortens by around 50–200 bp due to the end replication problem (ERP) of semiconservative DNA replication where DNA polymerase can synthesize only the leading strand continuously while the lagging strand is synthesized with the help of an RNA primer and short DNA fragments (Okazaki fragments). Those are finally stitched together by DNA ligase while the most distal RNA primer cannot be replaced by new DNA and its removal thus leaves an ss overhang of around 100–200 nucleotides [9]. In addition, oxidative stress can accelerate telomere shortening [10]. Telomeres shorten to the point of a minimum length when they cause a DNA damage response (DDR) that can lead to cellular senescence or apoptosis [4][11][12]. In the absence of additional modifications, cells can survive and remain senescent for years, and that is regarded as a tumor suppressor mechanism in long-lived species such as humans. Senescent cells release various types of molecules and have a senescence-associated secretory phenotype (SASP) that can target neighbouring cells and promote age-related disorders [13][14]. As a result, with progressing age, there is a steady build-up of senescent cells in various tissues. In contrast, removing those senescent cells from the organism either by genetic means or the use of senolytics is able to delay and ameliorate many age-related conditions in animal models [15][16].
The enzyme telomerase is able to counteract telomere shortening by adding telomeric repeats de novo to telomeres. It thereby increases the proliferative capacity of cells with high amounts of telomerase such as embryonic stem cells and cancer cells [17]. Telomerase is a RNA-dependent DNA polymerase and reverse transcriptase consisting of the reverse transcriptase protein (TERT) and a RNA component (TERC) that contains the template region for telomere synthesis. Telomeres gradually shorten in the absence of telomerase activity (TA) due to the ERP. In most human somatic cells there is no TA because TERT is not expressed while TERC is generally present.
2. The CRISPR/Cas System
Despite tremendous advances in the understanding of functions and dysfunctions of telomeres and telomerase, the inability to influence and detect changes directly inside biological systems has been an essential hurdle in their investigation. However, a solution to this matter has recently been developed—the clustered regularly interspaced short palindromic repeats (CRISPR)-associated system (Cas). For the first time it was reported in the early 2000s as an adaptive bacterial immune system that targets and destroys invading bacterial and viral DNA in prokaryotes [18]. It has since gained widespread acceptance. This finding generated evolutionary curiosity when it was identified because of its genomic CRISPR and Cas protein. However, it took an additional eight years to find and acknowledge an application of this system. Different groups presented ways of editing DNA in vitro using the CRISPR/Cas system in 2013 and 2014 [19][20][21].
This method is composed of two fundamental categories—guiding and effecting. An ssRNA molecule known as single guide RNA (sgRNA) serves as the guiding portion responsible for specificity [22]. This sgRNA component addresses a genomic area by complementing a specific DNA sequence and is linked to numerous Cas proteins, the most prevalent of which is Cas9. This protein exhibits double-strand (ds) endonuclease activity in its natural state [20][23] (Figure 1). Donor DNA with the required sequence can be introduced into the target area once a cut in the DNA has been created [24]. By pairing endonuclease activity with the RNA guide, genetic information can be changed in vitro and in vivo in a highly precise and carefully controlled manner.
