Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mai Abdel Haleem A. AbuSalah | + 4302 word(s) | 4302 | 2022-03-09 04:49:50 | | | |
| 2 | Dean Liu | -22 word(s) | 4280 | 2022-03-22 03:03:07 | | | | |
| 3 | Dean Liu | Meta information modification | 4280 | 2022-03-25 04:01:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abusalah, M. Nucleic Acid-Based COVID-19 Therapy Targeting Cytokine Storms. Encyclopedia. Available online: https://encyclopedia.pub/entry/20777 (accessed on 07 February 2026).
Abusalah M. Nucleic Acid-Based COVID-19 Therapy Targeting Cytokine Storms. Encyclopedia. Available at: https://encyclopedia.pub/entry/20777. Accessed February 07, 2026.
Abusalah, Mai. "Nucleic Acid-Based COVID-19 Therapy Targeting Cytokine Storms" Encyclopedia, https://encyclopedia.pub/entry/20777 (accessed February 07, 2026).
Abusalah, M. (2022, March 21). Nucleic Acid-Based COVID-19 Therapy Targeting Cytokine Storms. In Encyclopedia. https://encyclopedia.pub/entry/20777
Abusalah, Mai. "Nucleic Acid-Based COVID-19 Therapy Targeting Cytokine Storms." Encyclopedia. Web. 21 March, 2022.
Copy Citation
One of the promising therapeutic strategies to combat COVID-19 is nucleic acid-based therapeutic approaches, including microRNAs (miRNAs). Nucleic acid-based therapeutics (miRNAs included) have a latent ability to break the COVID-19 infection in general and quell the cytokine storm in particular.
COVID-19
cytokine storm
TNA
miRNA
siRNA
mRNA vaccine
1. COVID-19 Nucleic Acid-Based Therapeutics
Due to the periodical emergence of new versatile CoVs, which is considered a severe health concern [1], as is the case with SARS-CoV-2, and especially the new emerging variants with more lethality and transmissibility, the urgent development of novel efficient therapies is highly recommended. The variant of concern (VOC-202012/1) named B.1.1.7 (Alpha), originating in southeast England in 2020, was reported with high mortality rates from 2.5 to 4.1 per 1000 detected cases as compared to other circulating variants in England [2]. These variants also can escape the immune system via multiple recorded mutations, mainly E484K [3]. Delta (B.1.617.2) is presently the most prevalent COVID-19 variant in the U.S. [4]. This variant was discovered for the first time in India in December of 2020 [5]. In addition, this particular variant expanded to over 98 countries throughout the world in a matter of months, becoming the dominant variant in most countries, including India, the U.K., Israel, and the U.S. [6]. The Delta variant currently accounts for more than 83% of COVID-19 cases recorded in the U.S. [7] and it was found to be 40–60% more transmissible than Alpha, about twice as transmissible as the initial SARS-CoV-2 Wuhan strain [8]. Some monoclonal antibody therapies and antibodies produced by COVID-19 vaccination may be less successful against this variant [4]. On 16 July 2021, the Centers for Disease Control and Prevention reported a 35% rise in hospitalizations and a 69.3% seven-day average increase in new COVID-19 cases infected with a Delta variant [7].
New variants are continually developing, and improved surveillance systems will inevitability pick up on more of them as they get more advanced [9][10]. The new variant (B.1.1.529) was identified as a variant of concern and was given the name of Omicron on 26 November 2021 based on the suggestion of the WHO’s Technical Advisory Group on Virus Evolution (TAG-VE) [9][10]. When compared to other variants of the virus, such as Delta, it is not yet known if infection with Omicron causes more severe disease. In South Africa, preliminary data suggests that the rate of hospitalizations is increasing; however, this may be due to an increase in the general number of persons becoming infected rather than a result of a specific infection with Omicron, according to the available data [9]. Besides that, another highly mutated variant that spread rapidly, and was given the IHU name (B.1.640), was reported recently. So far, this variant has been discovered primarily in France, though it has also been discovered in a number of other countries as well [11]. The B.1.640 variant is not a new variant. For at least three months now, it has been detected. The week-old study by researchers from Méditerranée Infection in Marseille, which is part of France’s Instituts hospitalo-universitaires (IHU, or University Hospital Institutes), triggered a sudden discussion about this variant [11]. In comparison to the spread of Omicron, this variant is far less concerning. The most recent detection of this variant was on 25 December 2021, according to the available data. After that, no additional cases have been discovered in the global databases [11]. The exponential versatility of SARS-CoV-2 has hindered the control efforts implanted to reduce its infectivity. The emergence of new variants made current vaccines less effective, even in the recent rapid development and progression of therapeutic approaches, which urges the need to focus more on effective strategies to combat this unprecedented global pandemic.
Among the promising strategies to combat SARS-CoV-2 is nucleic acid-based therapeutics. Therapeutic nucleic acids (TNAs) include many pioneering technologies such as antisense oligonucleotides (ASOs), micro RNAs (miRNAs), small interfering RNAs (siRNAs), clustered regularly interspaced short palindromic repeats–CRISPR-associated protein (CRISPR–Cas), and mRNA vaccines [12]. Figure 1 shows the mechanism of action and criteria of each TNAs. TNAs are capable of targeting specific sequences of interest in the viral genome with a focus on the highly conserved sequence, such as targeting the highly conserved RNA-dependent RNA polymerase (RdRp) gene in the open reading frame 1ab (ORF1ab) region and the N gene by CRISPR-associated RNAs (crRNAs) deployed by the Cas13d system [13]. These antiviral therapeutics can inhibit viral gene expression [14] either during or after transcription [15]. Hence, nucleic acid-based therapeutics present great potential in combating SARS-CoV-2 infection. The most needed feature nowadays is scalable, rapid production; hence, nucleic acid-based therapeutics that possess this feature have the potential to be applied globally. Based on the published articles [12][16][17][18][19], researchers believe that mRNA vaccines, DNA vaccines, CRISPR, and ASO are more stable than the siRNA approach. Still, there have been no statistically significant comparative studies published to date. Despite that, researchers believe that the CRISPR systems will be more expensive and possibly harmful than all other approaches in the future. However, researchers think that ASO and mRNA would be the most cost-effective method.
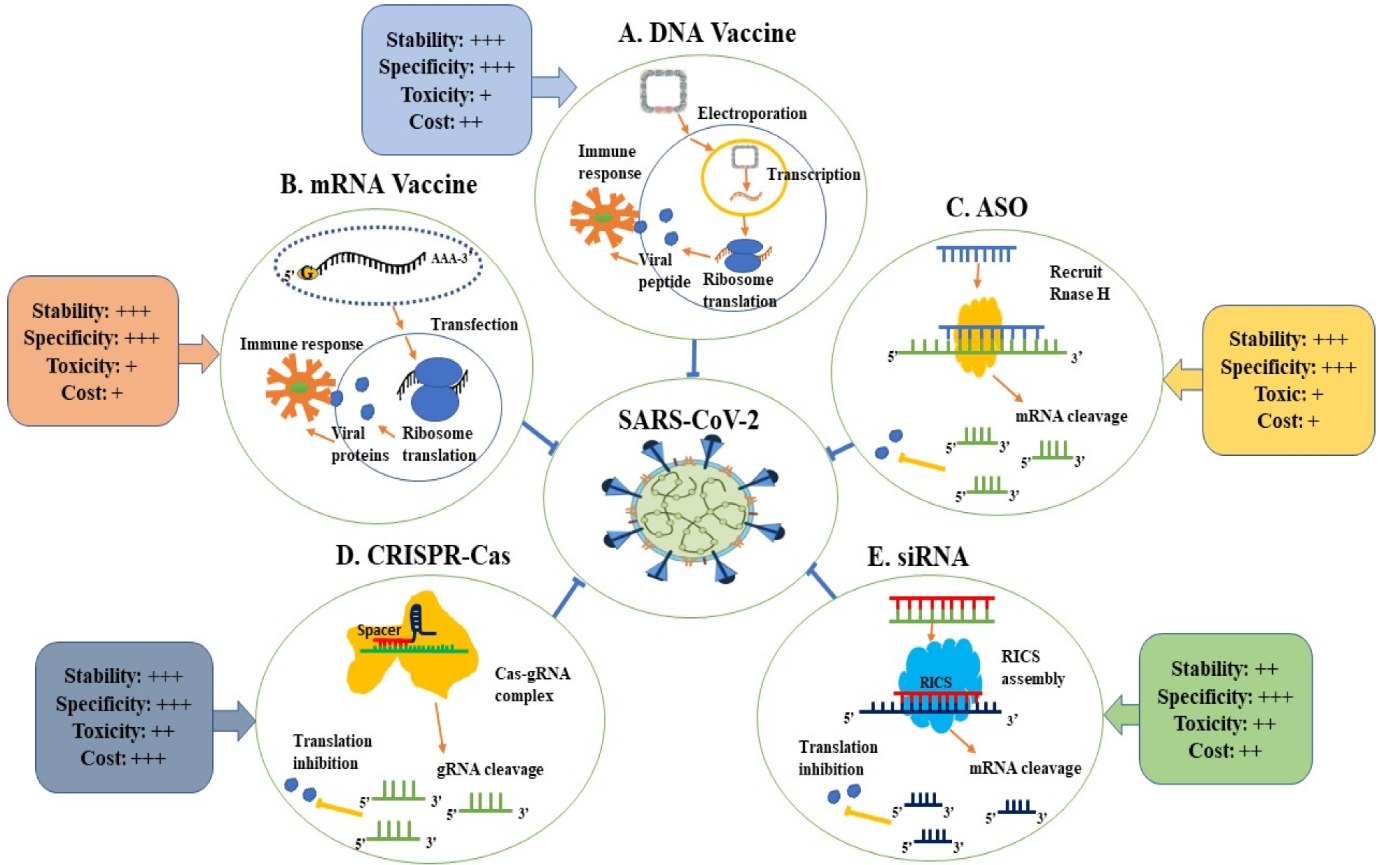
Figure 1. Applications of therapeutic nucleic acids in the fight against SARS-CoV-2.
