
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gianluca Tosini | + 2136 word(s) | 2136 | 2022-03-02 02:59:02 | | | |
| 2 | Bruce Ren | Meta information modification | 2136 | 2022-03-21 02:09:40 | | |
Video Upload Options
The retinal pigment epithelium (RPE) is a single layer of cells located between the choriocapillaris vessels and the light-sensitive photoreceptors in the outer retina. The RPE performs physiological processes necessary for the maintenance and support of photoreceptors and visual function. Among the many functions performed by the RPE, the timing of the peak in phagocytic activity by the RPE of the photoreceptor outer segments that occurs 1–2 h. after the onset of light has captured the interest of many investigators and has thus been intensively studied. Several studies have shown that this burst in phagocytic activity by the RPE is under circadian control and is present in nocturnal and diurnal species and rod and cone photoreceptors. Previous investigations have demonstrated that a functional circadian clock exists within multiple retinal cell types and RPE cells. However, the anatomical location of the circadian controlling this activity is not clear. Experimental evidence indicates that the circadian clock, melatonin, dopamine, and integrin signaling play a key role in controlling this rhythm.
1. Introduction
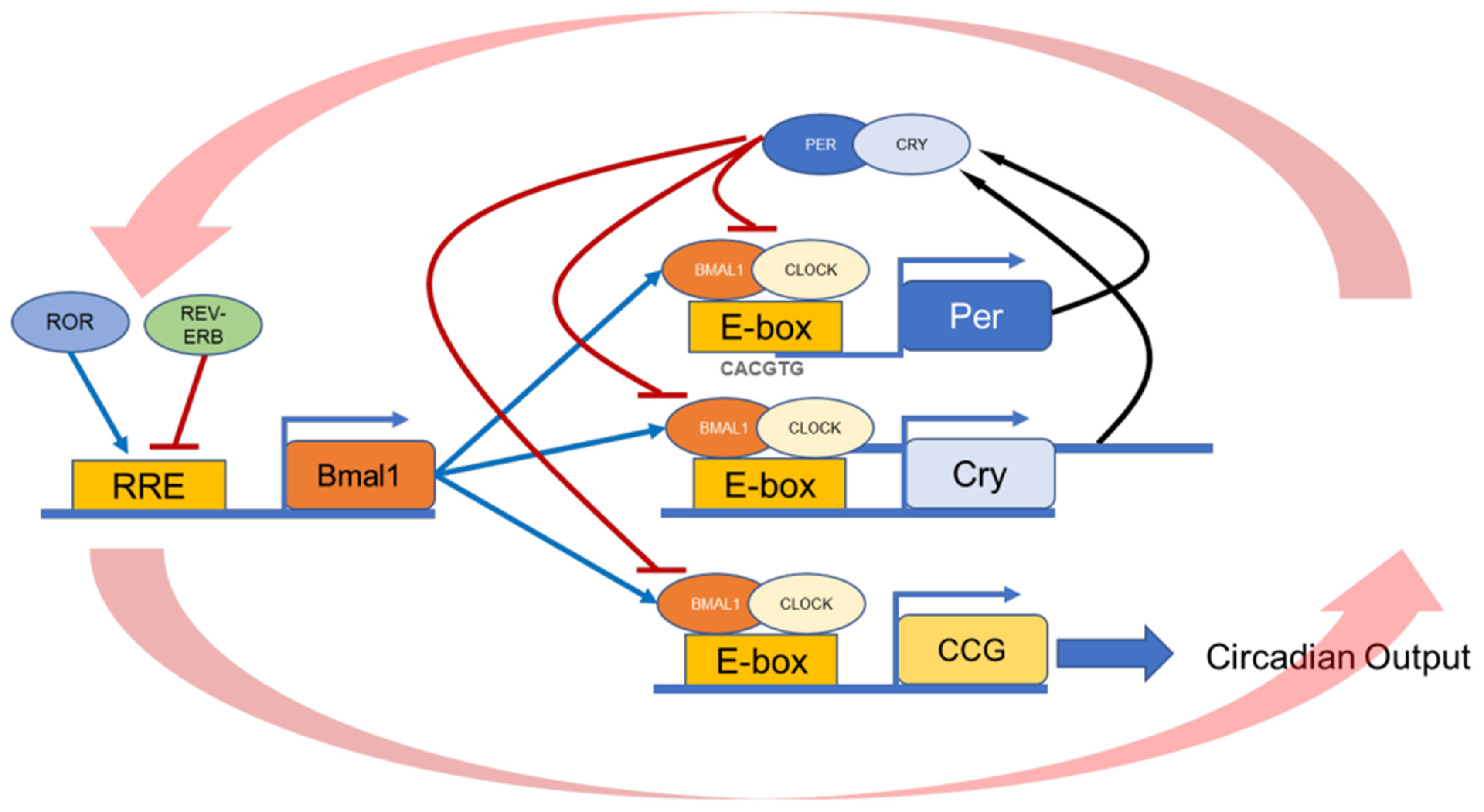
2. Regulation of RPE Function In Vivo
| Function in RPE | Animal Model | Human Retinal Disease | |
|---|---|---|---|
| MerTK receptor | Outer segment binding & internalization | RCS rat [43][44] Merkd mouse [45] |
Retinitis pigmentosa, rod-cone dystrophy [46][47][48][49][50] |
| Gas6, Protein S | MerTK ligands | Gas6 double KO and ProS1 [51] | Diabetic Retinopathy and macular edema [52] |
| ανβ5 integrin receptor | Outer segment binding, Control the diurnal rhythm in peak of phagocytosis |
β5−/− mouse [29] | unknown |