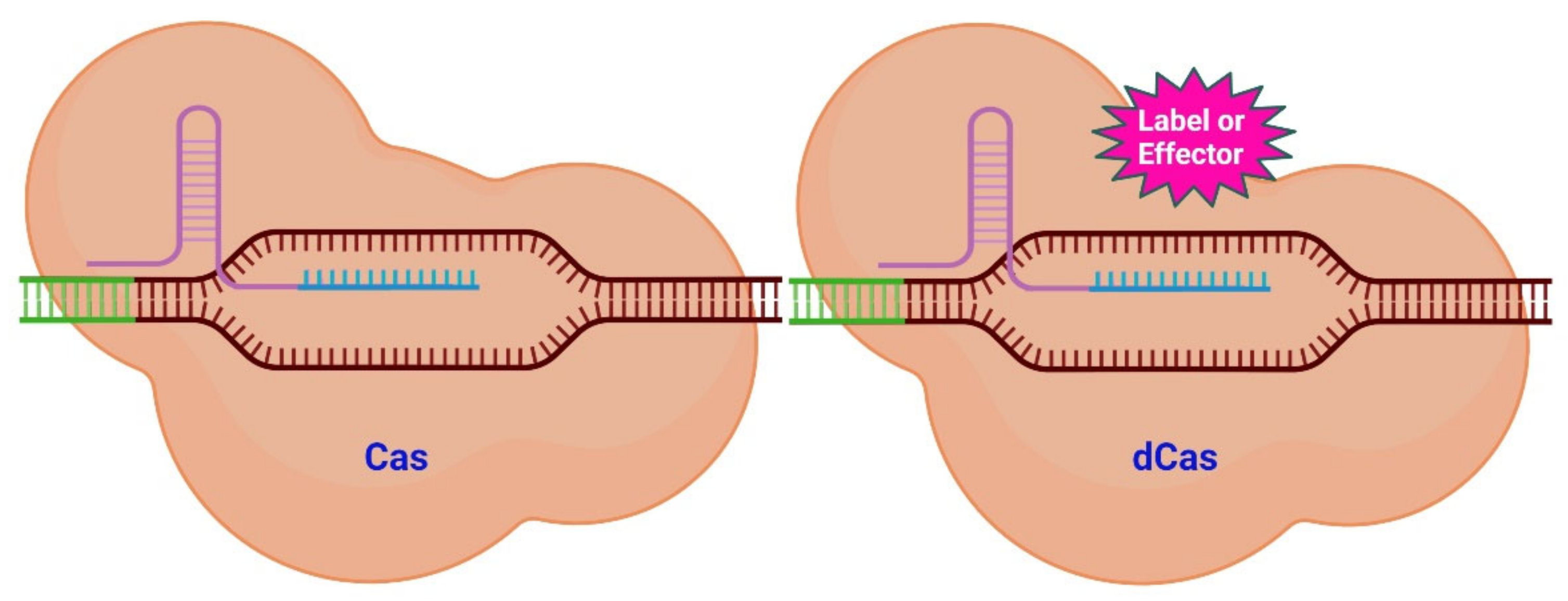
Figure 1. Scheme of CRISPR/Cas system variants. Targeting a specific genomic region (brown) with its sgRNA (lilac and turquois) is the function of the Cas system (left). The Cas protein will subsequently cut into the neighboring DNA region (green). The dCas system (right) uses a similar mechanism to that of the CAS system to target a genomic region. The dCas protein, however, lacks endonuclease activity. Numerous compounds may be fused to the dCas protein (pink) (labels and effectors). Labels place a fluorescent signal near to the target DNA, while effectors are able to alter the epigenetic state of the DNA [25].
However, the CRISPR-Cas system is not confined to only causing double-strand breaks (DSB). It is possible to modify the Cas protein to preserve its targeting capability while it loses its ability to cut DNA [22]. Cas/dead Cas (dCas) (the latter is catalytically inactive) can be used as it is or be modified with various functional groups. Molecules (labels and effectors) can be attached to dCas as shown in Figure 1. These molecules can then be placed closer together or be associated to specific areas of the genome.
Through innovation CRISPR has been developed into a powerful bio-analytical method to detect nucleic acids and diagnose various diseases [26][27][28][29][30]. A short CRISPR RNA directs CRISPR/Cas12a to select a target dsDNA and generates the Cas12a/crRNA/dsDNA ternary complex which cleaves the target dsDNA (cis-cleavage). Cas12a’s capacity to trans-cleave random ssDNA is triggered by the ternary complex formation following target DNA cleavage [26][31][32]. CRISPR therapies, which were discovered using CRISPR, are being developed into a potential therapy for malignancies and other genetic abnormalities [33].
RNA-guided immune mechanisms, such as CRISPR, are used by bacteria and archaea to fight off invaders such as viruses and plasmids [34]. In the presence of an adjacent protospacer motif (PAM) on the opposite strand, the sgRNA is able to direct CRISPR/Cas9 to a target location for cleavage [35], leading to DNA DSBs (Figure 2) [19][36]. The sequence-specific dsDNA binding and cleavage capabilities of the Cas9/sgRNA or dCas9/sgRNA systems have been used to develop biosensors for nucleic acid assays [28][35][37]. Because of characteristics such as adaptability, simplicity, specificity, and effectiveness, CRISPR/Cas9 technology has been extensively employed for genome editing (Figure 3) and has a significant potential for biomedical studies [38][39].