Based on the endogenous gene silencing mechanism at the translational level, known as RNA interference (RNAi) via small dsRNA molecules [20], which involve RNA-induced silencing complex (RISC) [21], effective therapeutic strategies could be developed against the SARS-CoV-2. However, RNAi application is associated with off-target effects [22] as well as delivery issues [23]. The gene-editing siRNAs are one of the TNAs that can be applied in the fight against SARS-CoV-2. This therapy can knock out the gene of interest without inducing an interferon response [24]. Therefore, a database was developed where it will help develop siRNAs that offer a prediction of off-target binding [25] as well as carriers of nanoparticles or viral vectors were developed, which will help in delivering siRNAs [26]. Nine siRNA, potentially conserved targets were identified bioinformatically in the SARS-COV-2 genome (GenBank accession number, MN908947.3) for safe siRNAs application [27]. Targeting highly conserved regions, the siRNAs Hel1, Hel2, siUC7, and siUTR3 showed potent SARS-CoV-2 gene expression interference delivered via a stable lipid nanoparticle (LNP), known as stealth LNP (sLNP), in the shape of a DOTAP40C or DOTAP/MP3 LNP-siRNAs (dmLNP-siRNAs) formulation [28]. Several siRNAs developed for the SARS-COV virus could potentially be used against SARS-COV-2. However, six designed siRNAs for most of the virus mRNAs (siRNA 1, siRNA 2, siRNA 3, siRNA 4, siRNA 5, and siRNA 6) were reported to have a fair but less than acceptable level of proliferation inhibition [29].
Given that about 2600 human miRNAs have been listed in the miRNA registry (miRBase 22), they are estimated to affect more than 60% of all human protein-coding genes [30][31]. miRNAs are considered a promising TNA in fighting COVID-19 infection, particularly for modifying proteins that are inaccessible to other small molecules [32]. miRNA’s therapeutic mode of action is usually via either blocking cellular receptors, preventing viral replication, or inhibiting the function of the viral proteins [33]. The therapeutic application of miRNA can be in the form of miRNA mimics to interfere with the SARS-CoV-2 gene expression or miRNA inhibitors against SARS-CoV-2-related endogenous miRNAs [32]. The first approach involves using miRNA mimics to reduce the expression of proteins that have been inappropriately induced [32]. Precursor miRNAs (pre-miRNAs) are produced in the nucleus after processing by the dsRNA-specific endonuclease (Drosha) from primary miRNA transcripts. Upon entering the cytoplasm, pre-miRNAs are processed by the Dicer enzyme, resulting in mature miRNA formation [34]. A mature, single-stranded miRNA is coupled with RISC, which binds to the 3′-UTR of the target mRNA and prevents it from being translated [35]. Hence, miRNAs directly affect the translation process by inhibiting the process or causing mRNA degradation. As a result, they can control a wide range of cell processes, including cell differentiation, apoptosis, growth, development, and neurological diseases, by modifying protein levels [36]. In the field of gene silencing, microRNA mimic technology (miR-Mimic) is a promising innovation. The non-natural double-stranded RNA synthesized using this technology is designed to have a 5′ end with a motif that is partially complementary to a specified sequence in the 3′-UTR of the target gene. If this piece of RNA, which mimics an endogenous miRNA, is present in the cell, it can attach specifically to its target gene and cause post-transcriptional inhibition of the target gene [37].
The second approach is based on the development of miRNA antagonists, such as anti-miRs or antagomirs, and inhibitors to increase or rescue the expression of specific proteins that have been downregulated [32]. Antagomirs (also known as blockmirs) are synthetically designed molecules that are used to antagonize miRNAs oligonucleotides. Antagomirs are entirely complementary to the mature miRNA sequence and contain a variety of phosphonothioate moieties to increase their stability [37]. Steric blocking mechanism, as well as hybridization to miRNA, allows these anti-miRs to be used as an additional control and for treatment of certain cellular disorders. If researchers can identify the miRNA sequences involved in viral infections, the utilization of anti-miRs, as a promising therapy to disrupt the pathways that induce the up- or downregulation of cell proteins that result in the appearance of disease symptoms, will be achieved [37].
Recently, various miRNA-based therapies have shown therapeutic potential in clinical studies [38][39], which is expected to continue in the future. siRNAs are found to be more specific than miRNAs because miRNAs target multiple mRNAs relative to siRNAs, which target only one mRNA [40], but are less efficient than miRNAs [41]. Although miRNAs are susceptible to conserved target sequence mutations [42], the human miRNAs, miR-4699-3p, miR-299-5p, and miR-12132 bind to the altered N protein coding region via 28881-3 GGG/AAC mutations [43]. Hence, miRNA offers a versatile and innovative therapeutic approach, possibly targeting and controlling mutations of concern.
Several miRNAs, on the other hand, can be used as an effective antiviral agent against SARS-CoV-2. miR-200c inhibited ACE2 receptor expression in cardiomyocytes [44], exhibiting the promise of miRNA therapy. miR-98-5p blocks SARS-CoV-2 entry via TMPRSS2 expression reduction in lung epithelia [45]. miR-32 showed maximum TMPRSS2 gene suppression and significant TMPRSS2 expression reduction by miR-214 and miR-98, offering innovative tools to prevent SARS-CoV-2 entry [46]. Furthermore, 42 conserved miRNAs have been predicted to have antiviral properties against SARS-CoV-2 [47]. One hundred and twenty-eight low-expressed miRNAs in lung tissue are expected to target the SARS-CoV-2 genome and higher expression might suppress the infection [48]. miR-548c-5p, which suppresses the proliferation of colorectal cancer (CRC) [49], has been predicted to bind to 15 sites of the SARS-CoV-2 genome and exert potential therapeutic activity [50]. Multiple deduced antisense miRNAs were hypothesized to disrupt translation via binding to the 3′UTR, 5′UTR, and ORF9 regions of the SARS-CoV-2 genome [51]. Furthermore, the highly expressed miR-16, miR-200, and miR-24 in lung epithelia were highlighted with good prospects of mitigating COVID-19, likely in the form of miRNA mimics, as the former regulates inflammatory mediators and later two miRNAs downregulate ACE2 [32]. Figure 2 summarizes several host miRNAs targeting the SARS-CoV-2 genome or the cellular receptor in the host with COVID-19 therapeutic prospects.

Figure 2. Targeting the SARS-CoV-2 genome via several host miRNAs.