| MFG-E8 | ανβ5 integrin ligand Control the diurnal rhythm in peak of phagocytosis |
MFG-E8−/− mouse [30] | unknown |
| Dopamine receptor 2 | Controls the rhythm in RPE circadian clocks, light adaptation, peak of phagocytosis after light onset | D2R KO mouse [31] | unkown |
| Melatonin receptor 1 and 2 | Control the timing of the peak of phagocytosis | MT1 & MT2 KO mouse [34] | |
| RPE specific Bmal1 KO | Control the diurnal rhythm in peak of phagocytosis | RPEcre; Bmal1fl/fl [42] | unknown |
| Per1/Per2 global KO | Controls the amplitude of the peak of phagocytosis | Per1−/−Per2Brdm1 [41] | unknown |
3. Regulation of RPE Function In Vitro
References
- Felder, M.-P.; Buhr, E.D.; Dkhissi-Benyahya, O.; Hicks, D.; Peirson, S.N.; Ribelayga, C.P.; Sandu, C.; Spessert, R.; Tosini, G. Ocular Clocks: Adapting Mechanisms for Eye Functions and Health. Investig. Opthalmology Vis. Sci. 2018, 59, 4856–4870.
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873.
- Storch, K.-F.; Paz, C.; Signorovitch, J.; Raviola, E.; Pawlyk, B.; Li, T.; Weitz, C.J. Intrinsic Circadian Clock of the Mammalian Retina: Importance for Retinal Processing of Visual Information. Cell 2007, 130, 730–741.
- Sawant, O.B.; Horton, A.M.; Zucaro, O.F.; Chan, R.; Bonilha, V.L.; Samuels, I.S.; Rao, S. The Circadian Clock Gene Bmal1 Controls Thyroid Hormone-Mediated Spectral Identity and Cone Photoreceptor Function. Cell Rep. 2017, 21, 692–706.
- Baba, K.; Pozdeyev, N.; Mazzoni, F.; Contreras-Alcantara, S.; Liu, C.; Kasamatsu, M.; Martinez-Merlos, T.; Strettoi, E.; Iuvone, P.M.; Tosini, G. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc. Natl. Acad. Sci. USA 2009, 106, 15043–15048.
- Baba, K.; Piano, I.; Lyuboslavsky, P.; Chrenek, M.A.; Sellers, J.T.; Zhang, S.; Gargini, C.; He, L.; Tosini, G.; Iuvone, P.M. Removal of clock gene Bmal1 from the retina affects retinal development and accelerates cone photoreceptor degeneration during aging. Proc. Natl. Acad. Sci. USA 2018, 115, 13099–13104.
