Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Matthias Widbiller | + 1630 word(s) | 1630 | 2022-03-10 04:17:03 | | | |
| 2 | Rita Xu | Meta information modification | 1630 | 2022-03-17 10:00:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Widbiller, M. Human Amnion Epithelial Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/20677 (accessed on 07 February 2026).
Widbiller M. Human Amnion Epithelial Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/20677. Accessed February 07, 2026.
Widbiller, Matthias. "Human Amnion Epithelial Cells" Encyclopedia, https://encyclopedia.pub/entry/20677 (accessed February 07, 2026).
Widbiller, M. (2022, March 17). Human Amnion Epithelial Cells. In Encyclopedia. https://encyclopedia.pub/entry/20677
Widbiller, Matthias. "Human Amnion Epithelial Cells." Encyclopedia. Web. 17 March, 2022.
Copy Citation
hAECs were isolated from human placentas, and dental pulp stem cells (hDPSCs) and dentin matrix proteins (eDMPs) were obtained from human teeth. Both hAECs and hDPSCs were cultured with 10% FBS, eDMPs and an osteogenic differentiation medium (StemPro). Viability was assessed by MTT and cell adherence to dentin was evaluated by scanning electron microscopy.
human amnion epithelial cells
dental pulp stem cells
dentin matrix proteins
1. Introduction
Regenerative endodontics refers to biologically based treatment procedures, e.g., revitalization, for immature necrotic teeth [1]. It aims at the restoration of the pulp’s physiology, including its immune, sensory and secretory functions, to improve the long-term prognosis of the tooth [2]. Over the last two decades, in vivo studies have shown satisfactory clinical outcomes with healing of periapical lesions [3] and resolution of clinical symptoms [4][5], as well as root thickening and lengthening [4] or apical closure [6]. However, the newly formed hard tissue does not resemble dentin but an ectopic tissue similar to cementum [7] or osteodentin [8], while the soft tissue lacks the pulp’s characteristic organization and cells with a distinct odontoblast phenotype [9]. The absence of odontoblasts after revitalization and the formation of a tissue other than dentin might compromise both the capability of the treated teeth to react to future injuries and also their biomechanical performance [10].
Novel and more elaborate approaches are being tested to overcome the lack of regeneration after the classical approach of revitalization and to achieve more predictable histological outcomes [11]. In this context, tissue engineering relies on the delivery of stem cells and/or recombinant growth factors in a scaffold into the root canal to facilitate pulp regeneration. These methods can be subcategorized into the cell homing approach, which utilizes signaling molecules to induce the migration, proliferation and differentiation of stem cells from the periapical tissues [12][13], and the cell transplantation approach [14]. The latter relies on the delivery of stem cells able to form new pulp tissue in the root canal [15].
To be considered as regenerated dental pulp, the newly formed tissue in the root canal must be vascularized as well as innervated, contain a similar cell density and microarchitecture to natural pulp and give rise to new odontoblast cells located at the dentin–pulp interface that are able to secrete tubular dentin in the course of tooth development but also at later time points [16]. In the context of pulp regeneration, the re-establishment of an odontoblast layer seems to be crucial due to its central role in tooth physiology and pathology [17][18][19]. Located at the dentin–pulp interface, these cells are the first line of defense against a bacterial invasion [20], they release antimicrobial agents [21] and have an immunomodulatory potential [22], allowing the tooth to immediately respond to stimuli, e.g., by secretion of dentin [17]. They also possess sensory functions by transducing pH changes and pressure as well as other pain-related stimuli [18]. Thus, it is of great interest for dental pulp tissue engineering to identify cell sources that are capable of differentiating into odontoblasts.
Recent in vivo studies have shown that pulp regeneration is possible after stem cell transplantation [23][24]. Histology revealed newly differentiated odontoblast-like mineralizing cells in contact with dentin. This approach is based on the transplantation of previously isolated and expanded autologous dental pulp stem cells (hDPSCs) and has proven successful in clinical trials [14][25]. In vitro studies also display odontogenic differentiation and the mineralization potential of hDPSCs when cultured with dentin matrix proteins (eDMPs) [26]. Dental pulp stem cells express dentin sialoprotein and differentiate into odontoblast-like cells with cellular processes extending into the dentinal tubules when seeded into EDTA-conditioned dentin cylinders and transplanted subcutaneously into immunocompromised mice [27]. However, the cell transplantation approach using hDPSCs and other tooth-derived cell types is challenging due to the necessity of cell expansion to obtain a sufficient number of cells, the need for a donor tooth and the limited differentiation potential of the multipotent stem cells compared to pluripotent stem cells [28].
