Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jonti Evan Shepherd | + 3263 word(s) | 3263 | 2022-03-14 04:22:02 | | | |
| 2 | Rita Xu | Meta information modification | 3263 | 2022-03-23 04:33:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shepherd, J. Salt Stress in Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/20854 (accessed on 07 February 2026).
Shepherd J. Salt Stress in Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/20854. Accessed February 07, 2026.
Shepherd, Jonti. "Salt Stress in Plants" Encyclopedia, https://encyclopedia.pub/entry/20854 (accessed February 07, 2026).
Shepherd, J. (2022, March 22). Salt Stress in Plants. In Encyclopedia. https://encyclopedia.pub/entry/20854
Shepherd, Jonti. "Salt Stress in Plants." Encyclopedia. Web. 22 March, 2022.
Copy Citation
Salinization of soils and freshwater resources by natural processes and/or human activities has become an increasing issue that affects environmental services and socioeconomic relations. In addition, salinization jeopardizes agroecosystems, inducing salt stress in most cultivated plants (nutrient deficiency, pH and oxidative stress, biomass reduction), and directly affects the quality and quantity of food production. Depending on the type of salt/stress (alkaline or pH-neutral), specific approaches and solutions should be applied to ameliorate the situation on-site.
salt stress
neutral and alkaline salinity
plant–microbe associations
salinity and nanotechnology
1. Introduction
Increased concentrations of dissolved salts (ions) either in (i) water resources used for (fert)irrigational purposes (electrical conductivity in water: ECir > 1.0 mS/cm) or in (ii) soil solutions/extracts (electrical conductivity of saturated soil extracts: ECe > 2 mS/cm) usually induce one of the most widespread abiotic disorders in cultivated and native plant species, known as salt stress, after a mid-term period (e.g., one–two weeks) of exposure [1][2][3]. It is predicted that salt stress and related negative environmental implications will become even more critical, notably due to ongoing global climate change (e.g., more frequent and pronounced drought periods coupled with heat-stresses, underpinned evapotranspiration demands, over-increased average air temperature, rising sea levels, and the increasing tendency of generating grey waters, which are not purified to the appropriate level and are (re)used [4]. Accordingly, the most recent projections forecast the increase in irrigated agriculture from ~20% of totally cultivated land areas to 47% by 2030 [5], soliciting a difficult challenge to accomplish within a short period of time due to intense competition in the agri–domestic–industry triangle, posing markedly depleted and quality-constrained blue/green water resources [6].
Salt stress in agroecosystems disturbs crop food/feed yield production and quality due to a wide range of primary (osmotic stress, reduced nutrient uptake and growth) and more complex secondary salt-induced physiological disbalances (generation of reactive oxygen species and radicals which can damage proteins, membrane lipids, carbohydrates, and DNA structures) [1][3][7]. However, over-prolonged durations of salinity in permanent and unrecoverable soil degradation scenarios (e.g., dispersion of soil stable aggregates and structures, soil crusting, swamping, desertification) are realistic options depending on the geo-hydro-morphological and climatological conditions of salinity-exposed areas [2][8]. Based on the electrical conductivity of soil extracts (ECe), its pHH2O reaction and exchangeable sodium percentage (ESP) index, across terrestrial ecosystems, the most relevant are three common types of soil salinity [9]:
-
Saline soils (ECe > 4 mS/cm, pHH2O < 8.5, and ESP < 15)
-
Saline-alkaline or saline-sodic (ECe > 4 mS/cm, pHH2O < 8.5, and ESP > 15)
-
Alkaline or sodic soils (ECe > 4 mS/cm, pHH2O > 8.5, and ESP > 15).
It was confirmed that environmental implications could vary markedly among plant species and pedospheric conditions depending on salinity levels and intensity (duration). For instance, sodium chloride-induced salt stress, as one of the most common and widely elaborated (in controlled and natural conditions) abiotic stresses [10], is caused by relatively neutral salts, i.e., NaCl dissociation in the water matrix generates a neutral pH reaction (pH = 6.998; Section 2).
2. Neutral and Alkaline Salinity and Impacts to Plants
Table 1 presents some of the most relevant scenarios of salt-stressed rhizosphere conditions, both with neutral and alkaline salt types, based on the biogeochemical speciation approach. Briefly, particular salt types were separately dissolved in the rhizosphere solution, which corresponds to wide uncontaminated mineral soil conditions [11].
Table 1. Chemical speciation reactions for widely studied neutral and alkaline salt types dissolved in rhizosphere solution (after [12]).
