| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | roberto piciotti | + 4017 word(s) | 4017 | 2022-03-07 07:23:26 | | | |
| 2 | Conner Chen | -166 word(s) | 3851 | 2022-03-22 08:05:01 | | |
Video Upload Options
Non-alcoholic fatty liver disease (NAFLD) is characterized by an enhanced activation of the immune system, which predispose the evolution to nonalcoholic steatohepatitis (NASH) and hepatocellular carcinoma (HCC). Resident macrophages and leukocytes exert a key role in the pathogenesis of NAFLD. In particular, CD4+ effector T cells are activated during the early stages of liver inflammation and are followed by the increase of natural killer T cells and of CD8+ T cytotoxic lymphocytes which contribute to auto-aggressive tissue damage. To counteract T cells activation, programmed cell death 1 (PD-1) and its ligand PDL-1 are exposed respectively on lymphocytes and liver cells’ surface and can be targeted for therapy by using specific monoclonal antibodies, such as of Nivolumab, Pembrolizumab and Atezolizumab.
1.Introduction
Non-alcoholic fatty liver disease (NAFLD) is the commonest chronic liver disorder in Western countries and is defined by fat accumulation in more than 5% of hepatocytes in the absence of other causes of liver diseases [1]. NAFLD is usually a mild disease characterized by simple steatosis, however it may progress to more severe forms where inflammation (non-alcoholic steatohepatitis NASH) and fibrosis occur, possibly evolving to cirrhosis and hepatocellular carcinoma (HCC) [2]. In particular, HCC is currently the fourth leading cause of cancer [3]and it is estimated that NAFLD related cirrhosis and HCC will become the main indication for liver transplantation in the next years [4].
The pathogenesis of NAFLD is very complex and involves a "multiple hits" process where different insults act together to foster fat accumulation, inflammation and progressive liver damage[5]. Among all concurring factors, insulin resistance (IR), oxidative stress, hormones and cytokines released by adipose tissue and alterations in gut microbiota are key determinants. In addition, both genetic and epigenetic factors predispose to NAFLD and its advanced forms, including HCC [5].
Even though the pathogenesis and progression of NAFLD are multifactorial, inflammation is considered a key player in the evolution to advanced disease and HCC [6]. Indeed, the liver hosts several resident immune system cells, in particular resident macrophages called Kuppfer cells (KCs), natural killer cells (NK cells), natural killer T cells (NKT cells) and lymphocytes. In the presence of obesity, IR and lipotoxicity, the immune system is activated in the liver, with a corresponding grade of inflammation triggered by the “wound healing response”. When inflammatory signals persist, monocytes and neutrophils are recruited in the liver, as well as other lymphocytes with an increase in CD8+ occurs, with consequent further rise of the inflammatory response and activation of fibrogenesis and carcinogenesis cascade [7].
Different regulatory pathways counterbalance immune system activation. For example, the programmed cell death protein 1 (PD-1), a membrane protein expressed on all T cells, acts together with T-cell immunoglobulin mucin-3 (Tim-3) inhibiting their activity. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) competes with CD28 for binding of B7 molecules and results in a net negative signal for the proliferation and the survival of the T cell[8] . In this regard, if on the one hand a hyperactivated immune system contributes to progression from steatosis to NASH and fibrosis, on the other hand when downregulated, it favors hepatic carcinogenesis due to insufficient tumor surveillance.
Several studies have shown that the gut microbiota plays a key role in the pathogenesis of NAFLD [9][10]. Interestingly, dysfunctions in the gut are associated with NAFLD and NASH and lead to the release of pathogen-associated molecular patterns (PAMPs) and bacterial products from enterohepatic circulation and contributes to liver inflammation [11].
Thus, although targeting the immune system is emerging as a promising strategy for both NAFLD and HCC treatment, several questions are still unsolved. In addition, contrasting data are accumulating in literature concerning either in vitro and in vivo models as well as in patients.
2.The hepatic immunological milieu
The liver has a pivotal role in the modulation of the immune response maintaining immunotolerance to non-pathological antigens and responding to constant inflammatory stimuli. This implies the assessment of a complex immunological network and the establishment of important interactions between liver-resident cells and peripheral leukocytes. The liver, indeed, represents a milieu of innate and adaptive immune cells and accounts for the largest population of resident macrophages (Kupffer cells (KCs)) in the body, a high density of natural killer cells (NK cells), natural killer T cells (NKT cells) and of resident lymphocytes [12].