A potential cell source to overcome those obstacles might be the amnion [29], the innermost layer of the human placenta. It contains amniotic epithelial cells (hAECs), which are formed by day 8 after fertilization and therefore maintain the plasticity of pre-gastrulation cells. Thus, hAECs are able to differentiate into cells of all three embryological layers [30], whereas multipotent stem cells, such as hDPSCs, are only capable of differentiating into cell types of one germ layer [31]. Human AECs showed the expression of human embryonic and pluripotent stem cell markers [30], such as stage-specific embryonic antigen-4 (SSEA4), octamer-binding transcription factor 4 (OCT4) and nanog homebox (NANOG) [32]. Moreover, hAECs reportedly have antimicrobial properties [33], immunomodulating potential [34] and can induce angiogenesis [35], which makes a useful cell type for regenerative therapies [29]. Up to 300 million hAECs can be obtained from one human placenta by a simple isolation protocol [29] which may be either expanded, directly applied or cryopreserved, e.g., in cell banks [29], which would ease the provision of these pluripotent stem cells. They are already used to treat several medical conditions, e.g., liver diseases and Parkinson’s disease [36][37]. Moreover, amnion epithelial cells are not tumorigenic [32][38] and do not elicit an immune response upon heterologous transplantation, since they express very low levels of leukocyte antigens [30][32].