| Neutral Salt Type | pH | Prevalent Ions (%) | Precipitated Forms |
|---|---|---|---|
| Sodium chloride NaCl |
7.94 | Na+ 98; Cl− 98 |
Magnesite Dolomite Hydroxyapatite Calcite Huntite Vaterite Artinite |
| Potassium chloride KCl |
7.94 | K+ 98; Cl− 98 |
|
| Magnesium chloride Mg Cl2 |
7.93 | Mg2+ 77; Cl− 98; Mg-OC 10; MgSO4 4; MgHCO3+ 4; MgCl+ 1 |
|
| Calcium chloride CaCl2 |
7.93 | Ca2+ 78; Cl− 98; Ca-organo-complexed forms 6; CaSO4 6; CaHCO3+ 5; CaCl+ 1 |
|
| Sodium sulphate Na2SO4 |
7.94 | Na+ 98; SO42− 72; CaSO4− 16; MgSO4− 10 |
|
| Alkaline Salt Type | pH | Prevalent Ions (%) | |
| Sodium hydrogencarbonate NaHCO3 |
8.01 | Na+ 98; HCO3− 92; CaHCO3+ 2; CaCO3 1 |
|
| Sodium carbonate Na2CO3 |
8.08 | Na+ 98.2; HCO3− 92; CaHCO3+ 2.2; CaCO3 1.3 |
|
| Potassium carbonate K2CO3 |
8.03 | K+ 98; HCO3− 92; CaHCO3+ 2; CaCO3 1 |
|
| Magnesium carbonate MgCO3 |
8.07 | Mg2+ 75; Mg-organo-complexed forms 10; MgHCO3+ 5; MgSO4 4; HCO3− 91; MgHCO3+ 2 |
|
| Calcium carbonate CaCO3 |
8.07 | Ca2+ 76; Ca-organo-complexed forms 6; CaHCO3+ 6; CaSO4 6; HCO3− 90; CO32− 1 |
According to the modeling results, the calculated pH values were shifted to mid-alkaline reactions (7.9–8.0), with confirmed organo-complexation of Mg (approximately 10%) and Ca (approximately 6%) within the dissolved organic pools. In addition, predicted mineral precipitations were attributed mostly to Ca/Mg phosphate and carbonate minerals (e.g., brucite, dolomite, hydroxyapatite, huntite, vaterite; Table 1).
Both salt types, neutral and alkaline, in certain scenarios can induce relevant salt disorders, i.e., stresses. However, the stress induced from neutral salt (NaCl, MgCl2, Na2SO4, CaCl2) will greatly differ from that induced by alkaline salt (Na2CO3, NaHCO3, K2CO3) [1][13] (Table 1). Accordingly, it was shown that more neutral salts mostly disrupt macro/micronutrient homeostasis, causing adverse osmotic imbalances and damage [14]. In the presence of more alkaline salts, the negatively induced effects will be almost identical; however, additional adverse impacts will be further aggravated due to the increased (alkaline) pH reaction of surrounding media, different ionic strengths, and different biogeochemical reactions, along with chemical speciation (Table 1). For instance, under more alkaline rhizosphere conditions, the mobility and availability of certain essential nutrient chemical forms (free Ca, Mg, Cl, H2PO4−) can be markedly reduced because of precipitation reactions and consequently homeostasis imbalance [13][14][15] (Table 1). Furthermore, a high alkaline pH reaction can additionally damage the structure of the root cell membrane, disrupting its structural integrity and functionality [1][16][17] (Figure 1A–C). Additionally, significantly lower tolerances of plants to alkaline vs. neutral salt stress have been documented thus far [17][18][19].

However, it was shown that excessive salinity can impose crucial implications on trace element soil biogeochemistry (Table 1), consequently either improving or limiting the uptake and accumulation of trace elements. For instance, the authors of [22] recently detected that NaCl salinity-induced root exudates in halophytic mangrove plant species (Avicennia marina) are very effective in binding Cu2+, Mn2+, and Cd2+, which can limit not only the phytoavailability but also the transfer of metals in deeper aquatic systems. Additionally, the authors of [23] confirmed inhibited Cd uptake in two other mangrove species (Rhizophora apiculata and Avicennia alba) exposed to increased salinity. In contrast, it was confirmed that salinity can enhance the uptake and deposition of toxic Cd and/or essential phytonutrients (Cu, Zn, Mn) in the native halophyte Carpobrotus rossii [24]. Similar effects were also observed in different glycophytes, such as in edible amaranth cultivars [25][26], muskmelon [27], radish cultivars [10], and strawberry (Figure 1). Such implications can be explained by geochemical interrelations in the rhizosphere. Namely, increased ionic strength in the rhizosphere solution under the presence of dissolved Cl− can enhance trace element mobility (Table 1) via complexation reactions, accompanied by saturated reactive sites of the soil matrix through adsorption with Na+ [11].
To cope with excessive salt forms (Table 1) and different alkalinity- or salinity-induced disorders (Figure 1), higher plants, notably tolerant to excessive salinity, have developed a wide range of abiotic stress-adaptive strategies, including detoxification, regulating osmotic adjustment, maintaining cationic/anionic balance, scavenging reactive oxygen species, and synthesizing compatible solutes [13][28][29]. Unfortunately, most halophytes are still not as relevant as agricultural food production, although some, such as mangroves, represent irreplaceable ecological value in coastal mariculture as well as environmental protection from different anthropogenic and natural pressures (pollutions, salinization [22][23]), assured by invaluable genetic pools for possible bioengineering developments (Section 3).