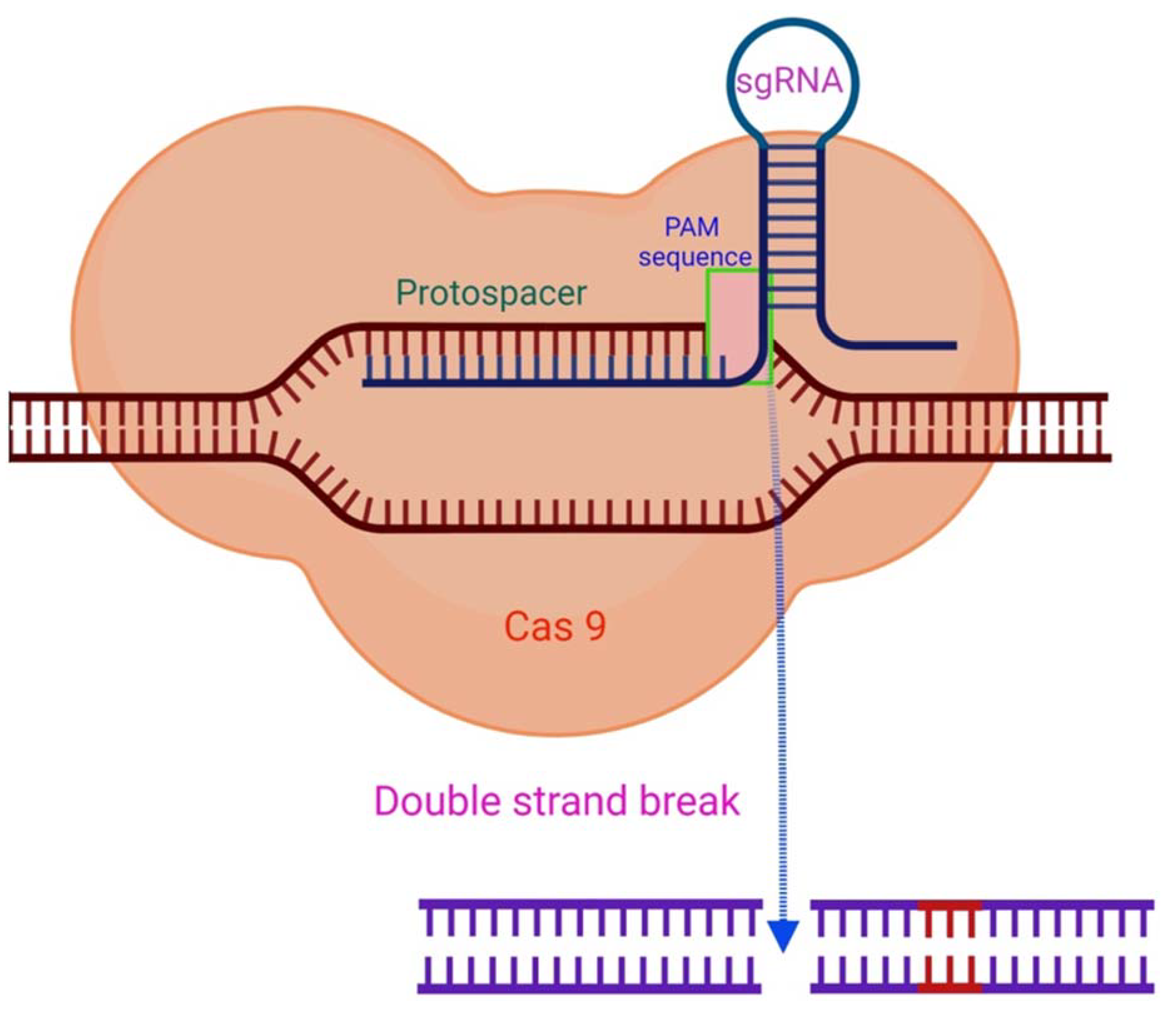
Figure 2. The CRISPR/Cas9 mechanism. By complementary base pairing, the sgRNA recognizes the target sequence (protospacer) in a host organism’s genome. The Cas9 nuclease causes a DSB in a PAM sequence region with a NGG (any nucleobase followed by two guanine nucleobase) sequence at the 3′position [35].