2. Isolation and Characterization of hAECs
As shown by the histological analysis, the first digestion of the amnion only partially detached hAECs (Figure 1A,B); however, the second digestion released nearly all cells (Figure 1C). Interestingly, the flow cytometric analysis of hAECs in culture revealed both epithelial (CD49f, CD326) and mesenchymal (CD105, CD44) surface antigens (Figure 1D,E).
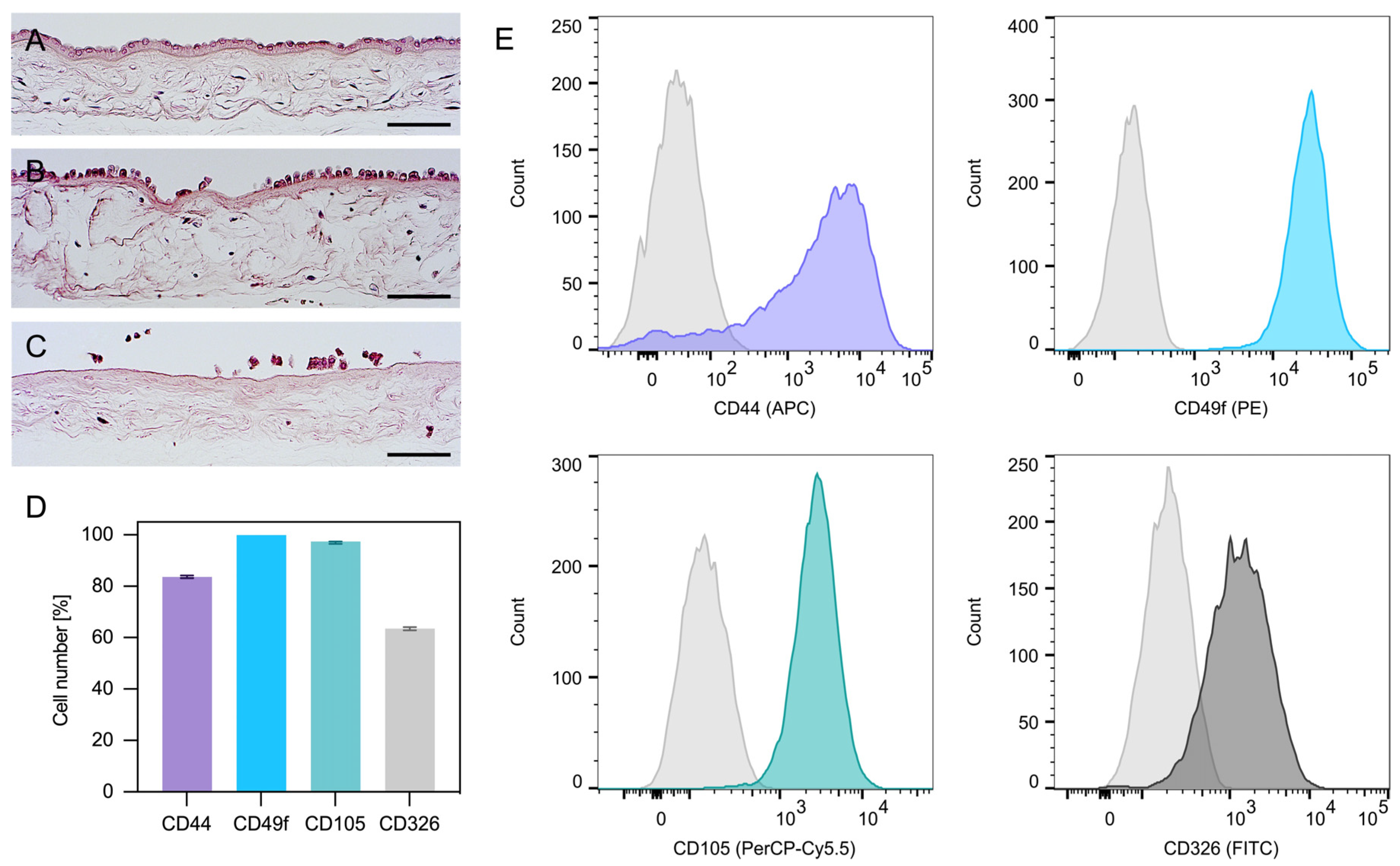
Figure 1. Amnion staining and expression profile of hAECs. Amnion before digestion (A) and after the first (B) and second digestion (C). The hAECs were attached to a collagen membrane forming a monolayer of columnar/cuboidal cells (hematoxylin and eosin; scale bars: 100 µm). Expression profile of hAECs determined by flow cytometry analysis (D). The hAECs in culture expressed both mesenchymal markers (CD44 and CD105) as well as epithelial markers (CD49f and CD326) (D,E).
3. Cell Viability
Human amnion epithelial cells showed a reduced viability compared to hDPSCs in all groups and at all time points (Figure 2A). Neither eDMPs nor StemPro had a significant impact on the viability of hAECs and hDPSCs at days 2 and 4; however, eDMP revealed a reduction at day 8 (Figure 2A).
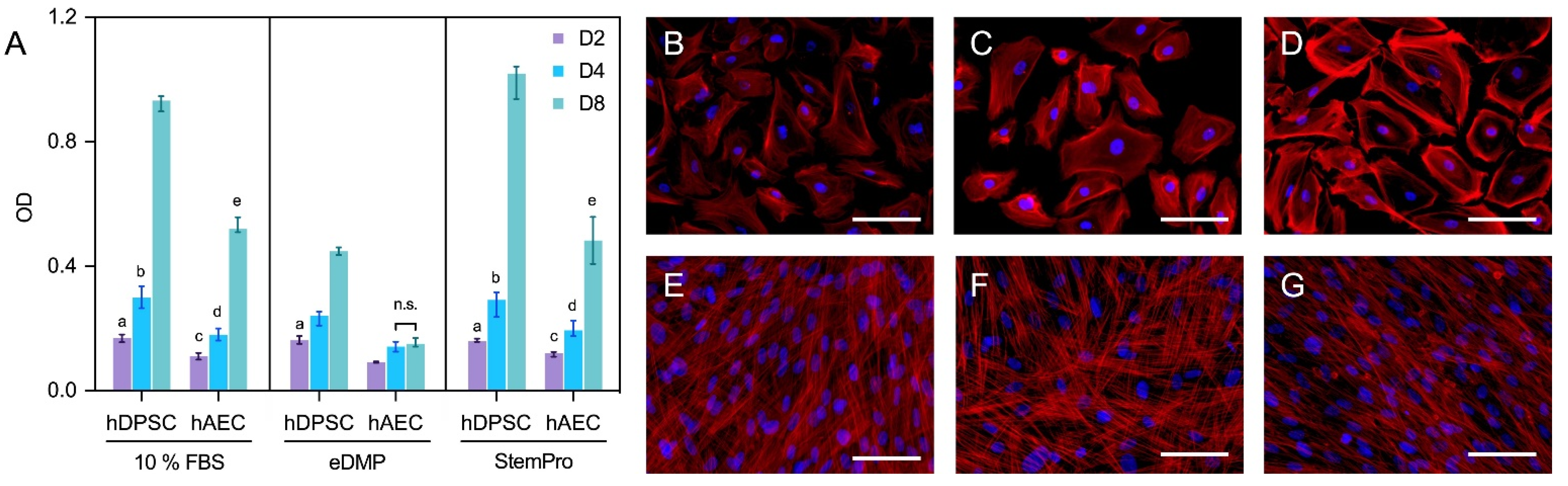
Figure 2. Viability and morphology of hAECs and hDPSCs. Cell viability of hAECs and hDPSCs cultured with eDMP and StemPro after 2, 4 and 8 days (A). Median values and 25–75% percentiles were calculated from three independent experiments