3. Sustainable Approaches and Solutions to Improve Plant Nutrition and Crop Production in Saline Conditions
It is possible to implement a wide range of sustainable preventive and proactive (reclamation) approaches separately and/or in combination to improve plant salt resistance and crop nutrition under salt-affected conditions (e.g., Figure 2). For instance, some halophytic strategies (traits) could be transferred to glycophytes (most cultivated crops, relatively sensitive to salinity), improving their resistance to salinity. Breeding and genetic approaches, such as the selection and creation of salt-resistant genotype(s), over (i) traditional breeding processes [30], (ii) marker-assisted selection [29][30][31][32], (iii) molecular and transgenic approaches [33], or (iv) genome editing (using the CRISPR/Cas9 tool) [34], have been the focus for an extended time, and some solutions have been successfully implemented for the alleviation of salt-affected crop production. However, certain constraints, such as high technological dependency, time-consuming procedures, unpredictable genetic gain, and extraordinarily diverse genotype–environment interactions (multi-collinearity [33]), still represent substantial limitation(s) in the progressive improvement of these approaches to increase salt tolerance in target crops.
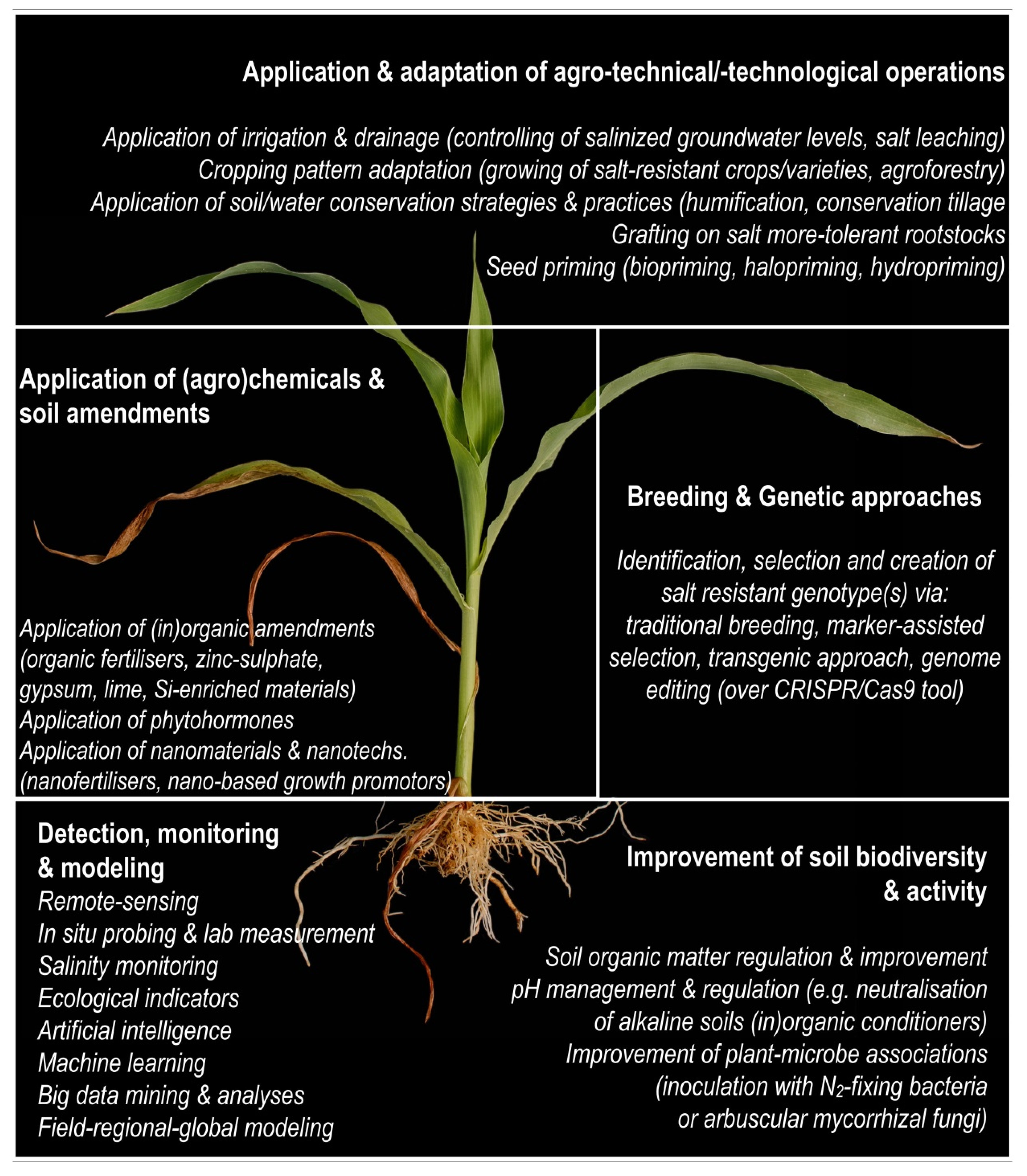
Figure 2. Solutions for salinization management and mitigating salt stress in plants.
Application and adaptation of specific agro/technical/technological operations can also ensure a wide spectrum of land, water, and crop management solutions for controlling and avoiding detrimental effects of salinity to crops [12]. Some of the most applicative strategies are controlled water management over the applications of (i) modern, low-pressurized, and localized irrigation [4], and if necessary, (ii) surface/underground drainage systems [35]. Both systems can help maintain salinized groundwater levels below the critical root zone level and leach concentrated salts from the rhizosphere.