- Baba, K.; Tosini, G. Aging Alters Circadian Rhythms in the Mouse Eye. J. Biol. Rhythm. 2018, 33, 441–445.
- Gianesini, C.; Hiragaki, S.; Laurent, V.; Hicks, D.; Tosini, G. Cone Viability Is Affected by Disruption of Melatonin Receptors Signaling. Investig. Opthalmology Vis. Sci. 2016, 57, 94–104.
- Ait-Hmyed, O.; Felder-Schmittbuhl, M.-P.; Garcia-Garrido, M.; Beck, S.C.; Seide, C.; Sothilingam, V.; Tanimoto, N.; Seeliger, W.M.; Bennis, M.; Hicks, D. Mice lacking Period 1 and Period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur. J. Neurosci. 2013, 37, 1048–1060.
- Hakkari, O.A.; Acar, N.; Savier, E.; Spinnhirny, P.; Bennis, M.; Felder-Schmittbuhl, M.; Mendoza, J.; Hicks, D. Rev-Erbα modulates retinal visual processing and behavioral responses to light. FASEB J. 2016, 30, 3690–3701.
- Kevany, B.M.; Palczewski, K. Phagocytosis of Retinal Rod and Cone Photoreceptors. Physiology 2010, 25, 8–15.
- Bok, D. The retinal pigment epithelium: A versatile partner in vision. J. Cell Sci. 1993, 1993, 189–195.
- Nguyen-Legros, J.; Hicks, D. Renewal of photoreceptor outer segments and their phagocytosis by theretinal pigment epithelium. Adv. Virus Res. 2000, 196, 245–313.
- LaVail, M.M. Chapter 44 Legacy of the RCS rat: Impact of a seminal study on retinal cell biology and retinal degenerative diseases. Prog. Brain Res. 2001, 131, 617–627.
- Lavail, M.M. Interaction of environmental light and eye pigmentation with inherited retinal degenerations. Vis. Res. 1980, 20, 1173–1177.
- LaVail, M.M. Rod Outer Segment Disk Shedding in Rat Retina: Relationship to Cyclic Lighting. Science 1976, 194, 1071–1074.
- Grace, M.S.; Chiba, A.; Menaker, M. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis. Neurosci. 1999, 16, 909–918.
- Besharse, J.C.; Hollyfield, J.G. Turnover of mouse photoreceptor outer segments in constant light and darkness. Investig. Ophthalmol. Vis. Sci. 1979, 18, 1019–1024.
- Teirstein, P.S.; Goldman, A.I.; O’Brien, P.J. Evidence for both local and central regulation of rat rod outer segment disc shedding. Investig. Ophthalmol. Vis. Sci. 1980, 19, 1268–1273.
- Terman, J.S.; Reme, C.E.; Terman, M. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993, 605, 256–264.
- Tosini, G.; Menaker, M. Circadian Rhythms in Cultured Mammalian Retina. Science 1996, 272, 419–421.
- Ruan, G.-X.; Allen, G.C.; Yamazaki, S.; McMahon, D.G. An Autonomous Circadian Clock in the Inner Mouse Retina Regulated by Dopamine and GABA. PLoS Biol. 2008, 6, e249-18.
- Tosini, G.; Davidson, A.J.; Fukuhara, C.; Kasamatsu, M.; Castanon-Cervantes, O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007, 21, 3866–3871.
- Jaeger, C.; Sandu, C.; Malan, A.; Mellac, K.; Hicks, D.; Felder-Schmittbuhl, M. Circadian organization of the rodent retina involves strongly coupled, layer-specific oscillators. FASEB J. 2015, 29, 1493–1504.
- Baba, K.; Sengupta, A.; Tosini, M.; Contreras-Alcantara, S.; Tosini, G. Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol. Vis. 2010, 16, 2605.