performed in triplicate (n = 9). Fluorescence microscopy of hAECs and hDPSCs cultured with different media after 7 days and stained with DAPI and phalloidin (B–G). Cells were cultured in DMEM with 10% FBS (B,E), with eDMP (C,F) and with StemPro (D,G). hAECs exhibit a cobblestone-like morphology (B–D) while hDPSCs exhibit a mesenchymal stem cell phenotype (E–G). (Scale bars: 50 µm).
4. Fluorescence Microscopy
Morphologically, the primary culture of the hAECs appeared homogenous with cobblestone-like morphology (Figure 2B–D), whereas hDPSCs were spindle-shaped and considerably smaller (Figure 2E–G). Overall, no relevant medium-dependent changes in cellular morphology were displayed by either cell type.
5. Cell Adhesion to Dentin
Representative scanning electron microscopic images of hDPSCs and hAECs on dentin disks are shown in Figure 3. Scanning electron microscope images revealed that hDPSCs (Figure 3A,B) and hAECs (Figure 3C,D) were homogeneously distributed on the dentin. Moreover, both cell types showed adhesion to dentin and spread their processes over the surface in both EDTA-conditioned (Figure 3B,D) and unconditioned (Figure 3A,C) dentin. EDTA-conditioned dentin exhibited a clean dentin surface where dentin tubules were visible, while tubules were covered with a smear layer in unconditioned disks. Both hDPSCs and hAECs extended processes to form cellular contacts (Figure 3B,D). Whereas the hDPSCs adhered to dentin appeared spindle-shaped (Figure 3A,B), the hAECs retained their typical cubic morphology (Figure 3C,D).