Multiple miRNAs in the host or SARS-CoV-2-encoded are associated with SARS-CoV-2 infection, where some might even inhibit the immune system [52]. These endogenous miRNAs might be a potential target for miRNA inhibitors to suppress SARS-CoV-2 infection. As such, cytokine storms can be reduced or suppressed via targeting the associated miRNAs, such as miR-125b, miR-138, miR-199a, and miR-21 [53]. The exosomal miR-424 expression is significantly elevated in SARS-CoV-2 infection, triggering thrombosis [54], which can also be an ideal target to reduce the concern of thrombotic complications. Numerous host miRNAs were found bioinformatically to interact with the viral genome. Many virus-derived miRNAs interact with the human genome, all of which present the opportunity to be targeted for interfering with SARS-CoV-2 infection [55]. miR-447b could also be a potential target as it binds to S protein RNA and facilitates viral entry [56]. Interestingly, miR-1307-3p was found with the highest expressed level in the lung tissue and showed high affinity to 3′-UTR of the SARS-CoV-2 genome [33], which makes it the model target to suppress the infection. Human miR-122 binds with high affinity to the SARS-CoV-2 genome, presenting a potential target for modulation [57]. Several databases offer insight into the therapeutic potential of miRNAs, such as the therapeutic target database (TTD) for COVID-19 drugs in clinical trials [58], IntaRNA for RNA–RNA interaction prediction [59], miRNA for expression profiles of miRNAs [60], psRNATarget for small RNA analysis [61], microRNA.org for miRNA target prediction [62], and miRTarBase for experimentally validated microRNA–target interactions [63][64]. By targeting host or viral-derived miRNAs and targeting mutated target sequences, the various miRNA strategies provide the optimum platform to be utilized against COVID-19 infection. Hence, miRNA-based therapy has great potential in dealing with the SARS-CoV-2 infection due to its versatile applications.
mRNA vaccines are considered one of the promising nucleic acid-based therapeutics due to their high potency, rapid, cheap manufacturing, and safe administration features [65]. Their manufacturing does not involve any living part of the organism; it is either conventional via in vitro transcription of plasmid DNA and adding a cap analog and a poly(A) tail or derived from alphavirus RNA replication to form self-amplifying mRNA (SAM) vaccines [66]. mRNA vaccines are synthesized in vitro from a DNA template to express the intended antigen in host cells [67]. The intended potent immune response is developed as mRNA is delivered to the host cell cytoplasm and encodes the desired antigen (Figure 3) [66]. mRNA vaccines have priority over conventional vaccines in many aspects, such as repeated administration [68] and safety concerns of conventional vaccines, especially live-attenuated vaccines, to cause infection [69]. In contrast, mRNA degradation in the cell reduces the potential risk of infection [70]. The enhancement ability of the introduced mRNA structure promotes the antibody lifespan, translation efficacy [71], flexibility, and neutralizing antibodies potency with just two doses [72]. Therefore, mRNA vaccines offer scalable, rapid manufacturing with little platform altering [73], and a fast-responding strategy to overcome the current pandemic dilemma [74]. Unfortunately, these vaccines still have some drawbacks, such as the need to store in extremely low temperatures, which hinders transportation (cold chain challenges) and storage [75], which can be slightly reduced via encapsulation with LNPs [76], escape immune system recognition via viral envelope glycosylation [77], and possible side effects such as local and systemic inflammatory responses [74]. Ultimately, mRNA vaccines still hold one of the keys to stop the SARS-CoV-2 infection.

Figure 3. Scheme of the mRNA vaccine’s mechanism of action. The delivery of mRNAs is accomplished via a carrier to improve mRNA distribution and stability in the cell, such as an LNP. Afterwards, the LNP–mRNA vaccines are injected intramuscularly. The mRNAs are released and translated by the host protein synthesis machinery after LNP–mRNAs enter the host cells. The produced proteins are degraded by proteasomes, resulting in peptides that are then linked to MHC I molecules and presented on the surface of host antigen-presenting cells. CD8+ T lymphocytes identify the peptide-MHC I complexes and respond with cellular immunological responses. MHC, major histocompatibility complex; ER, endoplasmic reticulum; UTR, untranslated region; ORF, open reading frame.
mRNA-1273 (Moderna COVID-19 vaccine) was one of the first developed mRNA vaccines against SARS-CoV-2, which is currently in Phase III clinical trials, which showed the production of a potent neutralizing antibody response [78] and the vaccine efficacy was reported as 94.1% [79]. This vaccine demonstrated early the effectiveness of mRNA vaccination in the neutralization of SARS-CoV-2. Although the emerging mutations of the S protein in the new variants have shown a small effect against neutralization via two Pfizer–BioNTech (BNT162b2) doses, where elicited, the sera-neutralization geometric mean titers (GMTs) were 0.81- to 1.46-fold against the USA-WA1/2020 virus [80]. A study conducted in Qatar showed an efficacy of 89.5% against the B.1.1.7 variant and 75.0% against the B.1.351 variant [81] as compared to 95% against the Wuhan virus in the USA [82], and 92.6% after the first dose only [83]. However, the BNT162b2 vaccine was shown to be effective against the B.1.1.7 variant [84]. In Israel, a study showed 91.5% efficacy after 14 days after a two-dose vaccination, where the B.1.1.7 variant was prevalent by 94·5% [85]. The BNT162b2 vaccine has been found to have no association with thrombocytopenic, thromboembolic, and hemorrhagic events, unlike the ChAdOx1 vaccine [86]. mRNA vaccines are highly versatile due to the ability to deliver the material of choice and the high degree of manipulation it allows [87]. Hence, mRNA vaccines show great promise by easily adjusting the introduced mRNA to meet the new mutated variants.
Among the other TNA strategies to combat SARS-CoV-2 is the employment of the CRISPR–Cas systems. CRISPR–Cas9 antiviral activity is conducted via expression disruption or direct neutralization of the viral genome [88]. Diagnostic systems have been developed based on the CRISPR system, which offers rapid, precise, and highly sensitive SARS-CoV-2 detection [89]. A prophylactic antiviral CRISPR in human cells (PAC-MAN) strategy was developed to prevent viral replication utilizing Cas13d to neutralize viral RNA [13]. CRISPR–Cas13 is also used in the CARVER (Cas13-aided viral expression and readout restriction) technology to neutralize RNA-based viruses [90]. ASO is another TNA strategy to eliminate the SARS-CoV-2 infection. ASO targets mRNA transcripts, small RNA, or long non-coding RNA [19]. Several ASOs have been designed to target regions of replication and transcription, such as the ORF1a and ORF1b regions in the SARS-CoV-2 genome, to inhibit COVID-19 infection [91]. These strategies can be deployed with other promising approaches, such as miRNA, to neutralize SARS-CoV-2 and reduce the cytokine storm.
2. Targeting the Cytokine Storm via Nucleic Acid-Based Approaches
Among the nucleic acid-based approaches, miRNAs hold great potential in attenuating the cytokine storm with versatility and high efficiency. Management of the cytokine storm in the SARS-CoV-2 infection could be accomplished via multiple mechanisms involving miRNAs, such as through miRNAs modulation of the involved inflammatory signaling pathways or even modulating the inflammatory response-related host miRNAs. As such, miRNA mimickers were hypothesized to be feasibly applied as cytokine storm anti-inflammatory agents by targeting the 3′UTR of pro-inflammatory mRNAs [92]. miR-26a-5p, miR-29b-3p, and miR-34a-5p were discovered to play a regulatory role in the endothelial dysfunction and inflammatory response of COVID-19. In addition, miR-26a-5p was reported to downregulate IL-6 and ICAM-1, while miR-29b-3p was found to downregulate IL-4 and IL-8, which are low-expressed during the infection [93]. Therefore, these three miRNAs could be the potential candidates to reduce the cytokine storm, especially miR-26a-5p, which targets IL-6 and present the opportunity to reduce mortality.