Spatiotemporal adaptation of cropping patterns (e.g., during high evapotranspiration demands, on the lowest terrain positions), such as growing salt-resistant crops/cultivars/varieties in a single, double, or multiple cropping system (agroforestry, combining forage and cereal crops), has been confirmed as a very effective option to alleviate soil salinity and co-occurring environmental constraints of (semi)arid (agro)ecosystems (Figure 2). For instance, mixed cropping (vs. mono-cropping) with two or more cultures simultaneously has been confirmed to be more effective in soil C and N restoration [36]. The same authors reported that mixed cropping demonstrated changing effects on crop growth, which depends upon the plant species. It also offers better protection against soil deterioration and the disruption of pests and pathogenic bacteria and fungus. In addition, this system helps to reduce water erosion and groundwater salinization/contamination. In many developing countries, intercropping has been effectively used in low-input harvesting systems for enhancing land use and cultivating water and nutrient regimes. In Bangladesh, farmers have introduced intercropping systems into sugarcane farming, which increases the midterm return (in 12–14 months) along with the total profit [37]. This mechanism boasts improved water uptake, better root absorptions, or balancing exploration over the soil profile. Salt-affected areas often overlap with water-stressed, organically depleted, and poorly structured sandy soils, which are knowingly compatible for implementation in land and water conservation practices (humification, reduced/zero tillage) and can additionally underpin crop nutrition under saline conditions (Figure 2).
Grafted crops, which combine within- or inter-species organisms at the rootstock and scion, are widely used in horticulture mostly to overcome different constraints and stresses, including salt stress. It was confirmed that grafting scions with more salt-tolerant rootstock varieties can improve the salt resistance of grafted plants over restrictions in salt (Na+, Cl−) uptake and enhance antioxidant enzymatic activities and hormonal adaptations to saline environments [38][39]. Grafting among genetically different and distant genera (species) is still widely unexplored but seems to be a very promising approach to improve many physiological reactions, such as salt-induced ones [40].
Soil amelioration with certain organic (more in Section 4) and/or inorganic (lime, gypsum, bottom and fly biomass/coal ash, saturated mud from sugar refineries) conditioners and amendments can also reclaim constrained lands and beneficially enhance crop production in such conditions in multiple ways. For example, applying an appropriate drainage system and providing quality irrigation water for leaching accumulated salts can be an effective measure for reclamation of saline soils. Still, saline-sodic and sodic soils (with elevated levels of Na+) usually demand the application of appropriate (Ca-/Mg-based) amendments to aid the amelioration [9][12]. Additionally, both Ca and Mg have the potential to further alleviate (sub)soil constraints over (i) stabilizing and improving the soil structure, (ii) reducing the sodium adsorption ratio (SAR), (iii) increasing flocculation, through improving soil structure, and thus decreasing clay dispersion, and (iv) improving water–air relations and many others (Figure 2).
Exogenous application of phytohormones has also been confirmed as a very effective and promising strategy against environmental stresses, including salt stress [41]. Phytohormone imbalance is very often a salt-induced phytoreaction. Phytohormones are a broad group of naturally occurring molecules or compounds (e.g., ethylene, auxin, gibberellins, cytokinins, strigolactones, brassinosteroids, abscisic/jasmonic/salicylic acid) which regulate plant growth and development under homeostasis and are irreplaceable in signaling transduction pathways during reaction stresses [42][43]. Recent studies suggest how phytohormones might be a crucial metabolic engineering target for creating salt stress-tolerant crop plants (see review in [43]). For instance, auxin can improve the growth performance in plants under salinity stress [44], albeit in some crops, salinity can reduce auxin levels. Moreover, exogenous addition of salicylic acid can effectively increase endogenous auxin and abscisic acid content and improve the growth performance in salt-stressed corn plants [45]. Similarly, the exogenous addition of jasmonic acid also seems to have the potential to alleviate salt-induced adverse impacts in plants. The latter is related to improved physiological properties (e.g., increase chlorophyll content and antioxidant enzymatic activity, reduce lipid peroxidation, improved K nutrition), which enhance plant growth and yield performance [41][46][47].
4. Salinity Amelioration by Organic Amendments
New research strategies that promote the benefits of different organic amendments for plant growth in saline/sodic soils report about the reduction of oxidative and osmotic stress, improving the conductance and stomatal density and the seed germination rate, prompting an increase in microbial activities [48], and many others. Implementing organic materials demonstrated significant benefits, improving the saline soil biome by enriching it with compost, green manure, poultry manure, and sugarcane remnants (press-mud) [49][50]. These organic amendments heighten the dissolution percentage of calcite (CaCO3) via the increased formation of carbonic acid while improving the binding of the small particles, effectively forming substantially sizable aggregates that remain unwavering within water [51]. This method is effective in both calcareous as well as non-calcareous soils because the large-sized individual organic particles create channels in poorly structured saline or sodic soils, and thus aide in ameliorating the soil permeability while leaching Na+ from the cation exchange sites over the soil profile [52]. The selection of a sustainable reclamation technique and organic material is an extremely important factor that should be determined via the analysis of both site-specific geographical and soil physicochemical parameters [53]. Among a wide range of soil organic amendments, biochar has been intensively studied recently as effectively improving the physicochemical and biological properties of saline/sodic soils.