- Baba, K.; DeBruyne, J.P.; Tosini, G. Dopamine 2 Receptor Activation Entrains Circadian Clocks in Mouse Retinal Pigment Epithelium. Sci. Rep. 2017, 7, 1–9.
- Bobu, C.; Craft, C.M.; Masson-Pevet, M.; Hicks, D. Photoreceptor Organization and Rhythmic Phagocytosis in the Nile RatArvicanthis Ansorgei: A Novel Diurnal Rodent Model for the Study of Cone Pathophysiology. Investig. Opthalmology Vis. Sci. 2006, 47, 3109–3118.
- Krigel, A.; Felder-Schmittbuhl, M.-P.; Hicks, D. Circadian-clock driven cone-like photoreceptor phagocytosis in the neural retina leucine zipper gene knockout mouse. Mol. Vis. 2010, 16, 2873–2881.
- Nandrot, E.F.; Kim, Y.; Brodie, S.; Huang, X.; Sheppard, D.; Finnemann, S.C. Loss of Synchronized Retinal Phagocytosis and Age-related Blindness in Mice Lacking αvβ5 Integrin. J. Exp. Med. 2004, 200, 1539–1545.
- Nandrot, E.; Anand, M.; Almeida, D.; Atabai, K.; Sheppard, D.; Finnemann, S.C. Essential role for MFG-E8 as ligand for vbeta5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12005–12010.
- Goyal, V.; DeVera, C.; Laurent, V.; Sellers, J.; Chrenek, M.A.; Hicks, D.; Baba, K.; Iuvone, P.M.; Tosini, G. Dopamine 2 Receptor Signaling Controls the Daily Burst in Phagocytic Activity in the Mouse Retinal Pigment Epithelium. Investig. Opthalmology Vis. Sci. 2020, 61, 10.
- Mao, Y.; Finnemann, S.C. Analysis of Photoreceptor Outer Segment Phagocytosis by RPE Cells in Culture. Program. Necrosis 2012, 935, 285–295.
- Ruggiero, L.; Connor, M.P.; Chen, J.; Langen, R.; Finnemann, S.C. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5-/- or Mfge8-/- mouse retina. Proc. Natl. Acad. Sci. USA 2012, 109, 8145–8148.
- Laurent, V.; Sengupta, A.; Sanchez, A.S.-B.; Hicks, D.; Tosini, G. Melatonin signaling affects the timing in the daily rhythm of phagocytic activity by the retinal pigment epithelium. Exp. Eye Res. 2017, 165, 90–95.
- Gibbs, D.; Kitamoto, J.; Williams, D.S. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc. Natl. Acad. Sci. USA 2003, 100, 6481–6486.
- Law, A.-L.; Ling, Q.; Hajjar, K.A.; Futter, C.E.; Greenwood, J.; Adamson, P.; Wavre-Shapton, S.T.; Moss, S.E.; Hayes, M.J. Annexin A2 Regulates Phagocytosis of Photoreceptor Outer Segments in the Mouse Retina. Mol. Biol. Cell 2009, 20, 3896–3904.
- Mustafi, D.; Kevany, B.M.; Genoud, C.; Bai, X.; Palczewski, K. Photoreceptor phagocytosis is mediated by phosphoinositide signaling. FASEB J. 2013, 27, 4585–4595.
- DeVera, C.; Tosini, G. Circadian analysis of the mouse retinal pigment epithelium transcriptome. Exp. Eye Res. 2020, 193, 107988.
- Louer, E.M.; Günzel, D.; Rosenthal, R.; Carmone, C.; Yi, G.; Stunnenberg, H.G.; Hollander, A.I.D.; Deen, P.M. Differential day-night expression of tight junction components in murine retinal pigment epithelium. Exp. Eye Res. 2020, 193, 107985.
- Chao, J.R.; Knight, K.; Engel, A.L.; Jankowski, C.; Wang, Y.; Manson, M.A.; Gu, H.; Djukovic, D.; Raftery, D.; Hurley, J.B.; et al. Human retinal pigment epithelial cells prefer proline as a nutrient and transport metabolic intermediates to the retinal side. J. Biol. Chem. 2017, 292, 12895–12905.