Many miRNAs were previously reported to modulate inflammatory mediators. Targeting these miRNAs to regulate the inflammatory response in the cytokine storm or directly applying these miRNAs could give rise to many novel therapeutics to reduce or suppress the cytokine storm and the COVID-19 infection. For instance, the mRNA 3′UTR of IL-1β, IL-6, and IL-8 could be targeted by numerous miRNAs (Table 1) [92]. The most common miRNAs that regulate the COVID-19–ACE2 interaction networks were miR-27a-3p, miR-10b-5p, miR-302c-5p, miR-587, miR-124-3p, and miR-16-5p [94], which offer great potential in reducing the cytokine storm of COVID-19 (Table 1). miR-16-5p was found to target multiple binding sites across coronavirus species [32][95], binds to 15 binding sites on the SARS-CoV-2 genome, is abundant in the alveolar A549 cells [32], regulate the COVID-19–ACE2 interaction networks [94], and downregulate the expression of IL-1β, IL-6, and TNF-α [96][97]; hence, it can be ideal choice to quell the storming cytokines of COVID-19. Another good candidate for the storm might be miR-125a, which downregulates TNF-α [98]. The cytokine storm could be attenuated by targeting the major player in the cytokine induction process, IL-17, via RNAi applications, including miRNA [99]—most notably, miR-129, which targeted human IL-17A, IL-17D, and IL-17RB [100]. MiR-302a reduces the cytokine storm in influenza A virus (IAV) infection [101]; hence, it is considered another good candidate for the COVID-19 cytokine storm attenuation. Even nutraceutical agents were found to possess therapeutic potential in treating the cytokine storm as they can regulate host miRNAs involved in the inflammatory response [102]. In addition, other cytokine downregulatory agents could be considered as potential targets [103], such as miR-146a, which downregulate TNF-α, IL-6, and IL-8; as well as miR-146b, which downregulate IL-1β [104] and miR-199a, which downregulate at least TNF-α, IL-1β, and IL-6 in alveoli [105]. Multiple-host miRNAs induce inflammatory mediators rather than downregulate them, and could be targeted by inhibitors to reduce or eliminate the cytokine storm. MiR-125b was found to elevate the expression of TNF-α and IL-8 [106], which could be a fitting miRNA inhibitor target [103]. One of the promising miRNA inhibitors would be the antagomirs for the safe, scalable, specific, and efficient silencing capability of endogenous miRNAs [103]. Therefore, TNAs, especially miRNA therapeutics, hold the key to quell the cytokine storm and stomping COVID-19.
Table 1. List of miRNAs that target the 3′UTRs of IL-1β, IL-6, and IL-8 mRNAs, and the recent findings amid the COVID-19 pandemic.
| mRNA | miRNA Binding SITES | Sequences of miRNA Binding Sites * | Recent Findings | References |
|---|---|---|---|---|
| IL-1ß | miR-376c-3p | 43-AACAUAGAGGAAAUUCCACGU-63 | miR-7 and miR-429 target RPS6KB1 mRNA and inhibit the viral replication.miR-101 and miR-7 target the mTOR mRNA and inhibit the viral replication.miR-21, miR-155 and miR-126 were reported as potential prognostic factor of COVID-19 based on in vivo study miR126-3p and miR-21-5p were reported as potential biomarkers based on in vivo study |
[34][37][107][108] |
| miR-155-5p | 4-UUAAUGCUAAUCGUGAUAGGGGUU-27 | |||
| miR-181c-3p | 65-AACCAUCGACCGUUGAGUGGAC-86 | |||
| miR-587 | 16-UUUCCAUAGGUGAUGAGUCAC-36 | |||
| miR-101-3p | 47-UACAGUACUGUGAUAACUGAA-67 | |||
| miR-10b-5p | 27-UACCCUGUAGAACCGAAUUUGUG-49 | |||
| miR-126-3p | 52-UCGUACCGUGAGUAAUAAUGCG-73 | |||
| miR-128-3p | 50-UCACAGUGAACCGGUCUCUUU-70 | |||
| miR-129–2-3p | 57-AAGCCCUUACCCCAAAAAGCAU-78 | |||
| miR-203a-3p | 65-GUGAAAUGUUUAGGACCACUAG-86 | |||
| miR-34a-5p | 22-UGGCAGUGUCUUAGCUGGUUGU-43 | |||
| miR-34c-5p | 13-AGGCAGUGUAGUUAGCUGAUUGC-35 | |||
| miR-375-5p | 5-GCGACGAGCCCCUCGCACAAACC-27 | |||
| miR-375-3p | 40-UUUGUUCGUUCGGCUCGCGUGA-61 | |||
| miR-429 | 51-UAAUACUGUCUGGUAAAACCGU-72 | |||
| miR-449a | 16-UGGCAGUGUAUUGUUAGCUGGU-37 | |||
| miR-7-5p | 24-UGGAAGACUAGUGAUUUUGUUGUU-47 | |||
| miR-21-5p | 8-UAGCUUAUCAGACUGAUGUUGA-29 | |||
| miR-204-5p | 33-UUCCCUUUGUCAUCCUAUGCCU-54 | |||
| IL-6 | miR-155-5p | 4-UUAAUGCUAAUCGUGAUAGGGGUU-27 | miR-98-5p targets and inhibits IL-6 gene expression, in turn influencing several proinflammatory cytokines, including TNF-α, IL-1β, and IL-10. miR-7 and miR-16 target RPS6KB1 mRNA and inhibit the viral replication.miR-100, let-7, miR-7 and miR-99a target the mTOR mRNA and inhibit the viral replication. Upregulation of miR-124-3p causes the degradation of Ddx58, thereby leading to a decrease in viral replication. miR-125a-3p inhibits the cleavage of the S gene miR-138-5p inhibits the cleavage of the ORF1a/b polyprotein gene miR-21-3p expressed in respiratory epithelial cells in the trachea and lung tissues, which targets the binding site of 6 different coronavirus, including SARS-CoV-2 and SARS miR-21 and miR-155 were reported as potential prognostic factor of COVID-19 based on in vivo study |
[34][37][109][107] |
| miR-125a-3p | 53-ACAGGUGAGGUUCUUGGGAGCC-74 | |||
| miR-149-5p | 15-UCUGGCUCCGUGUCUUCACUCCC-37 | |||
| miR-192-5p | 24-CUGACCUAUGAAUUGACAGCC-44 | |||
| miR-590-3p | 56-UAAUUUUAUGUAUAAGCUAGU-76 | |||
| miR-100-5p | 13-AACCCGUAGAUCCGAACUUGUG-34 | |||
| miR-671-5p | 29-AGGAAGCCCUGGAGGGGCUGGAG-51 | |||
| miR-20a-5p | 8-UAAAGUGCUUAUAGUGCAGGUAG-30 | |||
| let-7b-5p | 6-UGAGGUAGUAGGUUGUGUGGUU-27 | |||
| miR-16-5p | 14-UAGCAGCACGUAAAUAUUGGCG-35 | |||
| miR-376a-5p | 7-GUAGAUUCUCCUUCUAUGAGUA-28 | |||
| miR-335-5p | 16-UCAAGAGCAAUAACGAAAAAUGU-38 | |||
| miR-98-5p | 22-UGAGGUAGUAAGUUGUAUUGUU-43 | |||
| miR-124-3p | 53-UAAGGCACGCGGUGAAUGCCAA-74 | |||
| miR-1-3p | 53-UGGAAUGUAAAGAAGUAUGUAU-74 | |||
| miR-34a-5p | 22-UGGCAGUGUCUUAGCUGGUUGU-43 | |||
| miR-99a-5p | 13-AACCCGUAGAUCCGAUCUUGUG-34 | |||