Identical to non-saline soils, salt-affected soils benefit from the addition of biochar due to the freshly provided habitat created from the biochar, encompassing the ability to sustain vast multitudes of soil microorganisms, providing essential living elements to be compounded with the gained organic carbons and nutrients. Moreover, biochar stabilizes the soil structure, enhancing physical properties by balancing both the air porosity and water content, in relation to the cation ion exchange capacity [49]. Average types of biochar’s will increase the rate at which salt leaching occurs, effectively remediating the site for the immediate use of crop farming. Additionally, soil organic carbons aide in binding soil aggregates for a sustained long-term capacity in comparison to some other types of organic amendments stemming from non-degradable molecular makeups [53]. Biochar application improves the total porosity and water-holding capacity of salt-affected soils, but the effect appears to depend primarily on the feedstock type in combination with the organic material that is being used as the base source for the final product [52]. The reason for this is because biochar is created via the burning of organic materials in conditions either lacking or without O2, presenting a product that is a C-rich material achieved utilizing temperatures ranging from 300 to 1000 °C [54]. Due to the different organic constitutes that biochar is composed of, not all types will expend influences similar to that of one particular soil type, as well as no individual biochar can be effective within all (saline) soils [55]. This can be explained by examining biochar that is created utilizing non-woody raw materials, such as plant residues and numerous types of manure that are ample in nutrient content while rendering a less stable C with a higher pH than biochar generated from dry plant mass [56]. Thus, using biochar as a soil amendment in saline soils will effectively ameliorate the soil profile for superior growing conditions as various studies have proven the application in mitigating damages caused by salt stress [57]. Beneficial implications under biochar application are accomplished by: (i) the reduction of transient N via the process of adsorption, (ii) the release of both macro- and micro-mineral nutrients, and (iii) the decrease in stress factors caused by osmosis accomplished via improved water availability within the soil [58]. Due to strong absorptive properties, extremely high porosity, cation exchange capacity, and large surface area, biochar bind potentially toxic salt ions (Na+) at different magnitudes [56]. Moreover, such properties allow the desorption of potentially beneficial ions into the soil, effectively ameliorating nutrient misbalances caused by salinity [12][54].
Growth parameters such as photosynthetic rate, stomatal conductance, and transpiration rate are confidently influenced by biochar treatments, suggesting that biochar will reduce the adverse effects of salinity pressure on plants [52]. In addition, biochar can improve vital components related to crop yield, such as shoot biomass, root length, as well as yield in potatoes [56], maize, and tomatoes [59], grown under salinity conditions. The vast reaching impact of biochar on both the production of biomass and growth of herbaceous species can be examined in studies that have allowed Prunella vulgaris and Abutilon theophrasti to become exposed to salinity stress, revealing that biomass and plant growth were positively affected in both plant species in comparison to the control. However, it should be noted that biochar did not have a significant influence over the photosynthetic boundaries in either species while under salinity stress factors [60]. It can be stated that the response from each individual plant species differs enough to create prerequisites in order for a specific type of biochar to be recommended. Photosynthetic parameters increased within amended soils, leading to the rate of which plant growth stimulation occurred [61]. These findings revealed that biochar is able to be utilized as a stable organic amendment to soils for the purpose of mitigating salinity in grain crops [56]. The primary reason was that the utilization of biochar that has been tested for each area of specific soils and crop types has shown that the reduction in water was due to induced stomatal closure and regulation of transpiration, causing a higher efficiency [48], and thus leading to the preservation of both water balance and leaf turgidity within saline soil biomes. Plants develop antioxidant defense systems to cope with salt stress induced by oxidative damage. In addition, it has been confirmed that the increase in antioxidant enzymes triggered by the activation of plant defense mechanisms can be regulated by the application of biochar [58]. At heightened natural salinity levels (EC 1.26–2.00 mmhos/cm), it was recorded that lower catalase (CAT) and peroxidase (POD) activity occurred within biochar treatments of a 5% capacity, while lower superoxide dismutase (SOD) activity was recorded at treatment capacities of 2.5% accordingly [54]. However, at the biochar dosage of 10% (vs. control), a nonsignificant impact on antioxidant enzymatic activities was recorded [54]. Thus, a small percentage of biochar amended into the soil can alleviate many of the salinity-induced harmful effects on antioxidant enzymes. However, at higher (>10%) biochar application rates, negative consequences related to the increase in antioxidant enzymes [59] could be expected, the main reason being that there is a negative impact from the addition of biochar on growth due to high salinity and N immobilization [60]. Overall, the application of biochar reduced plant Na uptake due to transient Na+ binding, again due to its high adsorption capacity, which is responsible for decreasing osmotic stress by enhancing the soil’s moisture content and releasing mineral nutrients into the soil solution [56]. This point to the improved K/Na ratio, through which enhancing potassium (K) availability will substantially increase the majority of grain type plant growth and yield under saline soil stress factors [58].
References
- Song, T.; Xu, H.; Sun, N.; Jiang, L.; Tian, P.; Yong, Y.; Yang, W.; Cai, H.; Cui, G. Metabolomic Analysis of Alfalfa (Medicago sativa L.) Root-Symbiotic Rhizobia Responses under Alkali Stress. Front. Plant Sci. 2017, 8, 1208.
- Ondrasek, G.; Jelovica Badovinac, I.; Peter, R.; Petravić, M.; Macan, J.; Rengel, Z. Humates and Chlorides Synergistically Increase Cd Phytoaccumulation in Strawberry Fruits, Heightening Health Risk from Cd in Human Diet. Expo. Health 2022.