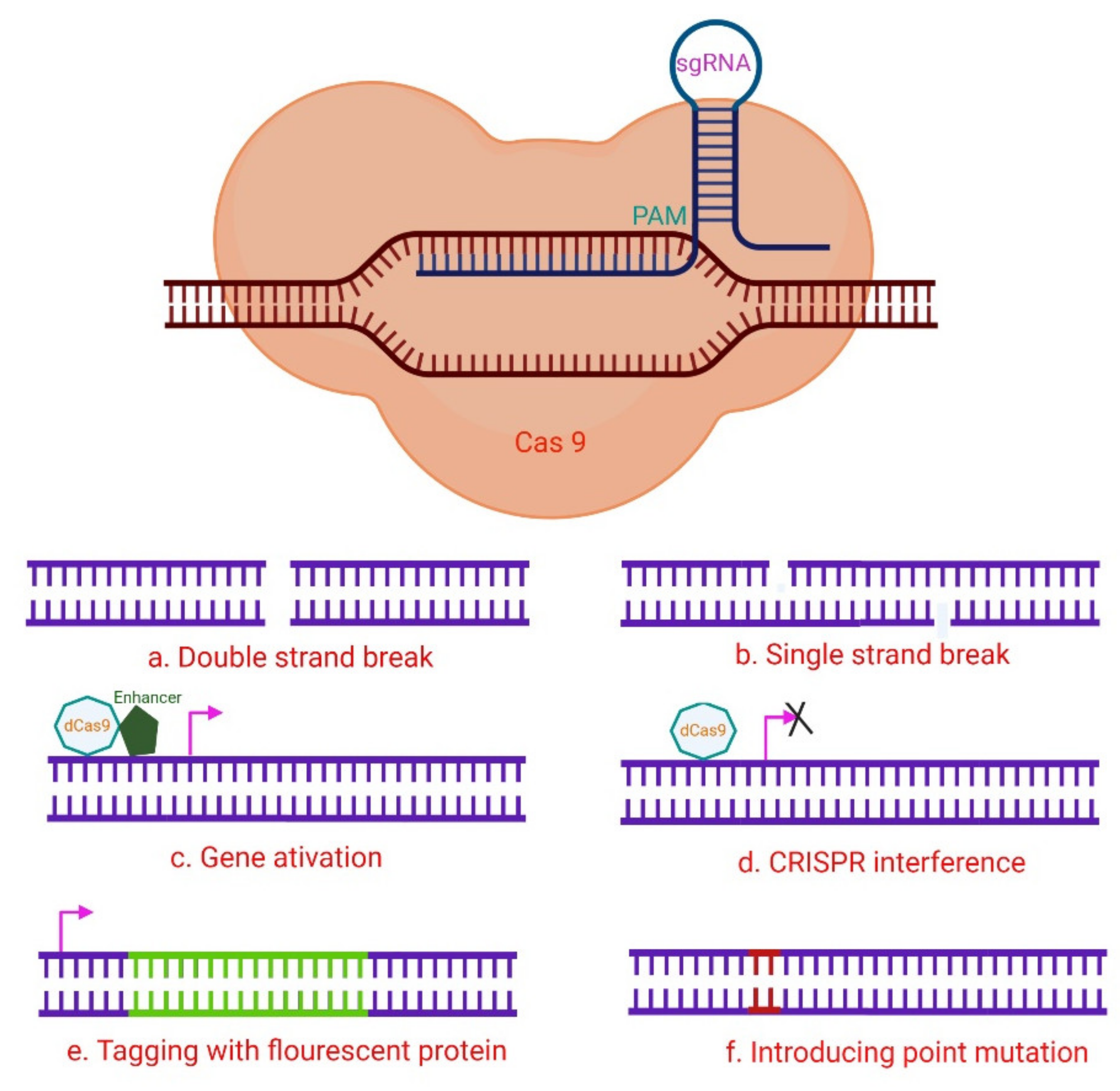
Figure 3. A hypothetical CRISPR/Cas9 experimental design. The numerous Cas9 variants available have made it easier to perform a variety of genomic modifications, including (a) induction of DNA DSB utilizing wild-type Cas9; (b) Cas9 nickase is used to induce DNA SSBs, in which two neighboring gRNAs target distinct strands, resulting in a DSB and subsequent NHEJ (non-homologous end joining) response; (c) The target gene is activated via CRISPR activation utilizing dCas9 and transcription activator fusion with transcription-activating proteins such as VP64 that target the promoter region. (d) By steric hindrance, CRISPR interference with dCas9 or dCas9-BFP decreases gene expression, and the dCas9-KRAB fusion epigenetically marks the gene for transcriptional repression. These methods can be used to stop gene transcription transiently. (e) A donor template that includes a fluorescent protein gene with homology regions can be used to drive homology-directed repair (HDR). By cutting at the target location with Cas9-WT or Cas9 nickase, it is possible to tag genes with fluorescent proteins. In another setting, the donor plasmid might include a selectable marker for determining whether knock-in events do occur. (f) Cas9-WT or Cas9 nickase also initiate the introduction of point mutations via HDR from an ss oligonucleotide [35].