| miR-191-5p | 16-CAACGGAAUCCCAAAAGCAGCUG-38 | |||
| miR-128-3p | 50-UCACAGUGAACCGGUCUCUUU-70 | |||
| miR-138-5p | 10-AGCUGGUGUUGUGAAUCAGGCCG-32 | |||
| miR-182-5p | 23-UUUGGCAAUGGUAGAACUCACACU-46 | |||
| miR-195-5p | 15-UAGCAGCACAGAAAUAUUGGC-35 | |||
| miR-203a-3p | 65-GUGAAAUGUUUAGGACCACUAG-86 | |||
| miR-205-5p | 34-UCCUUCAUUCCACCGGAGUCUG-55 | |||
| miR-21-3p | 46-CAACACCAGUCGAUGGGCUGU-66 | |||
| miR-21-5p | 8-UAGCUUAUCAGACUGAUGUUGA-29 | |||
| miR-221-3p | 65-AGCUACAUUGUCUGCUGGGUUUC-87 | |||
| miR-27a-3p | 51-UUCACAGUGGCUAAGUUCCGC-71 | |||
| miR-27a-5p | 10-AGGGCUUAGCUGCUUGUGAGCA-31 | |||
| miR-330-3p | 57-GCAAAGCACACGGCCUGCAGAGA-79 | |||
| miR-34b-5p | 13-UAGGCAGUGUCAUUAGCUGAUUG-35 | |||
| miR-375-5p | 5-GCGACGAGCCCCUCGCACAAACC-27 | |||
| miR-375-3p | 40-UUUGUUCGUUCGGCUCGCGUGA-61 | |||
| miR-429 | 51-UAAUACUGUCUGGUAAAACCGU-72 | |||
| miR-7-5p | 24-UGGAAGACUAGUGAUUUUGUUGUU-47 | |||
| miR-373-3p | 44-GAAGUGCUUCGAUUUUGGGGUGU-66 | |||
| miR-372-3p | 42-AAAGUGCUGCGACAUUUGAGCGU-64 | |||
| miR-302a-3p | 44-UAAGUGCUUCCAUGUUUUGGUGA-66 | |||
| miR-148b-3p | 63-UCAGUGCAUCACAGAACUUUGU-84 | |||
| miR-133a-3p | 53-UUUGGUCCCCUUCAACCAGCUG-74 | |||
| miR-122-5p | 15-UGGAGUGUGACAAUGGUGUUUG-36 | |||
| IL-8 | miR-195-5p | 15-UAGCAGCACAGAAAUAUUGGC-35 | Upregulation of miR-17 and miR-214 have an antiviral effect by binding to S-protein-encoding mRNA, hence cause inhibition of the viral replication. miRNA-145 downregulates ADAM17, which is a target of Jagged1/Notch1 signaling in vascular smooth muscle cells. miR-7, miR-17, miR-16 and miR-107 target RPS6KB1 mRNA and inhibit the viral replication. miR-101, let-7, miR-107, miR-7 and miR-99b target the mTOR mRNA and inhibit the viral replication. Upregulation of miR-124-3p causes the degradation of Ddx58, thereby leading to a decrease in viral replication. miR-99b-5p regulates immune reactions. miR-130a predicts the targets of QFPD, and QFPD, which may exert anti-SARS-CoV-2 activity miR-17-5p have antiviral roles against SARS-CoV-2 by targeting the ORF1ab and the S region miR-23a-3p has prognostic and therapeutic effects based on in vivo study miR-146a-5p was reported as potential biomarker based on in vivo study miR-29a-3p promote IL-8 and other pro-inflammatory cytokine expression, despite being inversely correlated with IL-8 in COVID-19 |
[34][37][110][93][108][111][112][113][114] |
| miR-20a-5p | 8-UAAAGUGCUUAUAGUGCAGGUAG-30 | |||
| miR-106a-5p | 13-AAAAGUGCUUACAGUGCAGGUAG-35 | |||
| miR-17-5p | 14-CAAAGUGCUUACAGUGCAGGUAG-36 | |||
| miR-30c-1-3p | 56-CUGGGAGAGGGUUGUUUACUCC-77 | |||
| miR-93-5p | 11-CAAAGUGCUGUUCGUGCAGGUAG-33 | |||
| miR-373-3p | 44-GAAGUGCUUCGAUUUUGGGGUGU-66 | |||
| miR-520c-3p | 54-AAAGUGCUUCCUUUUAGAGGGU-75 | |||
| miR-10a-3p | 63-CAAAUUCGUAUCUAGGGGAAUA-84 | |||
| miR-1225-5p | 1-GUGGGUACGGCCCAGUGGGGGG-22 | |||
| miR-23a-3p | 45-AUCACAUUGCCAGGGAUUUCC-65 | |||
| miR-23b-3p | 58-AUCACAUUGCCAGGGAUUACCAC-80 | |||
| miR-296-3p | 48-GAGGGUUGGGUGGAGGCUCUCC-69 | |||
| miR-302c-5p | 8-UUUAACAUGGGGGUACCUGCUG-29 | |||
| miR-302d-5p | 6-ACUUUAACAUGGAGGCACUUGC-27 | |||
| miR-450a-5p | 18-UUUUGCGAUGUGUUCCUAAUAU-39 | |||
| miR-493-5p | 16-UUGUACAUGGUAGGCUUUCAUU-37 | |||
| miR-499a-3p | 70-AACAUCACAGCAAGUCUGUGCU-91 | |||
| miR-519d-3p | 54-CAAAGUGCCUCCCUUUAGAGUG-75 | |||
| miR-520a-3p | 53-AAAGUGCUUCCCUUUGGACUGU-74 | |||
| miR-526b-3p | 51-GAAAGUGCUUCCUUUUAGAGGC-72 | |||
| miR-5582-3p | 47-UAAAACUUUAAGUGUGCCUAGG-68 | |||
| miR-587 | 16-UUUCCAUAGGUGAUGAGUCAC-36 | |||
| miR-664a-3p | 49-UAUUCAUUUAUCCCCAGCCUACA-71 | |||
| miR-1-3p | 53-UGGAAUGUAAAGAAGUAUGUAU-74 | |||
| miR-429 | 51-UAAUACUGUCUGGUAAAACCGU-72 | |||
| miR-34a-5p | 22-UGGCAGUGUCUUAGCUGGUUGU-43 | |||
| miR-155-5p | 4-UUAAUGCUAAUCGUGAUAGGGGUU-27 | |||
| let-7b-5p | 6-UGAGGUAGUAGGUUGUGUGGUU-27 | |||
| miR-124-3p | 53-UAAGGCACGCGGUGAAUGCCAA-74 | |||
| miR-126-3p | 52-UCGUACCGUGAGUAAUAAUGCG-73 | |||
| miR-16-5p | 14-UAGCAGCACGUAAAUAUUGGCG-35 | |||
| miR-27a-3p | 51-UUCACAGUGGCUAAGUUCCGC-71 | |||
| miR-335-5p | 16-UCAAGAGCAAUAACGAAAAAUGU-38 | |||
| miR-1291 | 14-UGGCCCUGACUGAAGACCAGCAGU-37 | |||
| miR-138-5p | 10-AGCUGGUGUUGUGAAUCAGGCCG-32 | |||
| miR-101-3p | 47-UACAGUACUGUGAUAACUGAA-67 | |||
| miR-107 | 50-AGCAGCAUUGUACAGGGCUAUCA-72 | |||
| miR-129–2-3p | 57-AAGCCCUUACCCCAAAAAGCAU-78 | |||
| miR-130a-3p | 55-CAGUGCAAUGUUAAAAGGGCAU-76 | |||
| miR-146a-5p | 21-UGAGAACUGAAUUCCAUGGGUU-42 | |||
| miR-147a | 47-GUGUGUGGAAAUGCUUCUGC-66 | |||
| miR-194-5p | 15-UGUAACAGCAACUCCAUGUGGA-36 | |||
| miR-203a-3p | 65-GUGAAAUGUUUAGGACCACUAG-86 | |||
| miR-21-3p | 46-CAACACCAGUCGAUGGGCUGU-66 | |||
| miR-21-5p | 8-UAGCUUAUCAGACUGAUGUUGA-29 | |||
| miR-210-3p | 66-CUGUGCGUGUGACAGCGGCUGA-87 | |||
| miR-212-3p | 71-UAACAGUCUCCAGUCACGGCC-91 | |||
| miR-214-3p | 71-ACAGCAGGCACAGACAGGCAGU-92 | |||
| miR-221-3p | 65-AGCUACAUUGUCUGCUGGGUUUC-87 | |||
| miR-29a-5p | 4-ACUGAUUUCUUUUGGUGUUCAG-25 | |||
| miR-29a-3p | 42-UAGCACCAUCUGAAAUCGGUUA-63 | |||
| miR-30d-5p | 6-UGUAAACAUCCCCGACUGGAAG-27 | |||
| miR-376a-5p | 7-GUAGAUUCUCCUUCUAUGAGUA-28 | |||
| miR-671-5p | 29-AGGAAGCCCUGGAGGGGCUGGAG-51 | |||
| miR-7-5p | 24-UGGAAGACUAGUGAUUUUGUUGUU-47 | |||
| miR-941 | 47-CACCCGGCUGUGUGCACAUGUGC-69 | |||
| miR-99b-5p | 7-CACCCGUAGAACCGACCUUGCG-28 | |||
| miR-520f-3p | 55-AAGUGCUUCCUUUUAGAGGGUU-76 | |||
| miR-372-3p | 42-AAAGUGCUGCGACAUUUGAGCGU-64 | |||
| miR-148b-3p | 63-UCAGUGCAUCACAGAACUUUGU-84 | |||
| miR-133a-3p | 53-UUUGGUCCCCUUCAACCAGCUG-74 | |||
| miR-9-5p | 16-UCUUUGGUUAUCUAGCUGUAUGA-38 | |||
| miR-30a-5p | 6-UGUAAACAUCCUCGACUGGAAG-27 |
* All sequences of the miRNA binding site were retrieved from https://mirbase.org/ (Accessed on 19 January 2022). Abbreviations: RPS6KB1, ribosomal protein S6 kinase B1; mTOR, mammalian target of rapamycin; ORF, open reading frame; ADAM 17, a disintegrin and metalloprotease 17; QFPD, Qingfei Paidu Decoction.