- Zhao, G.; Zhao, Y.; Lou, W.; Su, J.; Wei, S.; Yang, X.; Wang, R.; Guan, R.; Pu, H.; Shen, W. Nitrate Reductase-Dependent Nitric Oxide Is Crucial for Multi-Walled Carbon Nanotube-Induced Plant Tolerance against Salinity. Nanoscale 2019, 11, 10511–10523.
- Ondrašek, G. Irrigation in Agroecosystems; IntechOpen: London, UK, 2019.
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050. Summary Version; Licence: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018; p. 60.
- Ondrasek, G. Drought—Detection and Solutions; IntechOpen: London, UK, 2020.
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930.
- Ondrasek, G.; Rengel, Z.; Veres, S. Soil Salinisation and Salt Stress in Crop Production. In Abiotic Stress in Plants—Mechanisms and Adaptations; IntechOpen: London, UK, 2011.
- Horney, R.D.; Taylor, B.; Munk, D.S.; Roberts, B.A.; Lesch, S.M.; Plant, R.E. Development of Practical Site-Specific Management Methods for Reclaiming Salt-Affected Soil. Comput. Electron. Agric. 2005, 46, 379–397.
- Ondrasek, G.; Romic, D.; Rengel, Z. Interactions of Humates and Chlorides with Cadmium Drive Soil Cadmium Chemistry and Uptake by Radish Cultivars. Sci. Total Environ. 2020, 702, 134887.
- Ondrasek, G.; Rengel, Z. The Role of Soil Organic Matter in Trace Element Bioavailability and Toxicity. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 403–423.
- Ondrasek, G.; Rengel, Z. Environmental Salinization Processes: Detection, Implications & Solutions. Sci. Total Environ. 2021, 754, 142432.
- Guo, R.; Shi, L.X.; Yan, C.; Zhong, X.; Gu, F.X.; Liu, Q.; Xia, X.; Li, H. Ionomic and Metabolic Responses to Neutral Salt or Alkaline Salt Stresses in Maize (Zea Mays L.) Seedlings. BMC Plant Biol. 2017, 17, 41.
- Yang, C.; Chong, J.; Li, C.; Kim, C.; Shi, D.; Wang, D. Osmotic Adjustment and Ion Balance Traits of an Alkali Resistant Halophyte Kochia Sieversiana during Adaptation to Salt and Alkali Conditions. Plant Soil 2007, 294, 263–276.
- Yang, C.W.; Xu, H.H.; Wang, L.L.; Liu, J.; Shi, D.C.; Wang, D.L. Comparative Effects of Salt-Stress and Alkali-Stress on the Growth, Photosynthesis, Solute Accumulation, and Ion Balance of Barley Plants. Photosynthetica 2009, 47, 79–86.
- Wang, H.; Ahan, J.; Wu, Z.H.; Shi, D.C.; Liu, B.; Yang, C.W. Alteration of nitrogen metabolism in rice variety ‘Nipponbare’ induced by alkali stress. Plant Soil 2012, 355, 131–147.
- Wang, X.; Chen, W.; Zhou, Y.; Han, J.; Zhao, J.; Shi, D.; Yang, C. Comparison of Adaptive Strategies of Alfalfa (Medicago Sativa L.) to Salt and Alkali Stresses. AJCS 2012, 6, 309–315.
- Yang, C.W.; Jianaer, A.; Li, C.Y.; Shi, D.C.; Wang, D.L. Comparison of the Effects of Salt-Stress and Alkali-Stress on Photosynthesis and Energy Storage of an Alkali-Resistant Halophyte Chloris Virgata. Photosynthetica 2008, 2, 273–278.
- Guo, R.; Shi, L.X.; Ding, X.M.; Hu, Y.; Tian, S.Y.; Yan, D.F.; Shao, S.; Gao, Y.; Liu, R.; Yang, Y.F. Effects of Saline and Alkaline Stress on Germination, Seedling Growth, and Ion Balance in Wheat. Agron. J. 2010, 102, 1252–1260.
- Ondrašek, G.; Romić, D.; Romić, M.; Duralija, B.; Mustač, I. Strawberry Growth and Fruit Yield in a Saline Environment. Agric. Conspec. Sci. 2006, 71, 155–158.
- Ondrasek, G.; Rengel, Z.; Maurović, N.; Kondres, N.; Filipović, V.; Savić, R.; Blagojevic, B.; Tanaskovik, V.; Meriño-Gergichevich, C.; Romić, D. Growth and Element Uptake by Salt-Sensitive Crops under Combined NaCl and Cd Stresses. Plants 2021, 10, 1202.
- Zhu, C.Q.; Ghoto, K.; Gao, G.F.; Chen, J.; Hu, W.J.; Qiao, F.; Liu, J.Y.; Zheng, H.L. Trace Metals Complexation Behavior with Root Exudates Induced by Salinity from a Mangrove Plant Avicennia marina (Forsk.) Vierh. Bioremediat. J. 2019, 23, 82–93.
- Sari, I.; Din, Z.B. Effects of Salinity on the Uptake of Lead and Cadmium by Two Mangrove Species Rhizophora apiculata Bl. and Avicennia alba Bl. Chem. Ecol. 2012, 28, 365–374.