Moreover, compared to the periphery, a greater ratio of CD8+ T to CD4+ T cells is also reported in the liver [13] . CD8+T cells recognize and kill cells presenting antigens through the binding of MHC class I molecules and T-cell receptor (TCR) on cell surface. For a correct activation, CD8+ Ts need a second co-stimulatory signal by B7 expressed on antigen presenting cells (APCs) binding to CD28. CD4+ T cells recognizing the peptides presented by MHC class II molecules play an important role in protecting the liver from infections but are also involved in hepatocellular damage and autoimmunity [14]. In the liver, CD4+ T cells can acquire different cell states (i.e., TH1, TH2, and TH17cells) characterized by specific cytokine profiles, anyway during inflammation they can display a mixed phenotype showing simultaneously features of different polarization status [15].
CD8+ T and CD4+ T activity can be inhibited by specific negative regulators. In this regard, PD-1, a membrane protein exposed by all T cells, counteracts lymphocytes activation by engaging its ligands programmed cell death 1 ligand 1 (PD-L1) [16]. PD-1 is constitutively expressed in both humans and mice T cell population and its upregulation is directly promoted by interferon (IFN)-γ. PD-1/PD-L1 T cell inhibition occurs by interfering with TCR/CD28 signaling and leads to reduced pro-inflammatory cytokine production (e.g. IL-2, IFN-γ, and tumor necrosis factor (TNF)-α).
Important participants in liver immune response are also NKT cells able to kill target cells and to produce a wide spectrum of pro- and anti-inflammatory molecules (for example, IFN-γ, IL-4 and IL-17) [17]. Additionally, liver basal immune activity is also exerted by liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs) and hepatocytes which act as APCs triggering cytokine production [18]. Considering this, deregulation of liver’s tightly controlled immunological network may be a hallmark of tissue damage and may contribute to liver disease.
New evidence claims that immune cells, both innate and adaptive, are skewed toward a proinflammatory phenotype in the liver with NAFLD and NASH [19]. Indeed, activation of immune response and the release of proinflammatory cytokines promote damaging of hepatocyte and activation of HSCs while the early stages of liver inflammation in NAFLD and NASH are dominated by CD4+ effector T cells and followed by a cytotoxic CD8+ T cell response [20]. Therefore, adaptive immune cells are deemed to have a pivotal role in NASH progression and gain particular interest as novel target for liver disease treatment. Immune response is also mediated by the activation of innate immune receptors in hepatocytes which trigger a proinflammatory environment found to be involved in NAFLD pathogenesis and progression [21]. Therefore, toll-like receptors (TLRs)-2, TLR4, TLR5, and TLR9, which are involved in the liver inflammation, were found activated in NAFLD and linked to the increase of some inflammation-associated nuclear transcription factors such as IRF, NF-κB and peroxisome-proliferator- activated receptors (PPAR-α). Intracellular inflammasome activation in hepatocytes and cell death are also two key triggers of liver inflammation during NAFLD and NASH progression. Together they foster the production of the pro-inflammatory cytokines IL-1β and IL-18 and further enhance the downstream activation of intracellular adaptors and kinases including TNF-receptor-associated factors, TGF-β-activated kinase 1/JNK, and IκB kinases (IKKs)[8] . Furthermore, the oxidative stress in experimental obesity models have been found associated to T cell infiltration, fibrosis and HCC and is strongly promoted by STAT-1 and STAT-3 signaling in mice.