References
- Chen, Y.; Liu, Q.; Guo, D. Emerging coronaviruses: Genome structure, replication, and pathogenesis. J. Med. Virol. 2020, 92, 418–423.
- Challen, R.; Brooks-Pollock, E.; Read, J.M.; Dyson, L.; Tsaneva-Atanasova, K.; Danon, L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ 2021, 372, n579.
- Zhou, D.; Dejnirattisai, W.; Supasa, P.; Liu, C.; Mentzer, A.J.; Ginn, H.M.; Zhao, Y.; Duyvesteyn, H.M.E.; Tuekprakhon, A.; Nutalai, R.; et al. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell 2021, 184, 2348–2361.e6.
- Mayo Clinic. COVID-19 Variants: What’s the Concern? Available online: https://www.mayoclinic.org/diseases-conditions/coronavirus/expert-answers/covid-variant/faq-20505779 (accessed on 31 August 2021).
- Hodcroft, E. Variant: 21A (Delta). Available online: https://covariants.org/variants/21A.Delta (accessed on 31 August 2021).
- WHO. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 31 August 2021).
- Hagen, A. How Dangerous Is the Delta Variant (B.1.617.2)? Available online: https://asm.org/Articles/2021/July/How-Dangerous-is-the-Delta-Variant-B-1-617-2 (accessed on 31 August 2021).
- Abbott, B. Delta Variant Accounts for 83% of Known U.S. Covid-19 Cases. Available online: https://www.wsj.com/articles/delta-variant-accounts-for-83-of-known-covid-19-cases-11626807019 (accessed on 31 August 2021).
- WHO. Update on Omicron. Available online: https://www.who.int/news/item/28-11-2021-update-on-omicron (accessed on 19 January 2022).
- Geddes, L. What Do We Know about the New B.1.1.529 Coronavirus Variant and Should We Be Worried? Available online: https://www.gavi.org/vaccineswork/what-we-know-about-new-b11529-coronavirus-variant-so-far?gclid=Cj0KCQiAip-PBhDVARIsAPP2xc3Q_oSb2Vr7HHnELNZmKy2-vrIG_qeiugLkRy3k3mjodpSQfcg_T78aAiVVEALw_wcB (accessed on 19 January 2022).
- Sinha, A. ‘IHU’ Variant of COVID-19 Explained: Few Cases, Limited Spread. Available online: https://indianexpress.com/article/explained/ihu-variant-few-cases-limited-spread-7706641/ (accessed on 19 January 2022).
- Le, T.K.; Paris, C.; Khan, K.S.; Robson, F.; Ng, W.-L.; Rocchi, P. Nucleic acid-based technologies targeting coronaviruses. Trends Biochem. Sci. 2021, 46, 351–365.
- Abbott, T.R.; Dhamdhere, G.; Liu, Y.; Lin, X.; Goudy, L.; Zeng, L.; Chemparathy, A.; Chmura, S.; Heaton, N.S.; Debs, R.; et al. Development of CRISPR as an antiviral strategy to combat SARS-CoV-2 and influenza. Cell 2020, 181, 865–876.e12.
- Sridharan, K.; Gogtay, N.J. Therapeutic nucleic acids: Current clinical status. Br. J. Clin. Pharmacol. 2016, 82, 659–672.
- Gewirtz, A.M.; Sokol, D.L.; Ratajczak, M.Z. Nucleic acid therapeutics: State of the art and future prospects. Blood 1998, 92, 712–736.
- Yan, Z.P.; Yang, M.; Lai, C.L. COVID-19 Vaccines: A Review of the Safety and Efficacy of Current Clinical Trials. Pharmaceuticals 2021, 14, 406.
- Dash, P.; Mohapatra, S.; Ghosh, S.; Nayak, B. A Scoping Insight on Potential Prophylactics, Vaccines and Therapeutic Weaponry for the Ongoing Novel Coronavirus (COVID-19) Pandemic- A Comprehensive Review. Front. Pharmacol. 2020, 11, 590154.
- Flanagan, K.L.; Best, E.; Crawford, N.W.; Giles, M.; Koirala, A.; Macartney, K.; Russell, F.; Teh, B.W.; Wen, S.C. Progress and Pitfalls in the Quest for Effective SARS-CoV-2 (COVID-19) Vaccines. Front. Immunol. 2020, 11, 579250.
- Berber, B.; Aydin, C.; Kocabas, F.; Guney-Esken, G.; Yilancioglu, K.; Karadag-Alpaslan, M.; Caliseki, M.; Yuce, M.; Demir, S.; Tastan, C. Gene editing and RNAi approaches for COVID-19 diagnostics and therapeutics. Gene Ther. 2021, 28, 290–305.
- Kim, D.; Rossi, J. RNAi mechanisms and applications. BioTechniques 2008, 44, 613–616.
- Lee, Y.; Hur, I.; Park, S.-Y.; Kim, Y.-K.; Suh, M.R.; Kim, V.N. The role of PACT in the RNA silencing pathway. EMBO J. 2006, 25, 522–532.
- Settleman, J.; Sawyers, C.L.; Hunter, T. Challenges in validating candidate therapeutic targets in cancer. eLife 2018, 7, e32402.
- Deng, Y.; Wang, C.C.; Choy, K.W.; Du, Q.; Chen, J.; Wang, Q.; Li, L.; Chung, T.K.H.; Tang, T. Therapeutic potentials of gene silencing by RNA interference: Principles, challenges, and new strategies. Gene 2014, 538, 217–227.
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498.
- Medeiros, I.G.; Khayat, A.S.; Stransky, B.; Santos, S.; Assumpção, P.; de Souza, J.E.S. A small interfering RNA (siRNA) database for SARS-CoV-2. Sci. Rep. 2021, 11, 8849.
- Ghosh, S.; Firdous, S.M.; Nath, A. siRNA could be a potential therapy for COVID-19. EXCLI J. 2020, 19, 528–531.
- Chen, W.; Feng, P.; Liu, K.; Wu, M.; Lin, H. Computational identification of small interfering RNA targets in SARS-CoV-2. Virol. Sin. 2020, 35, 359–361.
- Idris, A.; Davis, A.; Supramaniam, A.; Acharya, D.; Kelly, G.; Tayyar, Y.; West, N.; Zhang, P.; McMillan, C.L.; Soemardy, C. A SARS-CoV-2 targeted siRNA-nanoparticle therapy for COVID-19. Mol. Ther. 2021, 29, 2219–2226.
- Niktab, I.; Haghparast, M.; Beigi, M.-H.; Megraw, T.L.; Kiani, A.; Ghaedi, K. Design of advanced siRNA therapeutics for the treatment of COVID-19. Meta Gene 2021, 29, 100910.
- Farr, R.; Rootes, C.; Rowntree, L.; Nguyen, T.; Hensen, L.; Kedzierski, L.; Cheng, A.; Kedzierska, K.; Au, G.; Marsh, G. Altered microRNA expression in COVID-19 patients enables identification of SARS-CoV-2 infection. PLoS Pathog. 2021, 17, e1009759.
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105.
- Hum, C.; Loiselle, J.; Ahmed, N.; Shaw, T.A.; Toudic, C.; Pezacki, J.P. MicroRNA mimics or inhibitors as antiviral therapeutic approaches against COVID-19. Drugs 2021, 81, 517–531.
- Balmeh, N.; Mahmoudi, S.; Mohammadi, N.; Karabedianhajiabadi, A. Predicted therapeutic targets for COVID-19 disease by inhibiting SARS-CoV-2 and its related receptors. Inform. Med. Unlocked 2020, 20, 100407.
- Narozna, M.; Rubis, B. Anti-SARS-CoV-2 Strategies and the Potential Role of miRNA in the Assessment of COVID-19 Morbidity, Recurrence, and Therapy. Int. J. Mol. Sci. 2021, 22, 8663.
- Han, J.; Lee, Y.; Yeom, K.-H.; Nam, J.-W.; Heo, I.; Rhee, J.-K.; Sohn, S.Y.; Cho, Y.; Zhang, B.-T.; Kim, V.N. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell 2006, 125, 887–901.
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712.
- Hu, J.; Stojanovic, J.; Yasamineh, S.; Yasamineh, P.; Karuppannan, S.K.; Hussain Dowlath, M.J.; Serati-Nouri, H. The potential use of microRNAs as a therapeutic strategy for SARS-CoV-2 infection. Arch. Virol. 2021, 166, 2649–2672.