3. Targeting Telomeres with CRISPR
The ability to apply CRISPR imaging directly to living biological systems is the real value of the technique. Other fluorescently labelling genomic approaches exist, however they are harmful to cells and may result in irreparable DNA damage [40]. In vitro recording of telomeres and other genomic components has been unachievable due to this restriction. Targeting telomeres for imaging is a new application of the CRISPR/dCas system. This method offers several benefits over previous systems with the majority of benefits being due to the CRISPR system’s dynamic and long-term persisting nature. Among the first imaging investigations performed with CRISPR was one performed by Chen and colleagues [41]. They used enhanced green fluorescent protein (EGFP) to detect telomeres in HEK293T, UMUC3, and HeLa cell lines. The authors identified telomere movements within these cells with a labelling efficacy and intensity similar to the well-known DNA FISH procedure.
Further optimization to this method may result in further improved labelling efficiency and specificity [42]. Labelled telomeres became much simpler to identify when the EGFP fluorescent tag was replaced with the brighter mClover fluorescent tag, which however, resulted in minor off-target impacts [43]. Although imaging of telomeres is not a unique concept, the CRISPR/Cas system’s exceptional accuracy and efficiency form a novel technique to track telomeres fast and effectively.
Shao and co-workers were the first to demonstrate that CRISPR-dCas labelling was minimally cytotoxic to cells and appropriate for long-term observations [44]. Their technique was utilized to quantify the relative movements of telomeres and centromeres during interphase within a five-minute timeframe [44]. This approach has recently been applied to transgenic mouse models [45]. By expressing dCas-GFP throughout a mouse, telomere guides might be inserted into particular tissues for labelling. The authors combined this method with CRISPR-interference of the TRF1 gene to detect telomere aggregation and fusion in real-time [45]. This approach can be applied to additional genes, allowing investigators to analyze changes in telomere dynamics following genetic modifications.
Inducible shelterin CRISPR/Cas9 knockout (KO) cells were used in a study conducted by Kim and colleagues to perform a thorough investigation of human telomeric dysfunction [46]. The authors developed inducible CRISPR KO human cell lines for the shelterin complex subunits TRF1, TRF2, RAP1, TIN2, and TPP1, as well as POT1. In mice, homozygous ablation of many of those subunits causes death [46]. The majority of human telomere regulator loss-of-function investigations utilized RNA.
Dai and co-authors created a CRISPR/Cas9 protein in tumor cells through an NF-κB-activated gene-expression (Nage) vector [47]. Due to sgRNA targeting telomeric DNA co-expressed in cells, Cas was able to cleave telomeric DNA, which resulted in tumor cell death. An adeno-associated virus (AAV) which contains the Cas9/sgRNA expression vector can be packaged and delivered intravenously into mice in order to inhibit tumor growth without generating side effects or toxicity [47].
DDR signaling initiates and maintains cellular senescence [11]. DDR signaling, genomic instability, and cellular senescence can all be triggered by telomere dysfunction, and the connections between these processes have been well-studied. Abdisalaam and co-workers employed a combination of biochemical and imaging approaches to induce DNA DSB selectively in telomeres, along with a highly regulatable CRISPR/Cas9 approach [48]. Moreover, the authors used micronucleus imaging, telomere immunofluorescence, fluorescence in situ hybridization (FISH), chromatin immuno-precipitation (ChIP), and the telomere shortest-length assay (TeSLA). They demonstrated that chromosomal mis-segregation caused by induction of DDR signaling in response to faulty telomeres resulted in cytosolic chromatin fragments, leading to an early senescence phenotype [48]. The authors found that cytosolic chromatin fragments were recognized by cyclic GMP–AMP synthase (cGAS), which activated the stimulator of interferon genes (STING) cytosolic DNA-sensing pathway and downstream interferon signaling. Not only did genetic and pharmacological alteration of cGAS diminish immunological signaling, but it also reduced telomere dysfunction-related premature cellular senescence [48].