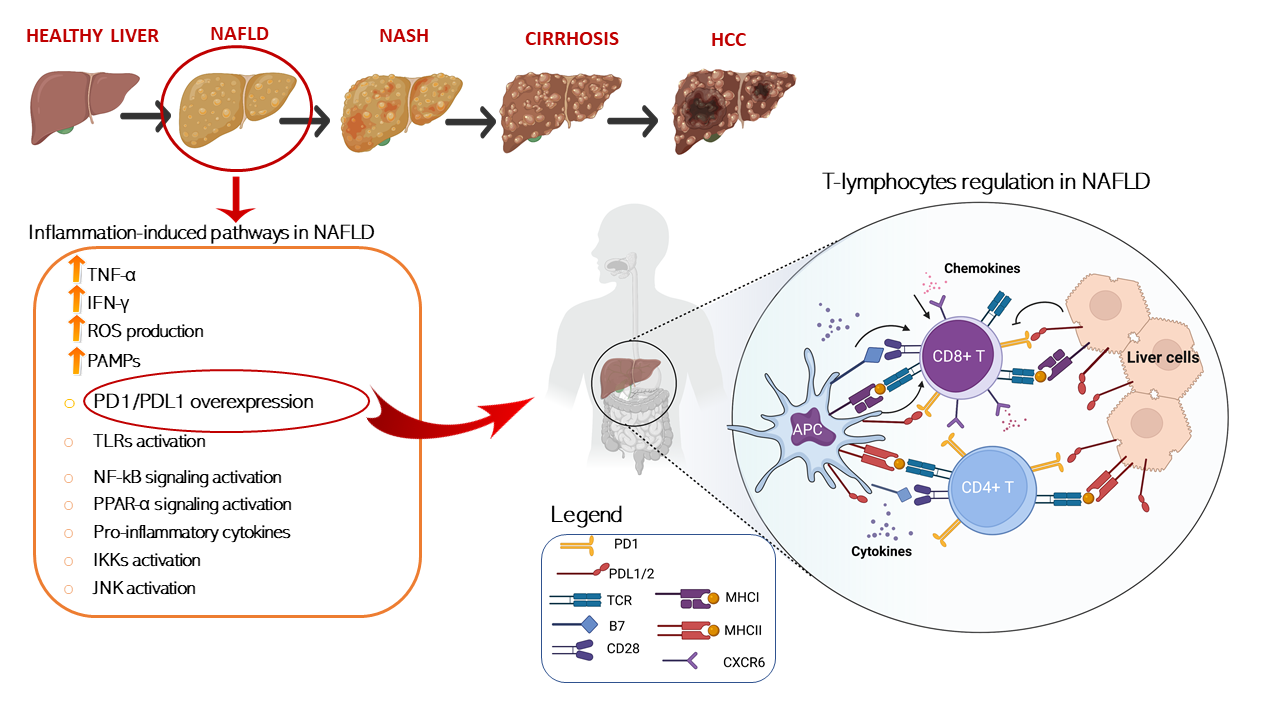
In this regard, several papers have recently investigated, in different NAFLD/NASH preclinical models, how dysregulation of immune cells promoted by pathological metabolic changes is directly involved in tissue-damage process and in the progression to HCC. A schematic representation of T cells immune regulation upon liver disease is shown in Figure 1.
Figure 1. Schematic representation of T cells immune regulation and inflammation-induced pathways upon liver disease. T cell activation requires two signals, the first signal is specific and implies T cell receptor recognition and binding to MHC/antigen presented by an antigen-presenting cell or by tissue specific immunized cells. The second signal is nonspecific and involves the B7 ligand, exposed on the antigen-presenting cells to bind its receptor, CD28, on T cells. On the other hand, T cell are mainly inhibited trough the binding of the exhaustion marker PD-1 which is expressed on cell surface binding to its ligand PD-L1/2 present on APC or exposed on liver cell in HCC setting. T cells activation is furtherly damped by increased levels of immunosuppressive cytokines such as IL-4, IL-8, and IL-10, accompanied by the suppression of immune activating cytokines IL-1, tumor necrosis factor alpha (TNF-a), and interferon gamma (IFN-γ) secreted by T cells. Conversely, IL- 5 which induces chemokine receptor CXCR6 upregulation by increasing CD8 T cells susceptibility to metabolic stimuli and triggering CD8+ auto-aggression of liver tissue. Antigen presenting cells (APCs); programmed cell death protein 1 (PD-1); programmed cell death 1 ligand 1/2 (PD-L1/2); T-cell receptor (TCR); major histocompatibility complexes I/II (MHC I/II); pathogen-associated molecular patterns (PAMPs); Toll-like receptors (TLR); nuclear factor kappa (NF-kB); peroxisome-proliferator- activated receptors (PPAR-α); IκB kinases (IKK); c-Jun N-terminal kinases (JNK).
3.Dysregulation of the immune system in experimental NAFLD
3.1. Fat accumulation induces liver CD4+T cell depletion in in vitro and in vivo models
Ma et al. have recently observed the lipid-induced loss of CD4+ T lymphocytes in NAFLD patients and preclinical models supporting the tight correlation between hepatic disease and the immune microenvironment [22] . Thus, mice trans-genetically induced through liver-specific MYC oncogene (MYC-ON) and fed a methionine–choline-deficient diet (MCD) developed NAFLD and were used to assess lipid metabolism dysregulation.
Remarkably, fewer CD4+ T lymphocytes, but not CD8+ T cells, were found in MYC-ON MCD mice compared to heathy controls. As cause, CD4+ T lymphocyte death was found to be induced by linoleic acid (C18:2) accumulation within the cells. Dose–response and time-course analysis revealed that CD4+ T lymphocytes were more susceptible to linoleic acid -induced cell death than CD8+ T lymphocytes and culturing of CD4+ T lymphocytes with FFA-depleted conditioned medium no longer caused their death in MYC-ON MCD mice.