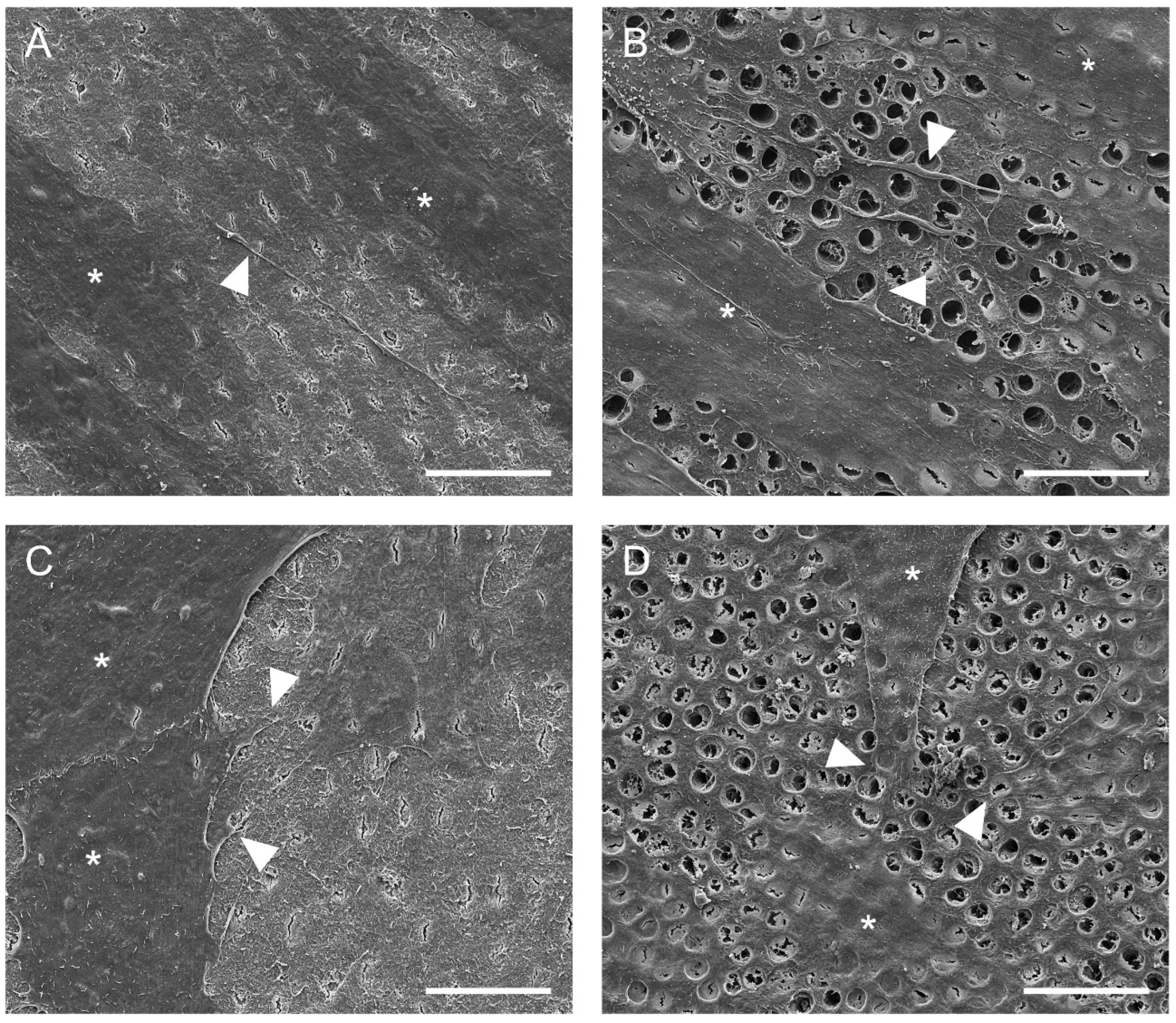
Figure 3. Adhesion of hDPSC and hAECs onto dentin surface. Representative SEM images of dentin surface with hDPSC (A,B) and hAECs (C,D) after 48 h (cells marked by asterisks). Cell adhesion and spreading on the surface of dentin was evident with (B,D) and without (A,C) EDTA conditioning. Some cytoplasmic processes (arrowheads) were evident in both cell types. (Scale bars: 20 µm).
6. Gene Expression
Genes associated with odontoblast differentiation and mineralization (collagen type I alpha 1 chain (COL1A1), bone morphogenetic protein 4 (BMP4), integrin binding sialoprotein (IBSP), nestin (NES) and bone gamma-carboxyglutamate protein or osteocalcin (BGLAP)) were either not expressed in hAECs or the expression was significantly downregulated in comparison to the hDPSCs (Figure 4A). However, genes associated with epithelial–mesenchymal transition were upregulated in hAECs compared to hDPSCs. Specifically, the insulin like growth factor binding protein 2 (IGFBP2) gene was significantly upregulated in hAECs cultured with StemPro or eDMPs at days 1 and 7, and S100 calcium binding protein A4 (S100A4) was considerably upregulated in hAECs at all time points (Figure 4A). Glutathione peroxidase 3 (GPX3), a gene associated with the reduction of hydrogen peroxide, which arises from oxidative stress, was significantly upregulated in hAECs in almost all groups and at all time points.