- Milićević, N.; Hakkari, O.A.; Bagchi, U.; Sandu, C.; Jongejan, A.; Moerland, P.D.; Brink, J.B.T.; Hicks, D.; Bergen, A.A.; Felder-Schmittbuhl, M. Core circadian clock genes Per1 and Per2 regulate the rhythm in photoreceptor outer segment phagocytosis. FASEB J. 2021, 35, e21722.
- DeVera, C.; Dixon, J.; Chrenek, M.A.; Baba, K.; Iuvone, P.M.; Tosini, G. The circadian clock in the retinal pigment epithelium controls the diurnal rhythm of phagocytic activity. bioRxiv 2020.
- Nandrot, E.; Dufour, E.M.; Provost, A.C.; Péquignot, M.O.; Bonnel, S.; Gogat, K.; Marchant, D.; Rouillac, C.; de Condé, B.S.; Bihoreau, M.-T.; et al. Homozygous Deletion in the Coding Sequence of the c-mer Gene in RCS Rats Unravels General Mechanisms of Physiological Cell Adhesion and Apoptosis. Neurobiol. Dis. 2000, 7, 586–599.
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; Lavail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651.
- Lu, Q.; Gore, M.; Zhang, Q.; Camenisch, T.; Boast, S.; Casagranda, F.; Lai, C.; Skinner, M.K.; Klein, R.; Matsushima, G.K.; et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 1999, 398, 723–728.
- Brea-Fernandez, A.J.; Pomares, E.; Brion, M.J.; Marfany, G.; Blanco, M.J.; Sanchez-Salorio, M.; Gonzalez-Duarte, R.; Carracedo, A. Novel splice donor site mutation in MERTK gene associated with retinitis pigmentosa. Br. J. Ophthalmol. 2008, 92, 1419–1423.
- Ksantini, M.; Lafont, E.; Bocquet, B.; Meunier, I.; Hamel, C.P. Homozygous Mutation in MERTK Causes Severe Autosomal Recessive Retinitis Pigmentosa. Eur. J. Ophthalmol. 2011, 22, 647–653.
- Mackay, D.S.; Henderson, R.H.; Sergouniotis, P.I.; Li, Z.; Moradi, P.; Holder, G.E.; Waseem, N.; Bhattacharya, S.S.; Aldahmesh, M.A.; Alkuraya, F.S.; et al. Novel mutations in MERTK associated with childhood onset rod-cone dystrophy. Mol. Vis. 2010, 16, 369–377.
- McHenry, C.L.; Liu, Y.; Feng, W.; Nair, A.R.; Feathers, K.L.; Ding, X.; Gal, A.; Vollrath, U.; Sieving, P.A.; Thompson, D. MERTK arginine-844-cysteine in a patient with severe rod-cone dystrophy: Loss of mutant protein function in transfected cells. Investig. Opthalmology Vis. Sci. 2004, 45, 1456–1463.
- Tschernutter, M.; Jenkins, S.A.; Waseem, N.H.; Saihan, Z.; E Holder, G.; Bird, A.C.; Bhattacharya, S.S.; Ali, R.R.; Webster, A.R. Clinical characterisation of a family with retinal dystrophy caused by mutation in the Mertk gene. Br. J. Ophthalmol. 2006, 90, 718–723.
- Prasad, D.; Rothlin, C.V.; Burrola, P.; Burstyn-Cohen, T.; Lu, Q.; de Frutos, P.G.; Lemke, G. TAM receptor function in the retinal pigment epithelium. Mol. Cell. Neurosci. 2006, 33, 96–108.
- Sugimoto, M.; Kondo, M.; Yasuma, T.; D’Alessandro-Gabazza, C.N.; Toda, M.; Imai, H.; Nakamura, M.; Gabazza, E.C. Increased expression of Protein S in eyes with diabetic retinopathy and diabetic macular edema. Sci. Rep. 2021, 11, 1–9.