Dysregulation in mitochondrial-derived ROS production and in beta-oxidation were identified as the critical mediator for CD4+ T lymphocyte death. Carnitine palmitoyltransferase 1 (CPT1), the rate-limiting enzyme in long-chain fatty acid oxidation (FAO), was found increased within CD4+ T mitochondria, in parallel with a decreased expression of several genes coding for components of the electron transport complex (ECT). As consequence, mitochondrial membrane potential, maintained by proper ETC activity, was significantly decreased by linoleic acid in CD4+ but not CD8+ T lymphocyte prompting ROS generation and resulting in CD4+ T cell death. Furthermore, after C18:2 treatment, the increase of both mitochondrial superoxide and caspase 3/7 activity confirmed that CD4+ T lymphocytes died through mitochondrial ROS-induced apoptosis. As proof, CPT1 knockdown blocked linoleic acid-induced mitochondrial ROS production in CD4+ t cells and the blockage of ROS production by using N-acetylcysteine (NAC) abrogated cell death in vitro in CD4+ T lymphocytes when incubated with hepatocytes from MCD-ON MYC mice and reversed in vivo the loss of hepatic CD4+ T lymphocytes in MCD-diet-fed mice [22].
3.2. CD8+ T cells and NKT cells mediate liver damage in in vitro and murine models: an insight into the PD-1/PD-L1 signaling pathway
In in vitro and preclinical models which recapitulate the key features of NASH, it has been assessed the hepatic accumulation of CD8+ T cells and their functions, thus unveiling the pivotal role played by them in liver disease progression. In a recent study, Dudek et al. demonstrated that in NASH mice CD8+ T liver cells are involved in auto-aggressive tissue damage through defined, non-redundant sequential activation steps [23]. NASH was induced in animals through the administration of a choline-deficient, high-fat diet (CD-HFD) for 9–12 months. Subsequently, chemokine receptor CXCR6, a prominent marker for liver residency and for tissue-resident and effector memory, was found overexpressed in CD8+ T cells and co-expressed with markers associated with CD8+ T exhaustion (such as PD-1) and indicative for their effector function such as Granzyme B (GzmB, TNF and IFN-γ [24].
Genome-wide transcriptome analysis and gene-set enrichment analysis (GSEA) on NASH liver CXCR6+ CD8 T cells have furtherly confirmed these findings in mice. Thus, genes associated with T cell effector function (as Bhlhe40 which encodes for granzymes) and activation, exhaustion (Pdcd1 and Tox) and tissue residency (S1pr1, Klf2 and Rgs1) were found overexpressed in this setting. FOXO1 was demonstrated through loss- and gain-of-function experiments to be an effective negative regulator of CXCR6 expression in CD8+ T. Namely, the inhibition of FOXO1 caused the upregulation of CXCR6, whereas the overexpression of FOXO1 reduced the expression of CXCR6 in CXCR6- CD8 T. In turn, FOXO1 expression is regulated by IL-15 signaling which mediates FOXO1 phosphorylation allowing the nuclear export and proteasomal degradation of cytosolic FOXO1 [30]. Notably, both serum and mRNA levels of IL-15 were higher in patients and in mice with NASH suggesting the pivotal role of IL-5 in the induction of CXCR6+ CD8+ T cells. As proof, treatment with IL-15 downregulated FOXO1 in mice CD8+ T cells thus upregulating CXCR6 and other CD8+ T inhibitors such as PD-1, GzmB and CD69 which are involved in the immune response. Moreover, serum IL-15 levels were found to be correlated with higher frequency of hepatic CXCR6+PD-1high CD8+ T cells and with low FOXO1 expression. In this setting, low FOXO1 activity rendered T cells responsive to environmental metabolic cues such as acetate and capable of killing hepatocytes triggering auto aggression and boosting chronic liver damage.
Accordingly, Wolf et al. claimed that CD8+ and NKT cells cooperatively induce liver damage in mice fed choline-deficient high-fat diet enhancing steatosis, NASH development, and transition to HCC. In particular, NKT cells mediate the lipid uptake via LTβR activation in hepatocytes inducing fat accumulation and leading to the onset of macrovesicular steatosis [25].
4.Dysregulation of the immune system in human NAFLD
4.1. CD8+ T cells and NKT cells mediate liver damage in human NAFLD: an insight into the PD-1/PD-L1 complex
As previously discussed, Wolf et al. demonstrated that CD8+ and NKT cells cooperatively induce liver damage in fed choline-deficient high-fat diet mice enhancing steatosis, NASH development, and transition to HCC. Again, analyzing liver histological specimens of patients with NASH, alcoholic and viral diseases and healthy controls, the authors showed a higher number of CD8+ and NKT in the samples of NASH subjects, as well as NASH induced HCC compared to healthy subjects [20]. Therefore, similarly to what observed in murine models, it could be speculated that in patients with NASH exists an interaction between CD8+ T cells, NKT cells, their secreted cytokines and hepatocytes, thus suggesting that targeting specific immune signaling pathways could potentially diminish the risk to develop liver damage and progression to HCC.