An improved approach in CHO (Chinese hamster ovary) and mouse A9 cells following microcell-mediated chromosome transfer (MMCT) to recipient cells utilizing a CRISPR/Cas9-induced homologous recombination (HR) was demonstrated by Uno et al. [49]. CRISPR/Cas9 and a circular targeting vector comprising two 3 kb HR arms were used to introduce EGFP into CHO cells. CRISPR/Cas9 and a linearized truncation vector with a single 7 kb HR arm at the 5′ end and a 1 kb artificial telomere at the 3′ end were used in CHO cells to accomplish telomere-associated truncation [49] For transgene insertion and telomere-related truncation, 6–11% of the targeting efficiency was obtained. A9 cells were used to confirm the transgene insertion (29%) [49]. The modified chromosomes can be transferred to other cells. As a result, chromosomal engineering using CHO and A9 cells as well as CRISPR/Cas9 for direct chromosome transfer is a rapid technique that will enable easier chromosome modification.
In the neuroblastoma cell line SH-SY5Y Kim and colleagues used the CRISPR-Cas9 tool to remove telomeres and promote senescence [50] Expression of Cas9 and guide RNA targeting telomere repeats ablated telomeres, resulting in reduced cell growth. Telomere deletion also impacted mitochondrial function in SH-SY5Y cells, resulting in lower mitochondrial respiration and cell survival [50]. Changes in the levels of Parkinson’s disease (PD)-associated proteins, such as PTEN-induced putative kinase 1 (PINK1), parkin, peroxisome proliferator-activated receptor coactivator- 1 alpha (PGC-1α), nuclear respiratory factor 1 (NRF1), and aminoacyl tRNA synthetase complex interacting multifunctional protein 2 (MFP-2), were demonstrated, lending support to the pathological relevance of cell senescence. Importantly, α-synuclein expression in the context of telomere elimination increased protein aggregation, suggesting a positive feedback loop between senescence/aging and PD pathogenesis [50]. The ablation of telomeres resulted in cellular alterations, including the loss of mitochondrial function and the accumulation of PD-related proteins as well as the reduction of cell survival. Because of these modifications, aging and PD-related features could be more easily replicated in cells.
Telomere disruption and destruction, as described above, can result in a variety of cellular abnormalities. The capacity of the CRISPR-Cas system to cut and insert genes enables in vivo studies of telomere deterioration in real-time. The activation of a telomeric repair mechanism mediated by the Rad51 gene was triggered by this technique to produce DSBs in telomeres [51].
Several studies investigated the relationship between telomeres and senescence [11][50][52][53]. It is also possible to employ CRISPR/Cas to generate more moderate changes in telomeres. Cells lost sister telomeres and had a lower replication capacity when a mutation to a subtelomeric CTCF (CCCTC-binding factor) binding region that is involved in telomere transcription generating TERRA (telomere repeat-encoding RNA) was introduced [54]. The creation of replication stress with thymidine or aphidicolin worsened this problem, resulting in a higher amount of apoptosis [54]. It has been shown that CTCF and TERRA sites are critical for proper replication and maintenance of telomeres and the overall integrity of chromosomes.
While the number of current studies on CRISPR/Cas-induced telomere deletion is limited, the technique’s ability to be applied to any cell type makes it possible to study a broad spectrum of disorders. In addition, the effect of senescence may be assessed across a wide range of settings in different tissues. Telomere shortening and its induced disorders (telomeropathies) may display new biological and mechanistic aspects that could help to better understand and characterize them.
References
- Lee, J.; Lau, S.; Meijer, E.; Hu, P. Living longer, with or without disability? A global and longitudinal perspective. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 162–167.
- Vaiserman, A.; Krasnienkov, D. Telomere length as a marker of biological age: State-of-the-art, open issues, and future perspectives. Front. Genet. 2021, 11, 1816.
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61.
- Aubert, G.; Lansdorp, P.M. Telomeres and aging. Physiol. Rev. 2008, 88, 557–579.
- Blackburn, E.H. Switching and signaling at the telomere. Cell 2001, 106, 661–673.
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Ann. Rev. Genet. 2008, 42, 301–334.
- Shay, J.W. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016, 6, 584–593.
- De Lange, T. Protection of mammalian telomeres. Oncogene 2002, 21, 532–540.
- Olovnikov, A.M. Telomeres, telomerase, and aging: Origin of the theory. Exp. Gerontol. 1996, 31, 443–448.
- von Zglinicki, T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002, 27, 339–344.
- Fagagna, F.D.A.D.; Reaper, P.M.; Clay-Farrace, L.; Fiegler, H.; Carr, P.; Von Zglinicki, T.; Saretzki, G.; Carter, N.P.; Jackson, S.P. A DNA damage checkpoint response in telomere-initiated senescence. Nature 2003, 426, 194–198.
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Duelling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153.
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118.