- Adijanto, J.; Philp, N.J. Cultured primary human fetal retinal pigment epithelium (hfRPE) as a model for evaluating RPE metabolism. Exp. Eye Res. 2014, 126, 77–84.
- Hu, J.; Bok, D. The use of cultured human fetal retinal pigment epithelium in studies of the classical retinoid visual cycle and retinoid-based disease processes. Exp. Eye Res. 2013, 126, 46–50.
- Mayerson, P.L.; Hall, M.O.; Clark, V.; Abrams, T. An improved method for isolation and culture of rat retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1599–1609.
- Sonoda, S.; Spee, C.; Barron, E.; Ryan, S.J.; Kannan, R.; Hinton, D.R. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat. Protoc. 2009, 4, 662–673.
- Godino, R.F.; Garland, D.L.; Pierce, E. Isolation, culture and characterization of primary mouse RPE cells. Nat. Protoc. 2016, 11, 1206–1218.
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, A Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–170.
- Nabi, I.; Mathews, A.; Cohen-Gould, L.; Gundersen, D.; Rodriguez-Boulan, E. Immortalization of polarized rat retinal pigment epithelium. J. Cell Sci. 1993, 104, 37–49.
- Kuznetsova, A.V.; Kurinov, A.M.; Aleksandrova, M.A. Cell Models to Study Regulation of Cell Transformation in Pathologies of Retinal Pigment Epithelium. J. Ophthalmol. 2014, 2014, 1–18.
- Fronk, A.H.; Vargis, E. Methods for culturing retinal pigment epithelial cells: A review of current protocols and future recommendations. J. Tissue Eng. 2016, 7, 27493715.
- Lakkaraju, A.; Umapathy, A.; Tan, L.X.; Daniele, L.; Philp, N.J.; Boesze-Battaglia, K.; Williams, D.S. The cell biology of the retinal pigment epithelium. Prog. Retin. Eye Res. 2020, 78, 100846.
- Pavan, B.; Frigato, E.; Pozzati, S.; Prasad, P.D.; Bertolucci, C.; Biondi, C. Circadian clocks regulate adenylyl cyclase activity rhythms in human RPE cells. Biochem. Biophys. Res. Commun. 2006, 350, 169–173.
- Yoshikawa, A.; Shimada, H.; Numazawa, K.; Sasaki, T.; Ikeda, M.; Kawashima, M.; Kato, N.; Tokunaga, K.; Ebisawa, T. Establishment of human cell lines showing circadian rhythms of bioluminescence. Neurosci. Lett. 2008, 446, 40–44.
- Milićević, N.; Mazzaro, N.; De Bruin, I.; Wils, E.; Brink, J.T.; Asbroek, A.T.; Mendoza, J.; Bergen, A.; Felder-Schmittbuhl, M.-P. Rev-Erbα and Photoreceptor Outer Segments modulate the Circadian Clock in Retinal Pigment Epithelial Cells. Sci. Rep. 2019, 9, 1–13.
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346.
- Baba, K.; Davidson, A.J.; Tosini, G. Melatonin Entrains PER2::LUC Bioluminescence Circadian Rhythm in the Mouse Cornea. Invest. Ophthalmol. Vis. Sci. 2015, 56, 4753–4758.
- Dunmire, J.; Dalvin, L.; Bouhenni, R.; Edward, D. Expression of Circadian Rhythm Genes in the Mouse Iris-Ciliary Body Complex. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1994.
- Tsuchiya, S.; Buhr, E.D.; Higashide, T.; Sugiyama, K.; Van Gelder, R.N. Light entrainment of the murine intraocular pressure circadian rhythm utilizes non-local mechanisms. PLoS ONE 2017, 12, e0184790.
- Evans, J.A.; Suen, T.-C.; Callif, B.L.; Mitchell, A.S.; Castanon-Cervantes, O.; Baker, K.M.; Kloehn, I.; Baba, K.; Teubner, B.J.W.; Ehlen, J.C.; et al. Shell neurons of the master circadian clock coordinate the phase of tissue clocks throughout the brain and body. BMC Biol. 2015, 13, 1–15.