Given the strict association between CD8+ and PD-1/PD-L1, recent studies focused on this signaling pathway, although the results are conflicting and not conclusive. Polymorphisms in the PD-1 gene have been associated with an increased risk of various types of cancers and some of them alter protein expression and function [26]. The PD-1 rs10204525 C > T and rs7421861 A > G variants boost PD-1 expression and have been associated with increased risk of esophageal cancer in Asian individuals . Furthermore, Kaplan-Meier survival curves showed that higher PD-1 gene expression contributed to worse survival of esophageal cancer patients [27].
In a cohort of 594 patients with NAFLD and 391 with NAFLD-HCC who were enrolled from three European sites (UK, Milan and Berna), the PD-1 rs7421861 was independently associated with NAFLD-HCC only in the UK cohort thus suggesting that the impact of genetic variants which modify the HCC milieu may differ according to ethnicity although pathways may be shared [27].
In a cohort of 134 Egyptian patients with biopsy proven NASH and NASH related HCC, the rs2282055 G>T genetic variant in the PD-L1 gene was associated with cancer [28]. In another study involving 167 HCC patients, PD-L1 hepatic levels were increased, paralleling CD8+ expression, and IFN gamma was found to be positively correlated with CD8+ and PD-L1 gene expression. These findings suggest that PD-L1 is not constitutively expressed by tumor cells but it may represent an adaptive mechanism of them to escape endogenous antitumor activity. However, there was no association between PD-L1 levels and HCC severity. Conversely, this study highlighted a better survival in patients with higher intra-tumoral expression of PD-L1 and CD8+, confirming the role of the cytotoxic T cell in the eradication of HCC [29]. In line with this report, Sideras et al. confirmed that low expression of PD-L1 and low CD8+ predict extremely poor HCC-specific survival in a cohort of 154 patients with resected liver tumors [30].
These results contrast with other evidences in smaller cohorts, where PD-L1 overexpression was associated with a poor prognosis in patients with HCC
5. Immune-related therapy in NAFLD and HCC
Reinforced by studies supporting a central role for the immune system in the regulation of NAFLD pathogenesis and its progression towards advanced forms, as well as carcinogenesis, a modulation of this system as therapeutic attempt was expected. Different possible targets have been selected, namely chemokine receptors, nuclear receptors, molecules expressed on immune system cells surface, tyrosin-kinases and anti-angiogenetic agents. Among all, the PD-1/PD-L1 complex has received great attention. However, while trials with drugs targeting this complex in HCC are accumulating, on the contrary data are available on the application of PD-1/PD-L1 blockage to prevent NAFLD progression to NASH or fibrosis.
5.1. Immunotherapy for NAFLD and HCC
Nowadays no pharmacological therapy has been approved for NAFLD, especially in the advanced forms of NASH and fibrosis [31]. However, since the immune response seems to play a key role in NASH and in its progression to HCC, several trials have explored the possibility to address it to treat patients.
Since HCC is emerging as one of the leading causes for cancer-related mortality, great efforts have been performed to identify an efficient curative strategy. Currently, the only proposed strategies for HCC are loco-regional treatments (i.e hepatic resection, ablation, transarterial chemoembolisation), inhibitors of tyrosin kinase/anti-angiogenetic agents, as Sorafenib, Regorafenib and Ramucirumab, and ultimately liver transplantation [32]. Very recently also Lenvatinib, an oral multikinase inhibitor, was confirmed as non-inferior to Sorafenib in untreated advanced hepatocellular carcinoma [33].
However, if on the one hand data on the efficacy of immunotherapy in patients with NASH and severe fibrosis are not conclusive yet, the results obtained in patients with HCC, developed on liver disease of different etiology, seem more promising. Indeed, given the established role of the immune system in carcinogenesis, in recent years immunotherapy, and in particular the immune checkpoint blockade, has been introduced in the treatment of HCC and may improve the prognosis of liver disease [34]. In this regard, checkpoint inhibitors such as anti-PD-1/ PDL-1 antibodies have been used to restore immune control of tumors by disrupting co-inhibitory receptors and enhancing anti-tumor T cell responses.
Despite the pathophysiological bases of this mechanism are clear and support the use of the PD-1/PD-L1 blockade, data in clinical practice are contrasting and to date the relationship between PD-L1 expression and host-tumor immunity in HCC is not well defined.
However, even if immunotherapy has been tested in this setting either as first line therapy or as adjuvant therapy after curative interventions, it may also contribute to “uncheck” the immune system to control HCC, thus worsening the underlying NASH.