- Tchkonia, T.; Zhu, Y.; Van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972.
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Verzosa, G.C.; Pezeshki, A.; et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016, 530, 184–189.
- Ogrodnik, M.; Evans, S.A.; Fielder, E.; Victorelli, S.; Kruger, P.; Salmonowicz, H.; Weigand, B.M.; Patel, A.D.; Pirtskhalava, T.; Inman, C.L.; et al. Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell 2021, 20, e13296.
- Chan, S.R.; Blackburn, E.H. Telomeres and telomerase. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 109–121.
- Mojica, F.J.M.; Diez-Villaseñor, C.; Garcia-Martinez, J.; Soria, E. Intervening Sequences of Regularly Spaced Prokaryotic Repeats Derive from Foreign Genetic Elements. J. Mol. Evol. 2005, 60, 174–182.
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826.
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and Applications of CRISPR-Cas9 for Genome Engineering. Cell 2014, 157, 1262–1278.
- Charpentier, E.; Marraffini, L.A. Harnessing CRISPR-Cas9 immunity for genetic engineering. Curr. Opin. Microbiol. 2014, 19, 114–119.
- Komor, A.C.; Badran, A.H.; Liu, D.R. CRISPR-Based Technologies for the Manipulation of Eukaryotic Genomes. Cell 2017, 168, 20–36.
- Gupta, R.M.; Musunuru, K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Investig. 2014, 124, 4154–4161.
- Ikeda, M.; Matsuyama, S.; Akagi, S.; Ohkoshi, K.; Nakamura, S.; Minabe, S.; Kimura, K.; Hosoe, M. Correction of a disease mutation using CRISPR/Cas9-assisted genome editing in Japanese black cattle. Sci. Rep. 2017, 7, 17827.
- Brane, A.C.; Tollefsbol, T.O. Targeting Telomeres and Telomerase: Studies in Aging and Disease Utilizing CRISPR/Cas9 Technology. Cells 2019, 8, 186.
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439.
- Li, S.Y.; Cheng, Q.X.; Wang, J.M.; Li, X.Y.; Zhang, Z.L.; Gao, S.; Cao, R.B.; Zhao, G.P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20.
- Zhou, W.; Hu, L.; Ying, L.; Zhao, Z.; Chu, P.K.; Yu, X.F. A CRISPR–Cas9-triggered strand displacement amplification method for ultrasensitive DNA detection. Nat. Commun. 2018, 9, 5012.
- Aman, R.; Mahas, A.; Mahfouz, M. Nucleic acid detection using CRISPR/Cas biosensing technologies. ACS Synth. Biol. 2020, 9, 1226–1233.
- Kaminski, M.M.; Abudayyeh, O.O.; Gootenberg, J.S.; Zhang, F.; Collins, J.J. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656.
- Li, S.Y.; Cheng, Q.X.; Liu, J.K.; Nie, X.Q.; Zhao, G.P.; Wang, J. CRISPR-Cas12a has both cis-and trans-cleavage activities on single-stranded DNA. Cell Res. 2018, 28, 491–493.
- Swarts, D.C.; Jinek, M. Mechanistic Insights into the cis-and trans-Acting DNase Activities of Cas12a. Mol. Cell 2019, 73, 589–600.
- Khan, F.A.; Pandupuspitasari, N.S.; Chun-Jie, H.; Ao, Z.; Jamal, M.; Zohaib, A.; Khan, F.A.; Hakim, M.R.; ShuJun, Z. CRISPR/Cas9 therapeutics: A cure for cancer and other genetic diseases. Oncotarget 2016, 7, 52541.
- Horvath, P.; Barrangou, R. CRISPR/Cas, the immune system of bacteria and archaea. Science 2010, 327, 167–170.
- Ceasar, S.A.; Rajan, V.; Prykhozhij, S.V.; Berman, J.N.; Ignacimuthu, S. Insert, remove or replace: A highly advanced genome editing system using CRISPR/Cas9. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2333–2344.
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 6213.
- Novikov, R.V.; Gribkova, A.K.; Kacher, J.G.; Zaytsev, P.A.; Armeev, G.A.; Gluhov, G.S.; Shaytan, A.K. Design of nucleic acid biosensors based on CRISPR/Cas systems and reporter split proteins. Mosc. Univ. Biol. Sci. Bull. 2021, 76, 52–58.
- Kaboli, S.; Babazada, H. CRISPR mediated genome engineering and its application in industry. Curr. Issues Mol. Biol. 2018, 26, 81–92.