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L. A microRNA-29 mimic (Remlarsen) represses extracellular matrix expression and fibroplasia in the skin. J. Investig. Dermatol. 2019, 139, 1073–1081.
- Seto, A.G.; Beatty, X.; Lynch, J.M.; Hermreck, M.; Tetzlaff, M.; Duvic, M.; Jackson, A.L. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 2018, 183, 428–444.
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as therapeutics for gene silencing. Mol. Ther. Nucleic Acids 2015, 4, e252.
- Iorio, M.V.; Croce, C.M. MicroRNA dysregulation in cancer: Diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol. Med. 2012, 4, 143–159.
- Hosseini Rad Sm, A.; McLellan, A.D. Implications of SARS-CoV-2 mutations for genomic RNA structure and host microRNA targeting. Int. J. Mol. Sci. 2020, 21, 4807.
- Maitra, A.; Sarkar, M.C.; Raheja, H.; Biswas, N.K.; Chakraborti, S.; Singh, A.K.; Ghosh, S.; Sarkar, S.; Patra, S.; Mondal, R.K.; et al. Mutations in SARS-CoV-2 viral RNA identified in Eastern India: Possible implications for the ongoing outbreak in India and impact on viral structure and host susceptibility. J. Biosci. 2020, 45, 76.
- Lu, D.; Chatterjee, S.; Xiao, K.; Riedel, I.; Wang, Y.; Foo, R.; Bär, C.; Thum, T. MicroRNAs targeting the SARS-CoV-2 entry receptor ACE2 in cardiomyocytes. J. Mol. Cell. Cardiol. 2020, 148, 46–49.
- Matarese, A.; Gambardella, J.; Sardu, C.; Santulli, G. miR-98 regulates TMPRSS2 expression in human endothelial cells: Key implications for COVID-19. Biomedicines 2020, 8, 462.
- Kaur, T.; Kapila, S.; Kapila, R.; Kumar, S.; Upadhyay, D.; Kaur, M.; Sharma, C. Tmprss2 specific miRNAs as promising regulators for SARS-CoV-2 entry checkpoint. Virus Res. 2021, 294, 198275.
- Sardar, R.; Satish, D.; Birla, S.; Gupta, D. Dataset of mutational analysis, miRNAs targeting SARS-CoV-2 genes and host gene expression in SARS-CoV and SARS-CoV-2 infections. Data Brief. 2020, 32, 106207.
- Chow, J.T.-S.; Salmena, L. Prediction and analysis of SARS-CoV-2-targeting microRNA in human lung epithelium. Genes 2020, 11, 1002.
- Ge, J.; Li, J.; Na, S.; Wang, P.; Zhao, G.; Zhang, X. miR-548c-5p inhibits colorectal cancer cell proliferation by targeting PGK1. J. Cell. Physiol. 2019, 234, 18872–18878.
- Fulzele, S.; Sahay, B.; Yusufu, I.; Lee, T.J.; Sharma, A.; Kolhe, R.; Isales, C.M. COVID-19 virulence in aged patients might be impacted by the host cellular microRNAs abundance/profile. Aging Dis. 2020, 11, 509–522.
- El-Nabi, S.H.; Elhiti, M.; El-Sheekh, M. A new approach for COVID-19 treatment by micro-RNA. Med. Hypotheses 2020, 143, 110203.
- Zhang, S.; Amahong, K.; Sun, X.; Lian, X.; Liu, J.; Sun, H.; Lou, Y.; Zhu, F.; Qiu, Y. The miRNA: A small but powerful RNA for COVID-19. Brief. Bioinform. 2021, 22, 1137–1149.
- Fani, M.; Zandi, M.; Ebrahimi, S.; Soltani, S.; Abbasi, S. The role of miRNAs in COVID-19 disease. Future Virol. 2021, 16, 301–306.
- Gambardella, J.; Sardu, C.; Morelli, M.B.; Messina, V.; Castellanos, V.; Marfella, R.; Maggi, P.; Paolisso, G.; Wang, X.; Santulli, G. Exosomal microRNAs drive thrombosis in COVID-19. medRxiv 2020, 142.
- Saçar Demirci, M.D.; Adan, A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. PeerJ 2020, 8, e9369.
- Gallagher, T.M.; Buchmeier, M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology 2001, 279, 371–374.
- Alam, T.; Lipovich, L. miRCOVID-19: Potential targets of human miRNAs in SARS-CoV-2 for RNA-based drug discovery. Non-Coding RNA 2021, 7, 18.
- Wang, Y.; Zhang, S.; Li, F.; Zhou, Y.; Zhang, Y.; Wang, Z.; Zhang, R.; Zhu, J.; Ren, Y.; Tan, Y.; et al. Therapeutic target database 2020: Enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020, 48, D1031–D1041.
- Mann, M.; Wright, P.R.; Backofen, R. IntaRNA 2.0: Enhanced and customizable prediction of RNA-RNA interactions. Nucleic Acids Res. 2017, 45, W435–W439.
- Ritchie, W.; Flamant, S.; Rasko, J.E.J. mimiRNA: A microRNA expression profiler and classification resource designed to identify functional correlations between microRNAs and their targets. Bioinformatics 2010, 26, 223–227.
- Dai, X.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2011, 39, W155–W159.
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008, 36, D149–D153.
- Chou, C.-H.; Shrestha, S.; Yang, C.-D.; Chang, N.-W.; Lin, Y.-L.; Liao, K.-W.; Huang, W.-C.; Sun, T.-H.; Tu, S.-J.; Lee, W.-H.; et al. miRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018, 46, D296–D302.
- Huang, H.-Y.; Lin, Y.-C.-D.; Li, J.; Huang, K.-Y.; Shrestha, S.; Hong, H.-C.; Tang, Y.; Chen, Y.-G.; Jin, C.-N.; Yu, Y.; et al. miRTarBase 2020: Updates to the experimentally validated microRNA-target interaction database. Nucleic Acids Res. 2020, 48, D148–D154.
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279.
- Yi, C.; Yi, Y.; Li, J. mRNA vaccines: Possible tools to combat SARS-CoV-2. Virol. Sin. 2020, 35, 259–262.
- McNamara, M.A.; Nair, S.K.; Holl, E.K. RNA-based vaccines in cancer immunotherapy. J. Immunol. Res. 2015, 2015, 794528.
- Rosenberg, Y.; Sack, M.; Montefiori, D.; Labranche, C.; Lewis, M.; Urban, L.; Mao, L.; Fischer, R.; Jiang, X. Pharmacokinetics and immunogenicity of broadly neutralizing HIV monoclonal antibodies in macaques. PLoS ONE 2015, 10, e0120451.
- Kamboj, M.; Sepkowitz, K.A. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect. Control. Hosp. Epidemiol. 2007, 28, 702–707.
- Lim, B.; Lee, K. Stability of the osmoregulated promoter-derived prop mRNA is posttranscriptionally regulated by RNase III in Escherichia coli. J. Bacteriol. 2015, 197, 1297–1305.
- Pardi, N.; Weissman, D. Nucleoside modified mRNA vaccines for infectious diseases. Methods Mol. Biol. 2017, 1499, 109–121.
- Schlake, T.; Thess, A.; Thran, M.; Jordan, I. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. 2019, 76, 301–328.
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. Npj Vaccines 2020, 5, 11.
- Wang, F.; Kream, R.M.; Stefano, G.B. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e924700.
- Crommelin, D.J.A.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the cold reality of mRNA vaccine stability. J. Pharm. Sci. 2021, 110, 997–1001.
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016, 7, 319–334.
- Vigerust, D.J.; Shepherd, V.L. Virus glycosylation: Role in virulence and immune interactions. Trends Microbiol. 2007, 15, 211–218.
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416.
- Xie, X.; Liu, Y.; Liu, J.; Zhang, X.; Zou, J.; Fontes-Garfias, C.R.; Xia, H.; Swanson, K.A.; Cutler, M.; Cooper, D.; et al. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera. Nat. Med. 2021, 27, 620–621.
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A.; National Study Group for, C.-V. Effectiveness of the BNT162b2 COVID-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Skowronski, D.M.; Serres, G.D. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2021, 384, 1576–1578.