As first line therapy, different monoclonal antibodies anti PD-L1 have been tested as As first line therapy, different monoclonal antibodies anti PD-L1 have been tested as camrelizumab, cemiplimab and avelumab [35][36][37]. All of them have been experimented in phase I-II studies in patients previously exposed to systemic therapies and in small cohorts.
More promising results were expected for nivolumab, pembrolizumab and atezolizumab. These PD-1/PD-L1 inhibitors have been combined with anti-antiangiogenic agents, such as lenvatinib, apatinib and bevacizumab in phase I and II studies.
KEYNOTE-240 trial which evaluated the overall survival in patients who received pembrolizumab and previously treated with Sorafenib and the CheckMate-459 one which compared the survival of patients treated with Nivolumab vs Sorfenib, failed to give positive results [38], even though firstly obtained in phase I/II studies as depicted in Table 1. It is important to underlie that these trials showed a response only in a certain proportion of patients, mainly those with a viral etiology of liver disease and possibly in those in whom increased expression of PD-L1 determined an abundant CD8+ infiltration with consequent neoplasm control [39].
Recently, the role of nivolumab in HCC was also explored in combination with ipilimumab, a target specific monoclonal antibody against CTLA-4. The combination of the two immune checkpoint inhibitors was approved by US FDA in patients who were in-tolerant to sorafenib or with advanced disease after the promising results achieved in the cohort 4 of the CheckMate-040 trial. Conversely, combination of CTLA-4 and PD-1 blockade are supposed to act synergistically and to be involved in the inhibition of effector T-cell and NK cell activation in peripheral tissues and in induction of Treg cell differentiation. Therefore, when combined they results in increased response rates and higher survival rates [40].
To date, no adjuvant treatment has been proven to improve survival of patients with an early-stage HCC following loco-regional treatments or resection.
5.2. HCC in the context of NAFLD may not benefit of checkpoint inhibition
As discussed above, emerging data identified in NASH an unfavorable prognostic factor towards response to this type of immuno-therapy compared to liver diseases which precede cancer onset deriving from different etiologies.
This hypothesis is supported by evidences driven by experimental models where treatment with Nivolumab and Pembrolizumab gave poor results in mice with NASH characterized by PD-1 overexpression. Pfister et al. confirmed this data by performing single cell RNA-seq analysis in leukocytes population of diet-induced NASH mice [41] where they observed an increased number of CD8+PD-1+ T cells expressing markers of exhaustion, suggesting their involvement in liver damage. In concordance, mice depleted of CD8+ T cells had a reduced liver damage and lower incidence of HCC. Furthermore, an increase of PD-L1 expression in hepatocytes and non-parenchymal cells was also found and directly correlated with the severity of liver disease. In line with these data, the administration of anti-PD-1 immunotherapy aggravated liver damage and increased the number of hepatic CD8+PD-1+ T cells. In keeping with these findings, a higher number of activated hepatic CD8+ T cells which express effector cytokines (IFN-γ, TNF-a) together with both progressive liver damage and HCC incidence were observed in Pdcd1−/− mice suggesting the unfavorable effects of anti-PD-1 treatment in NASH which support the tissue-damage role exerted from CD8+PD-1+ T lymphocytes.
Despite the negative impact of NAFLD/NASH on the response to immunotherapy has been confirmed also in pharmacological trials on patients with HCC a recent meta-analysis of 3 phase III trials comprising a total of 1,656 patients with unresectable HCC found that immunotherapy improved overall survival only in patients with viral HCC compared to non-viral ones [42]. This evidence was sustained by another meta-analysis involving 8 studies comprising more than 3700 patients[43] . Even worse, the checkpoint inhibition of PD-1/PD-L1 was linked to a decreased survival in patients NAFLD related HCC [42]. Conversely, no differences related to etiology were observed in patients treated with tyrosine kinase inhibitors /anti–vascular endothelial growth factor.
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73-84, doi:10.1002/hep.28431.
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313-1321, doi:10.1002/hep.20701.
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123-133, doi:10.1002/hep.29466.
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, doi:10.1016/j.metabol.2015.12.012
- Dongiovanni, P.; Romeo, S.; Valenti, L. Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World J Gastroenterol 2014, 20, 12945-12955, doi:10.3748/wjg.v20.i36.12945
- Peiseler, M.; Tacke, F. Inflammatory Mechanisms Underlying Nonalcoholic Steatohepatitis and the Transition to Hepatocellular Carcinoma. Cancers (Basel) 2021, 13, doi:10.3390/cancers13040730
- Jia, K.; He, Y.; Dziadziuszko, R.; Zhao, S.; Zhang, X.; Deng, J.; Wang, H.; Hirsch, F.R.; Zhou, C.; Yu, H.; et al. T cell immunoglobulin and mucin-domain containing-3 in non-small cell lung cancer. Translational lung cancer research 2019, 8, 895-906, doi:10.21037/tlcr.2019.11.17
- Meroni, M.; Longo, M.; Dongiovanni, P. The Role of Probiotics in Nonalcoholic Fatty Liver Disease: A New Insight into Therapeutic Strategies. Nutrients 2019, 11, doi:10.3390/nu11112642.