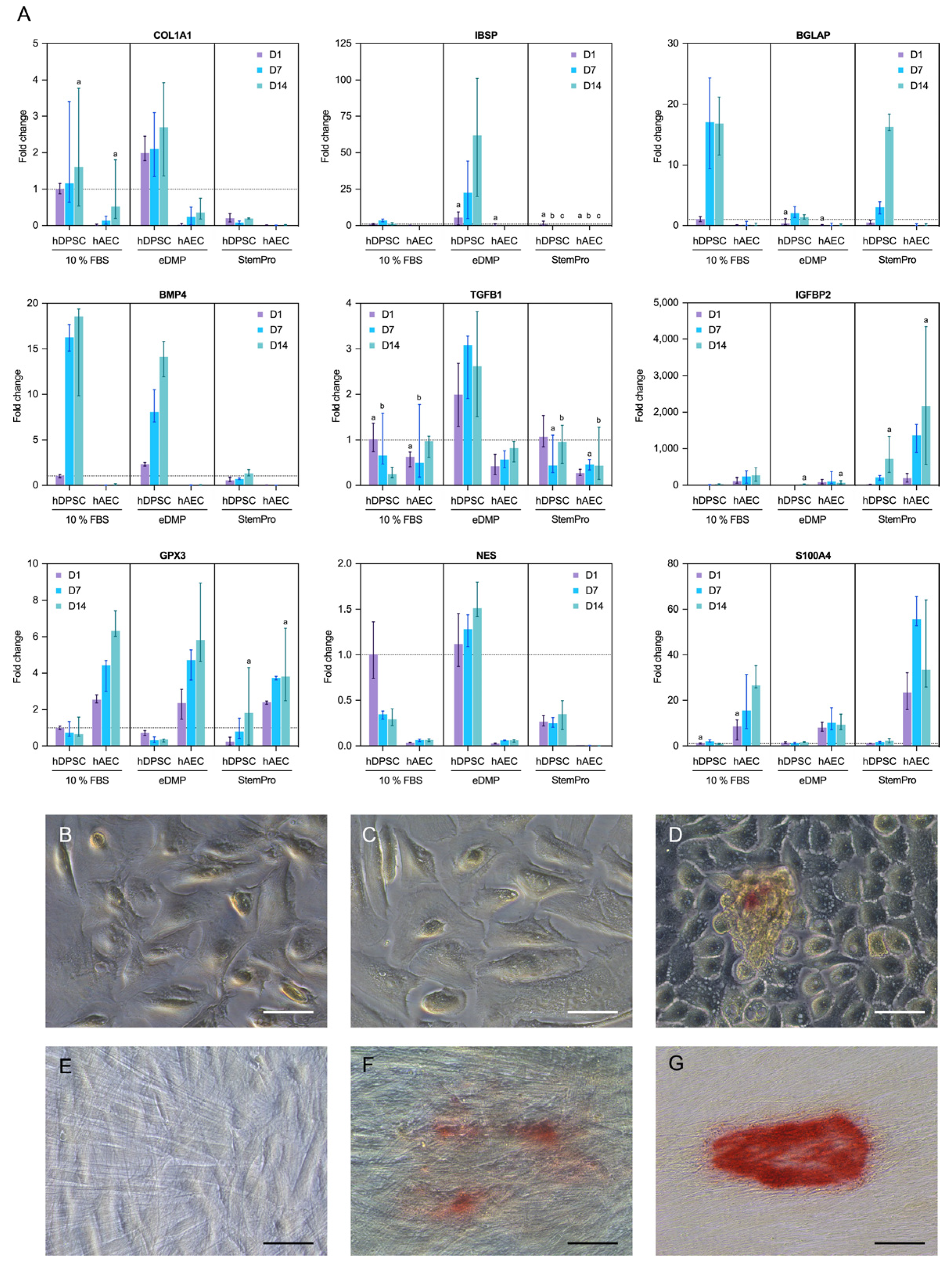
Figure 4. Expression of odontogenic and mineralization-associated genes. Effect of eDMPs and StemPro on expression of odontogenic and mineralization-associated marker genes (COL1A1, BMP4, IBSP, IGFBP-2, NES, TGFB1 and BGLAP) in hAECs and hDPSCs at days 1, 7 and 14 (A). Genes indicative of epithelial–mesenchymal transition (S100A4) and protection against oxidative damage (GPX3) are also depicted (A). Target gene expressions are depicted relative to the untreated control (hDPSCs with 10% FBS at day 1) and median values were calculated from two independent experiments in duplicated samples (n = 4). Non-significant differences between hAECs and hDPSCs for each medium and follow-up point are marked with lowercase letters (a, b, c). The effect of eDMPs and StemPro on mineralization of hAECs (B–D) and hDPSCs (E–G) using Alizarin Red staining assay. Calcium deposits were evident in hAECs cultured with StemPro (D) and hDPCS cultured with eDMPs (F) and StemPro (G). (Scale bars: 50 µm).
References
- Murray, P.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390.