- Muik, A.; Wallisch, A.-K.; Sänger, B.; Swanson, K.A.; Mühl, J.; Chen, W.; Cai, H.; Maurus, D.; Sarkar, R.; Türeci, Ö. Neutralization of SARS-CoV-2 lineage B. 1.1. 7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science 2021, 371, 1152–1153.
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829.
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021, 27, 1290–1297.
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 2018, 9, 1963.
- Soppe, J.A.; Lebbink, R.J. Antiviral goes viral: Harnessing CRISPR/Cas9 to combat viruses in humans. Trends Microbiol. 2017, 25, 833–850.
- Rahimi, H.; Salehiabar, M.; Barsbay, M.; Ghaffarlou, M.; Kavetskyy, T.; Sharafi, A.; Davaran, S.; Chauhan, S.C.; Danafar, H.; Kaboli, S.; et al. CRISPR systems for COVID-19 diagnosis. ACS Sens. 2021, 6, 1430–1445.
- Freije, C.A.; Myhrvold, C.; Boehm, C.K.; Lin, A.E.; Welch, N.L.; Carter, A.; Metsky, H.C.; Luo, C.Y.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Programmable inhibition and detection of RNA viruses using Cas13. Mol. Cell 2019, 76, 826–837.e11.
- Barrey, E.; Burzio, V.; Dhorne-Pollet, S.; Eléouët, J.-F.; Delmas, B. Think Different with RNA Therapy: Can Antisense Oligonucleotides Be Used to Inhibit Replication and Transcription of SARS-Cov-2? Preprints 2020, 2020040412.
- Gasparello, J.; Finotti, A.; Gambari, R. Tackling the COVID-19 “cytokine storm” with microRNA mimics directly targeting the 3′UTR of pro-inflammatory mRNAs. Med. Hypotheses 2021, 146, 110415.
- Centa, A.; Fonseca, A.S.; da Silva Ferreira, S.G.; Azevedo, M.L.V.; Vaz de Paula, C.B.; Nagashima, S.; Machado-Souza, C.; dos Santos Miggiolaro, A.F.R.; Baena, C.P.; de Noronha, L.; et al. Deregulated miRNA expression is associated with endothelial dysfunction in post-mortem lung biopsies of COVID-19 patients. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 320, L405–L412.
- Wicik, Z.; Eyileten, C.; Jakubik, D.; Simões, S.N.; Martins, D.C.; Pavão, R.; Siller-Matula, J.M.; Postula, M. ACE2 interaction networks in COVID-19: A physiological framework for prediction of outcome in patients with cardiovascular risk factors. J. Clin. Med. 2020, 9, 3743.
- Nersisyan, S.; Engibaryan, N.; Gorbonos, A.; Kirdey, K.; Makhonin, A.; Tonevitsky, A. Potential role of cellular miRNAs in coronavirus-host interplay. PeerJ 2020, 8, e9994.
- Yamada, K.; Takizawa, S.; Ohgaku, Y.; Asami, T.; Furuya, K.; Yamamoto, K.; Takahashi, F.; Hamajima, C.; Inaba, C.; Endo, K.; et al. MicroRNA 16-5p is upregulated in calorie-restricted mice and modulates inflammatory cytokines of macrophages. Gene 2020, 725, 144191.
- Ye, E.-A.; Liu, L.; Jiang, Y.; Jan, J.; Gaddipati, S.; Suvas, S.; Steinle, J.J. miR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling. J. Neuroinflamm. 2016, 13, 305.
- Sun, C.-M.; Wu, J.; Zhang, H.; Shi, G.; Chen, Z.-T. Circulating miR-125a but not miR-125b is decreased in active disease status and negatively correlates with disease severity as well as inflammatory cytokines in patients with Crohn’s disease. World J. Gastroenterol. 2017, 23, 7888–7898.
- Uludağ, H.; Parent, K.; Aliabadi, H.M.; Haddadi, A. Prospects for RNAi therapy of COVID-19. Front. Bioeng. Biotechnol. 2020, 8, 916.
- Mai, J.; Virtue, A.; Maley, E.; Tran, T.; Yin, Y.; Meng, S.; Pansuria, M.; Jiang, X.; Wang, H.; Yang, X.-F. MicroRNAs and other mechanisms regulate interleukin-17 cytokines and receptors. Front. Biosci. (Elite Ed.) 2012, 4, 1478–1495.
- Chen, X.; Zhou, L.; Peng, N.; Yu, H.; Li, M.; Cao, Z.; Lin, Y.; Wang, X.; Li, Q.; Wang, J.; et al. MicroRNA-302a suppresses influenza A virus-stimulated interferon regulatory factor-5 expression and cytokine storm induction. J. Biol. Chem. 2017, 292, 21291–21303.
- Desjarlais, M.; Wirth, M.; Lahaie, I.; Ruknudin, P.; Hardy, P.; Rivard, A.; Chemtob, S. Nutraceutical targeting of inflammation-modulating microRNAs in severe forms of COVID-19: A novel approach to prevent the cytokine storm. Front. Pharmacol. 2020, 11, 602999.
- Gangemi, S.; Tonacci, A. AntagomiRs: A novel therapeutic strategy for challenging COVID-19 cytokine storm. Cytokine Growth Factor Rev. 2021, 58, 111–113.
- Chen, B.-B.; Li, Z.-H.; Gao, S. Circulating miR-146a/b correlates with inflammatory cytokines in COPD and could predict the risk of acute exacerbation COPD. Medicine 2018, 97, e9820.
- Liu, Y.; Guan, H.; Zhang, J.-L.; Zheng, Z.; Wang, H.-T.; Tao, K.; Han, S.-C.; Su, L.-L.; Hu, D. Acute downregulation of miR-199a attenuates sepsis-induced acute lung injury by targeting SIRT1. Am. J. Physiol. Cell Physiol. 2018, 314, C449–C455.
- Hu, H.-L.; Nie, Z.-Q.; Lu, Y.; Yang, X.; Song, C.; Chen, H.; Zhu, S.; Chen, B.-B.; Huang, J.; Geng, S.; et al. Circulating miR-125b but not miR-125a correlates with acute exacerbations of chronic obstructive pulmonary disease and the expressions of inflammatory cytokines. Medicine 2017, 96, e9059.
- Garg, A.; Seeliger, B.; Derda, A.A.; Xiao, K.; Gietz, A.; Scherf, K.; Sonnenschein, K.; Pink, I.; Hoeper, M.M.; Welte, T.; et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 468–475.
- Sabbatinelli, J.; Giuliani, A.; Matacchione, G.; Latini, S.; Laprovitera, N.; Pomponio, G.; Ferrarini, A.; Svegliati Baroni, S.; Pavani, M.; Moretti, M.; et al. Decreased serum levels of the inflammaging marker miR-146a are associated with clinical non-response to tocilizumab in COVID-19 patients. Mech. Ageing Dev. 2021, 193, 111413.
- Marchi, R.; Sugita, B.; Centa, A.; Fonseca, A.S.; Bortoletto, S.; Fiorentin, K.; Ferreira, S.; Cavalli, L.R. The role of microRNAs in modulating SARS-CoV-2 infection in human cells: A systematic review. Infection, genetics and evolution. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2021, 91, 104832.
- Khan, M.A.-A.-K.; Sany, M.R.U.; Islam, M.S.; Islam, A.B.M.M.K. Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front. Genet. 2020, 11, 765.
- Kalhori, M.R.; Saadatpour, F.; Arefian, E.; Soleimani, M.; Farzaei, M.H.; Aneva, I.Y.; Echeverria, J. The Potential Therapeutic Effect of RNA Interference and Natural Products on COVID-19: A Review of the Coronaviruses Infection. Front. Pharmacol. 2021, 12, 616993.
- Chen, Z.-M.; Fu, J.-F.; Shu, Q.; Chen, Y.-H.; Hua, C.-Z.; Li, F.-B.; Lin, R.; Tang, L.-F.; Wang, T.-L.; Wang, W. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J. Pediatr. 2020, 16, 240–246.
- Wang, J.; Zhu, M.; Ye, L.; Chen, C.; She, J.; Song, Y. MiR-29b-3p promotes particulate matter-induced inflammatory responses by regulating the C1QTNF6/AMPK pathway. Aging 2020, 12, 1141–1158.
- Plowman, T.; Lagos, D. Non-Coding RNAs in COVID-19: Emerging Insights and Current Questions. Non-Coding RNA 2021, 7, 54.
More
Information
Subjects:
Infectious Diseases; Respiratory System
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
810
Revisions:
3 times
(View History)
Update Date:
25 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