- Guohong, L.; Qingxi, Z.; Hongyun, W. Characteristics of intestinal bacteria with fatty liver diseases and cirrhosis. Annals of Hepatology 2019, 18, 796-803, doi:https://doi.org/10.1016/j.aohep.2019.06.020
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 2015, 5, 8096, doi:10.1038/srep08096
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54-62, doi:10.1002/hep.21060
- Shuai, Z.; Leung, M.W.Y.; He, X.; Zhang, W.; Yang, G.; Leung, P.S.C.; Eric Gershwin, M. Adaptive immunity in the liver. Cellular & Molecular Immunology 2016, 13, 354-368, doi:10.1038/cmi.2016.4
- Shuai, Z.; Leung, M.W.; He, X.; Zhang, W.; Yang, G.; Leung, P.S.; Eric Gershwin, M. Adaptive immunity in the liver. Cell Mol Immunol 2016, 13, 354-368, doi:10.1038/cmi.2016.4
- Muscate, F.; Woestemeier, A.; Gagliani, N. Functional heterogeneity of CD4(+) T cells in liver inflammati. Semin Immunopathol 2021, 43, 549-561, doi:10.1007/s00281-021-00881-w
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 2018, 18, 153-167, doi:10.1038/nri.2017.108
- Ringelhan, M.; Pfister, D.; O'Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat Immunol 2018, 19, 222-232, doi:10.1038/s41590-018-0044-z
- Jenne, C.N.; Kubes, P. Immune surveillance by the liver. Nat Immunol 2013, 14, 996-1006, doi:10.1038/ni.2691
- Deng, C.; Chen, Y.; Li, W.; Peng, L.; Luo, X.; Peng, Y.; Zhao, L.; Wu, Q.; Zhang, W.; Zhang, X.; et al. Alteration of CD226/TIGIT immune checkpoint on T cells in the pathogenesis of primary Sjogren's syndrome. J Autoimmun 2020, 113, 102485, doi:10.1016/j.jaut.2020.102485
- Syn, W.K.; Oo, Y.H.; Pereira, T.A.; Karaca, G.F.; Jung, Y.; Omenetti, A.; Witek, R.P.; Choi, S.S.; Guy, C.D.; Fearing, C.M.; et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology 2010, 51, 1998-2007, doi:10.1002/hep.23599
- Cai, J.; Zhang, X.J.; Li, H. The Role of Innate Immune Cells in Nonalcoholic Steatohepatitis. Hepatology 2019, 70, 1026-1037, doi:10.1002/hep.30506
- Ma, C.; Kesarwala, A.H.; Eggert, T.; Medina-Echeverz, J.; Kleiner, D.E.; Jin, P.; Stroncek, D.F.; Terabe, M.; Kapoor, V.; ElGindi, M.; et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016, 531, 253-257, doi:10.1038/nature16969
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Huser, N.; Meiser, P.; Bayerl, F.; et al. Auto-aggressive CXCR6(+) CD8 T cells cause liver immune pathology in NASH. Nature 2021, 592, 444-449, doi:10.1038/s41586-021-03233-8.
- Prakash, M.D.; Munoz, M.A.; Jain, R.; Tong, P.L.; Koskinen, A.; Regner, M.; Kleifeld, O.; Ho, B.; Olson, M.; Turner, S.J.; et al. Granzyme B promotes cytotoxic lymphocyte transmigration via basement membrane remodeling. Immunity 2014, 41, 960-972
- Ye, L.-L.; Wei, X.-S.; Zhang, M.; Niu, Y.-R.; Zhou, Q. The Significance of Tumor Necrosis Factor Receptor Type II in CD8(+) Regulatory T Cells and CD8(+) Effector T Cells. Frontiers in immunology 2018, 9, 583-583, doi:10.3389/fimmu.2018.00583
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; Ringelhan, M.; Simonavicius, N.; Egger, M.; Wohlleber, D.; et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell 2014, 26, 549-564, doi:10.1016/j.ccell.2014.09.003.