- Zhang, C.; Sale, P.W.G.; Tang, C. Cadmium Uptake by Carpobrotus rossii (Haw.) Schwantes under Different Saline Conditions. Environ. Sci. Pollut. Res. 2016, 23, 13480–13488.
- He, B.Y.; Yu, D.P.; Chen, Y.; Shi, J.L.; Xia, Y.; Li, Q.S.; Wang, L.L.; Ling, L.; Zeng, E.Y. Use of Low-Calcium Cultivars to Reduce Cadmium Uptake and Accumulation in Edible Amaranth (Amaranthus mangostanus L.). Chemosphere 2017, 171, 588–594.
- Guo, S.H.; Hu, N.; Li, Q.S.; Yang, P.; Wang, L.L.; Xu, Z.M.; Chen, H.J.; He, B.Y.; Zeng, E.Y. Response of Edible Amaranth Cultivar to Salt Stress Led to Cd Mobilization in Rhizosphere Soil: A Metabolomic Analysis. Environ. Pollut. 2018, 241, 422–431.
- Ondrasek, G.; Romic, D.; Rengel, Z.; Romic, M.; Zovko, M. Erratum to “Cadmium Accumulation by Muskmelon under Salt Stress in Contaminated Organic Soil”. Sci. Total Environ. 2009, 407, 4785.
- Wang, Y.; Sun, G.; Suo, B.; Chen, G.; Wang, J.; Yan, Y. Effects of Na2CO3 and NaCl Stresses on the Antioxidant Enzymes of Chloroplasts and Chlorophyll Fluorescence Parameters of Leaves of Puccinellia tenuiflora (Turcz.) Scribn.et Merr. Acta Physiol. Plant. 2008, 30, 143–150.
- Ye, Y.; Medina-Velo, I.A.; Cota-Ruiz, K.; Moreno-Olivas, F. Can Abiotic Stresses in Plants Be Alleviated by Manganese Nanoparticles or Compounds? Ecotoxicol. Environ. Saf. 2019, 184, 109671.
- Mujeeb-Kazi, A.; Munns, R.; Rasheed, A.; Ogbonnaya, F.C.; Ali, N.; Hollington, P.; Dundas, I.; Saeed, N.; Wang, R.; Rengasamy, P.; et al. Breeding Strategies for Structuring Salinity Tolerance in Wheat. Adv. Agron. 2019, 155, 121–187.
- Bor, M.; Özdemir, F. Manipulating Metabolic Pathways for Development of Salt-Tolerant Crops. In Salinity Responses and Tolerance in Plants, Volume 1: Targeting Sensory, Transport and Signaling Mechanisms; Springer: Cham, Switzerland, 2018; pp. 235–256.
- Quan, R.; Wang, J.; Hui, J.; Bai, H.; Lyu, X.; Zhu, Y.; Zhang, H.; Zhang, Z.; Li, S.; Huang, R. Improvement of Salt Tolerance Using Wild Rice Genes. Front. Plant Sci. 2018, 8, 2269.
- Sarangi, S.; Mandal, C.; Dutta, S.; Mukherjee, P.; Mondal, R.; Kumar, S.P.J.; Choudhury, P.R.; Singh, V.P.; Tripathi, D.K.; Mandal, A.B. Microprojectile Based Particle Bombardment in Development of Transgenic Indica Rice Involving AmSOD Gene to Impart Tolerance to Salinity. Plant Gene 2019, 19, 100183.
- Farhat, S.; Jain, N.; Singh, N.; Sreevathsa, R.; Dash, P.K.; Rai, R.; Yadav, S.; Kumar, P.; Sarkar, A.K.; Jain, A.; et al. CRISPR-Cas9 Directed Genome Engineering for Enhancing Salt Stress Tolerance in Rice. Semin. Cell Dev. Biol. 2019, 96, 91–99.
- Ondrasek, G.; Rengel, Z.; Petosic, D.; Filipovic, V. Land and Water Management Strategies for the Improvement of Crop Production. Emerg. Technol. Manag. Crop Stress Toler. 2014, 2, 291–313.
- Amoah, A.A.; Miyagawa, S.; Kawakubo, N. Effect of Supplementing Inorganic Fertilizer with Organic Fertilizer on Growth and Yield of Rice-Cowpea Mixed Crop. Plant Prod. Sci. 2015, 15, 109–117. Available online: http://www.tandfonline.com/action/authorSubmission?journalCode=tpps20&page=instructions (accessed on 7 February 2022).
- Rahman, M.S.; Khatun, S.; Rahman, M.K. Sugarcane and Sugar Industry in Bangladesh: An Overview. Sugar Tech 2016, 18, 627–635.
- He, Y.; Zhu, Z.; Yang, J.; Ni, X.; Zhu, B. Grafting Increases the Salt Tolerance of Tomato by Improvement of Photosynthesis and Enhancement of Antioxidant Enzymes Activity. Environ. Exp. Bot. 2009, 66, 270–278.
- Xiaohui, F.; Kai, G.; Ce, Y.; Jinsong, L.; Huanyu, C.; Xiaojing, L. Growth and Fruit Production of Tomato Grafted onto Wolfberry (Lycium chinense) Rootstock in Saline Soil. Sci. Hortic. 2019, 255, 298–305.