- Cvek, M. Prognosis of luxated non-vital maxillary incisors treated with calcium hydroxide and filled with gutta-percha. A retrospective clinical study. Dent. Traumatol. 1992, 8, 45–55.
- Tong, H.J.; Rajan, S.; Bhujel, N.; Kang, J.; Duggal, M.; Nazzal, H. Regenerative Endodontic Therapy in the Management of Nonvital Immature Permanent Teeth: A Systematic Review—Outcome Evaluation and Meta-analysis. J. Endod. 2017, 43, 1453–1464.
- Lin, J.; Zeng, Q.; Wei, X.; Zhao, W.; Cui, M.; Gu, J.; Lu, J.; Yang, M.; Ling, J. Regenerative Endodontics Versus Apexification in Immature Permanent Teeth with Apical Periodontitis: A Prospective Randomized Controlled Study. J. Endod. 2017, 43, 1821–1827.
- Jiang, X.; Liu, H.; Peng, C. Clinical and Radiographic Assessment of the Efficacy of a Collagen Membrane in Regenerative Endodontics: A Randomized, Controlled Clinical Trial. J. Endod. 2017, 43, 1465–1471.
- Nazzal, H.; Kenny, K.; Altimimi, A.; Kang, J.; Duggal, M.S. A prospective clinical study of regenerative endodontic treatment of traumatized immature teeth with necrotic pulps using bi-antibiotic paste. Int. Endod. J. 2018, 51, e204–e215.
- Zhu, W.; Zhu, X.; Huang, G.T.-J.; Cheung, G.S.P.; Dissanayaka, W.; Zhang, C. Regeneration of dental pulp tissue in immature teeth with apical periodontitis using platelet-rich plasma and dental pulp cells. Int. Endod. J. 2013, 46, 962–970.
- Meschi, N.; Hilkens, P.; Lambrichts, I.; Van den Eynde, K.; Mavridou, A.; Strijbos, O.; De Ketelaere, M.; Van Gorp, G.; Lambrechts, P. Regenerative endodontic procedure of an infected immature permanent human tooth: An immunohistological study. Clin. Oral Investig. 2015, 20, 807–814.
- Wang, X.; Thibodeau, B.; Trope, M.; Lin, L.M.; Huang, G.T.-J. Histologic Characterization of Regenerated Tissues in Canal Space after the Revitalization/Revascularization Procedure of Immature Dog Teeth with Apical Periodontitis. J. Endod. 2010, 36, 56–63.
- Bucchi, C.; Marcé-Nogué, J.; Galler, K.M.; Widbiller, M. Biomechanical performance of an immature maxillary central incisor after revitalization: A finite element analysis. Int. Endod. J. 2019, 52, 1508–1518.
- Orti, V.; Collart-Dutilleul, P.-Y.; Piglionico, S.; Pall, O.; Cuisinier, F.; Panayotov, I. Pulp Regeneration Concepts for Nonvital Teeth: From Tissue Engineering to Clinical Approaches. Tissue Eng. Part B Rev. 2018, 24, 419–442.
- Galler, K.M.; Widbiller, M. Perspectives for Cell-homing Approaches to Engineer Dental Pulp. J. Endod. 2017, 43, S40–S45.
- He, L.; Zhong, J.; Gong, Q.; Cheng, B.; Kim, S.G.; Ling, J.; Mao, J.J. Regenerative Endodontics by Cell Homing. Dent. Clin. 2017, 61, 143–159.
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61.
- Ducret, M.; Fabre, H.; Celle, A.; Mallein-Gerin, F.; Perrier-Groult, E.; Alliot-Licht, B.; Farges, J.-C. Current challenges in human tooth revitalization. Bio.-Med. Mater. Eng. 2017, 28, S159–S168.
- Huang, G.T.-J. Dental pulp and dentin tissue engineering and regeneration advancement and challenge. Front. Biosci. 2011, 3, 788–800.
- Couve, E.; Osorio, R.; Schmachtenberg, O. Reactionary Dentinogenesis and Neuroimmune Response in Dental Caries. J. Dent. Res. 2014, 93, 788–793.
- Couve, E.; Osorio, R.; Schmachtenberg, O. The Amazing Odontoblast. J. Dent. Res. 2013, 92, 765–772.