- Kim, S.; Ji, S.; Koh, H.R. CRISPR as a Diagnostic Tool. Biomolecules 2021, 11, 1162.
- Ge, J.; Wood, D.K.; Weingeist, D.M.; Prasongtanakij, S.; Navasumrit, P.; Ruchirawat, M.; Engelward, B.P. Standard fluorescent imaging of live cells is highly genotoxic. J. Quant. Cell Sci. 2013, 83, 552–560.
- Chen, B.; Gilbert, L.A.; Cimini, B.A.; Schnitzbauer, J.; Zhang, W.; Li, G.W.; Park, J.; Blackburn, E.H.; Weissman, J.S.; Qi, L.S.; et al. Dynamic imaging of genomic loci in living human cells by an optimized CRISPR/Cas system. Cell 2013, 155, 1479–1491.
- Deng, W.; Shi, X.; Tjian, R.; Lionnet, T.; Singer, R.H. CASFISH: CRISPR/Cas9-mediated in situ labeling of genomic loci in fixed cells. Proc. Natl. Acad. Sci. USA 2015, 112, 11870–11875.
- Zhang, S.; Song, Z. Aio-Casilio: A robust CRISPR–Cas9–Pumilio system for chromosome labeling. J. Mol. Hist. 2017, 48, 293–299.
- Shao, S.; Zhang, W.; Hu, H.; Xue, B.; Qin, J.; Sun, C.; Sun, Y.; Wei, W.; Sun, Y. Long-term dual-color tracking of genomic loci by modified sgRNAs of the CRISPR/Cas9 system. Nucleic Acids Res. 2016, 4, e86.
- Duan, J.; Lu, G.; Hong, Y.; Hu, Q.; Mai, X.; Guo, J.; Si, X.; Wang, F.; Zhang, Y. Live imaging and tracking of genome regions in CRISPR/dCas9 knock-in mice. Genome Biol. 2018, 19, 192.
- Kim, H.; Li, F.; He, Q.; Deng, T.; Xu, J.; Jin, F.; Coarfa, C.; Putluri, N.; Liu, D.; Songyang, Z. Systematic analysis of human telomeric dysfunction using inducible telosome/shelterin CRISPR/Cas9 knockout cells. Cell Discov. 2017, 3, 17034.
- Dai, W.; Wu, J.; Wang, D.; Wang, J. Cancer gene therapy by NF-κB-activated cancer cell-specific expression of CRISPR/Cas9 targeting telomeres. Gene Ther. 2020, 27, 266–280.
- Abdisalaam, S.; Bhattacharya, S.; Mukherjee, S.; Sinha, D.; Srinivasan, K.; Zhu, M.; Akbay, E.A.; Sadek, H.A.; Shay, J.W.; Asaithamby, A. Dysfunctional telomeres trigger cellular senescence mediated by cyclic GMP-AMP synthase. J. Biol. Chem. 2020, 295, 11144–11160.
- Uno, N.; Hiramatsu, K.; Uno, K.; Komoto, S.; Kazuki, Y.; Oshimura, M. CRISPR/Cas9-induced transgene insertion and telomere-associated truncation of a single human chromosome for chromosome engineering in CHO and A9 cells. Sci. Rep. 2017, 7, 12739.
- Kim, H.; Ham, S.; Jo, M.; Lee, G.H.; Lee, Y.; Shin, J.; Lee, Y. CRISPR-Cas9 mediated telomere removal leads to mitochondrial stress and protein aggregation. Int. J. Mol. Sci. 2017, 18, 2093.
- Mao, P.; Liu, J.; Zhang, Z.; Zhang, H.; Liu, H.; Gao, S.; Rong, Y.; Zhao, Y. Homologous recombination-dependent repair of telomeric DSBs in proliferating human cells. Nat. Commun. 2016, 7, 12154.
- Carneiro, M.C.; Henriques, C.M.; Nabais, J.; Ferreira, T.; Carvalho, T.; Ferreira, M.G. Short Telomeres in Key Tissues Initiate Local and Systemic Aging in Zebrafish. PLoS Genet. 2016, 12, e1005798.
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging 2016, 8, 3–11.
- Beishline, K.; Vladimirova, O.; Tutton, S.; Wang, Z.; Deng, Z.; Lieberman, P.M. CTCF driven TERRA transcription facilitates completion of telomere DNA replication. Nat. Commun. 2017, 8, 2114.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revision:
1 time
(View History)
Update Date:
24 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