- Khaldun, A.B.M.; Huang, W.; Lv, H.; Liao, S.; Zeng, S.; Wang, Y. Comparative Profiling of MiRNAs and Target Gene Identification in Distant-Grafting between Tomato and Lycium (Goji Berry). Front. Plant Sci. 2016, 7, 1475.
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Jasmonates: Mechanisms and Functions in Abiotic Stress Tolerance of Plants. Biocatal. Agric. Biotechnol. 2019, 20, 101210.
- Peleg, Z.; Blumwald, E. Hormone Balance and Abiotic Stress Tolerance in Crop Plants. Curr. Opin. Plant Biol. 2011, 14, 290–295.
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and Their Metabolic Engineering for Abiotic Stress Tolerance in Crop Plants. Crop J. 2016, 4, 162–176.
- Egamberdieva, D. Alleviation of Salt Stress by Plant Growth Regulators and IAA Producing Bacteria in Wheat. Acta Physiol. Plant. 2009, 31, 861–864.
- Fahad, S.; Bano, A. Effect of Salicylic Acid on Physiological and Biochemical Characterization of Maize Grown in Saline Area. Pak. J. Bot. 2012, 44, 1433–1438.
- Ghassemi-Golezani, K.; Hosseinzadeh-Mahootchi, A. Improving Physiological Performance of Safflower under Salt Stress by Application of Salicylic Acid and Jasmonic Acid. WALIA J. 2015, 31, 104–109.
- Faghih, S.; Ghobadi, C.; Zarei, A. Response of Strawberry Plant Cv. ‘Camarosa’ to Salicylic Acid and Methyl Jasmonate Application Under Salt Stress Condition. J. Plant Growth Regul. 2017, 36, 651–659.
- Ahmed, A.; Kurian, J.; Raghavan, V. Biochar Influences on Agricultural Soils, Crop Production, and the Environment: A Review. Environ. Rev. 2016, 24, 495–502.
- Liu, D.; Ding, Z.; Ali, E.F.; Kheir, A.M.S.; Eissa, M.A.; Ibrahim, O.H.M. Biochar and Compost Enhance Soil Quality and Growth of Roselle (Hibiscus Sabdariffa L.) under Saline Conditions. Sci. Rep. 2021, 11, 8739.
- Ondrasek, G.; Bakić Begić, H.; Zovko, M.; Filipović, L.; Meriño-Gergichevich, C.; Savić, R.; Rengel, Z. Biogeochemistry of Soil Organic Matter in Agroecosystems & Environmental Implications. Sci. Total Environ. 2019, 658, 1559–1573.
- Ibrahim, M.E.H.; Ali, A.Y.A.; Zhou, G.; Elsiddig, A.M.I.; Zhu, G.; Nimir, N.E.A.; Ahmad, I. Biochar Application Affects Forage Sorghum under Salinity Stress. Chil. J. Agric. Res. 2020, 80, 317–325.
- Chaganti, V.N.; Crohn, D.M. Evaluating the Relative Contribution of Physiochemical and Biological Factors in Ameliorating a Saline-Sodic Soil Amended with Composts and Biochar and Leached with Reclaimed Water. Geoderma 2015, 259–260, 45–55.
- Carolina Feitosa de Vasconcelos, A. Biochar Effects on Amelioration of Adverse Salinity Effects in Soils. In Applications of Biochar for Environmental Safety; IntechOpen: London, UK, 2020.
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar Application for the Remediation of Salt-Affected Soils: Challenges and Opportunities. Sci. Total Environ. 2018, 625, 320–335.
- Ekebafe, M.O.; Ekebafe, L.O.; Maliki, M. Utilisation of Biochar and Superabsorbent Polymers for Soil Amendment. Sci. Prog. 2013, 96, 85–94.
- Mona, S.; Bhateria, R.; Deepak, B.; Kiran, B.; Nisha, R. Biochar for Reclamation of Saline Soils. In Microorganisms in Saline Environments: Strategies and Functions; Springer: Cham, Switzerland, 2019; pp. 451–466.
- Sun, J.; He, F.; Shao, H.; Zhang, Z.; Xu, G. Effects of Biochar Application on Suaeda Salsa Growth and Saline Soil Properties. Environ. Earth Sci. 2016, 75, 630.
- Xiaoqin, S.; Dongli, S.; Yuanhang, F.; Hongde, W.; Lei, G. Three-Dimensional Fractal Characteristics of Soil Pore Structure and Their Relationships with Hydraulic Parameters in Biochar-Amended Saline Soil. Soil Tillage Res. 2021, 205, 104809.
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar Increases Arbuscular Mycorrhizal Plant Growth Enhancement and Ameliorates Salinity Stress. Appl. Soil Ecol. 2015, 96, 114–121.
- Rhodes, C.J. Feeding and Healing the World: Through Regenerative Agriculture and Permaculture. Sci. Prog. 2013, 95, 345–446.
- Noori, Z.; Delavar, M.A.; Safari, Y. Applying Biochar and Mineral Amendments to Remediate the Chemical Properties of a Saline-Sodic Soil. J. Sci. Technol. Agric. Nat. Resour. 2021, 24, 21–36.
More
Information
Subjects:
Agriculture, Dairy & Animal Science; Area Studies
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
23 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