- Farges, J.-C.; Keller, J.-F.; Carrouel, F.; Durand, S.H.; Romeas, A.; Bleicher, F.; Lebecque, S.; Staquet, M.-J. Odontoblasts in the dental pulp immune response. J. Exp. Zoöl. Part B Mol. Dev. Evol. 2009, 312, 425–436.
- Farges, J.-C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediat. Inflamm. 2015, 2015, 230251.
- Farges, J.-C.; Bellanger, A.; Ducret, M.; Aubert-Foucher, E.; Richard, B.; Alliot-Licht, B.; Bleicher, F.; Carrouel, F. Human odontoblast-like cells produce nitric oxide with antibacterial activity upon TLR2 activation. Front. Physiol. 2015, 6, 185.
- Farges, J.-C.; Carrouel, F.; Keller, J.-F.; Baudouin, C.; Msika, P.; Bleicher, F.; Staquet, M.-J. Cytokine production by human odontoblast-like cells upon Toll-like receptor-2 engagement. Immunobiology 2011, 216, 513–517.
- Itoh, Y.; Sasaki, J.I.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp Regeneration by 3-dimensional Dental Pulp Stem Cell Constructs. J. Dent. Res. 2018, 97, 1137–1143.
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete Pulp Regeneration after Pulpectomy by Transplantation of CD105+ Stem Cells with Stromal Cell-Derived Factor-1. Tissue Eng. Part A 2011, 17, 1911–1920.
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227.
- Widbiller, M.; Eidt, A.; Lindner, S.R.; Hiller, K.-A.; Schweikl, H.; Buchalla, W.; Galler, K.M. Dentine matrix proteins: Isolation and effects on human pulp cells. Int. Endod. J. 2018, 51, e278–e290.
- Galler, K.M.; D’Souza, R.; Federlin, M.; Cavender, A.C.; Hartgerink, J.; Hecker, S.; Schmalz, G. Dentin Conditioning Codetermines Cell Fate in Regenerative Endodontics. J. Endod. 2011, 37, 1536–1541.
- Miki, T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am. J. Reprod. Immunol. 2018, 80, e13003.
- Gramignoli, R.; Srinivasan, R.C.; Kannisto, K.; Strom, S.C. Isolation of Human Amnion Epithelial Cells According to Current Good Manufacturing Procedures. Curr. Protoc. Stem Cell Biol. 2016, 37, 1–13.
- Ilancheran, S.; Michalska, A.; Peh, G.; Wallace, E.M.; Pera, M.; Manuelpillai, U. Stem Cells Derived from Human Fetal Membranes Display Multilineage Differentiation Potential. Biol. Reprod. 2007, 77, 577–588.
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630.
- Yang, P.-J.; Yuan, W.-X.; Liu, J.; Li, J.-Y.; Tan, B.; Qiu, C.; Zhu, X.-L.; Qiu, C.; Lai, D.-M.; Guo, L.-H.; et al. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol. Sin. 2018, 39, 1305–1316.
- Nemr, W.; Bashandy, M.; Araby, E.; Khamiss, O. Molecular displaying of differential immunoresponse to various infections of amniotic epithelia. Am. J. Reprod. Immunol. 2017, 77, e12662.
- Motedayyen, H.; Fathi, F.; Fasihi-Ramandi, M.; Taheri, R.A. The effect of lipopolysaccharide on anti-inflammatory and pro-inflammatory cytokines production of human amniotic epithelial cells. Reprod. Biol. 2018, 18, 404–409.
- Zhu, D.; Muljadi, R.; Chan, S.T.; Vosdoganes, P.; Lo, C.; Mockler, J.C.; Wallace, E.; Lim, R. Evaluating the Impact of Human Amnion Epithelial Cells on Angiogenesis. Stem Cells Int. 2015, 2016, 4565612.
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem Cell Characteristics of Amniotic Epithelial Cells. Stem Cells 2005, 23, 1549–1559.
- Lim, R.; Hodge, A.; Moore, G.; Wallace, E.M.; Sievert, W. A Pilot Study Evaluating the Safety of Intravenously Administered Human Amnion Epithelial Cells for the Treatment of Hepatic Fibrosis. Front. Pharmacol. 2017, 8, 549.
- Miki, T.; Strom, S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. Rep. 2006, 2, 133–141.
More
Information
Subjects:
Cell & Tissue Engineering
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
687
Revisions:
2 times
(View History)
Update Date:
17 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