- Chihab, H.; Jadid, F.Z.; Foka, P.; Zaidane, I.; El Fihry, R.; Georgopoulou, U.; Marchio, A.; Elhabazi, A.; Chair, M.; Pineau, P.; et al. Programmed cell death-1 3'-untranslated region polymorphism is associated with spontaneous clearance of hepatitis B virus infection. J Med Virol 2018, 90, 1730-1738, doi:10.1002/jmv.25265
- Eldafashi, N.; Darlay, R.; Shukla, R.; McCain, M.V.; Watson, R.; Liu, Y.L.; McStraw, N.; Fathy, M.; Fawzy, M.A.; Zaki, M.Y.W.; et al. A PDCD1 Role in the Genetic Predisposition to NAFLD-HCC? Cancers (Basel) 2021, 13, doi:10.3390/cancers13061412
- El-Derany, M.O. Polymorphisms in Interleukin 13 Signaling and Interacting Genes Predict Advanced Fibrosis and Hepatocellular Carcinoma Development in Non-Alcoholic Steatohepatitis. Biology (Basel) 2020, 9, doi:10.3390/biology9040075
- Xie, Q.K.; Zhao, Y.J.; Pan, T.; Lyu, N.; Mu, L.W.; Li, S.L.; Shi, M.D.; Zhang, Z.F.; Zhou, P.H.; Zhao, M. Programmed death ligand 1 as an indicator of pre-existing adaptive immune responses in human hepatocellular carcinoma. Oncoimmunology 2016, 5, e1181252, doi:10.1080/2162402X.2016.1181252
- Sideras, K.; Biermann, K.; Verheij, J.; Takkenberg, B.R.; Mancham, S.; Hansen, B.E.; Schutz, H.M.; de Man, R.A.; Sprengers, D.; Buschow, S.I.; et al. PD-L1, Galectin-9 and CD8(+) tumor-infiltrating lymphocytes are associated with survival in hepatocellular carcinoma. Oncoimmunology 2017, 6, e1273309, doi:10.1080/2162402X.2016.1273309.
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. NAFLD fibrosis score (NFS) can be used in outpatient services to identify chronic vascular complications besides advanced liver fibrosis in type 2 diabetes. J Diabetes Complications 2020, 34, 107684, doi:10.1016/j.jdiacomp.2020.107684.
- Foerster, F.; Gairing, S.J.; Muller, L.; Galle, P.R. NAFLD-driven HCC: Safety and efficacy of current and emerging treatment options. Journal of hepatology 2021, doi:10.1016/j.jhep.2021.09.007.
- Hiraoka, A.; Kumada, T.; Tada, T.; Tani, J.; Kariyama, K.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; et al. Efficacy of lenvatinib for unresectable hepatocellular carcinoma based on background liver disease etiology: multi-center retrospective study. Sci Rep 2021, 11, 16663, doi:10.1038/s41598-021-96089-x
- Pinter, M.; Jain, R.K.; Duda, D.G. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol 2021, 7, 113-123, doi:10.1001/jamaoncol.2020.3381
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol 2020, 21, 571-580, doi:10.1016/s1470-2045(20)30011-5.
- Pishvaian, M.J.; Weiss, G.J.; Falchook, G.S.; Yee, N.; Gil-Martin, M.; Shahda, S.; Moreno, V.; Brana, I.; Crittenden, M.; Formenti, S.; et al. Cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with advanced or metastatic hepatocellular carcinoma (HCC): Data from an expansion cohort in a phase I study. Annals of Oncology 2018, 29, viii410, doi:10.1093/annonc/mdy288.024
- Lee, D.W.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Kim, Y.J.; Yoon, J.H.; Kim, T.Y.; Han, S.W.; Oh, D.Y.; Im, S.A.; et al. Phase II Study of Avelumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib. Clin Cancer Res 2021, 27, 713-718, doi:10.1158/1078-0432.ccr-20-3094.
- Yau, T.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Kang, Y.K.; Hou, M.M.; Numata, K.; Yeo, W.; Chopra, A.; Ikeda, M.; et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. Journal of hepatology 2019, 71, 543-552, doi:10.1016/j.jhep.2019.05.014
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492-2502, doi:10.1016/S0140-6736(17)31046-2
- D'Alessio, A.; Rimassa, L.; Cortellini, A.; Pinato, D.J. PD-1 Blockade for Hepatocellular Carcinoma: Current Research and Future Prospects. Journal of hepatocellular carcinoma 2021, 8, 887-897, doi:10.2147/JHC.S284440
- Pfister, D.; Nunez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450-456, doi:10.1038/s41586-021-03362-0
- Pfister, D.; Nunez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450-456, doi:10.1038/s41586-021-03362-0
- Haber, P.K.; Puigvehi, M.; Castet, F.; Lourdusamy, V.; Montal, R.; Tabrizian, P.; Buckstein, M.; Kim, E.; Villanueva, A.; Schwartz, M.; et al. Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002-2020). Gastroenterology 2021, 161, 879-898, doi:10.1053/j.gastro.2021.06.008