Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell & Tissue Engineering
hAECs were isolated from human placentas, and dental pulp stem cells (hDPSCs) and dentin matrix proteins (eDMPs) were obtained from human teeth. Both hAECs and hDPSCs were cultured with 10% FBS, eDMPs and an osteogenic differentiation medium (StemPro). Viability was assessed by MTT and cell adherence to dentin was evaluated by scanning electron microscopy.
- human amnion epithelial cells
- dental pulp stem cells
- dentin matrix proteins
1. Introduction
Regenerative endodontics refers to biologically based treatment procedures, e.g., revitalization, for immature necrotic teeth [1]. It aims at the restoration of the pulp’s physiology, including its immune, sensory and secretory functions, to improve the long-term prognosis of the tooth [2]. Over the last two decades, in vivo studies have shown satisfactory clinical outcomes with healing of periapical lesions [3] and resolution of clinical symptoms [4,5], as well as root thickening and lengthening [4] or apical closure [6]. However, the newly formed hard tissue does not resemble dentin but an ectopic tissue similar to cementum [7] or osteodentin [8], while the soft tissue lacks the pulp’s characteristic organization and cells with a distinct odontoblast phenotype [9]. The absence of odontoblasts after revitalization and the formation of a tissue other than dentin might compromise both the capability of the treated teeth to react to future injuries and also their biomechanical performance [10].
Novel and more elaborate approaches are being tested to overcome the lack of regeneration after the classical approach of revitalization and to achieve more predictable histological outcomes [11]. In this context, tissue engineering relies on the delivery of stem cells and/or recombinant growth factors in a scaffold into the root canal to facilitate pulp regeneration. These methods can be subcategorized into the cell homing approach, which utilizes signaling molecules to induce the migration, proliferation and differentiation of stem cells from the periapical tissues [12,13], and the cell transplantation approach [14]. The latter relies on the delivery of stem cells able to form new pulp tissue in the root canal [15].
To be considered as regenerated dental pulp, the newly formed tissue in the root canal must be vascularized as well as innervated, contain a similar cell density and microarchitecture to natural pulp and give rise to new odontoblast cells located at the dentin–pulp interface that are able to secrete tubular dentin in the course of tooth development but also at later time points [16]. In the context of pulp regeneration, the re-establishment of an odontoblast layer seems to be crucial due to its central role in tooth physiology and pathology [17,18,19]. Located at the dentin–pulp interface, these cells are the first line of defense against a bacterial invasion [20], they release antimicrobial agents [21] and have an immunomodulatory potential [22], allowing the tooth to immediately respond to stimuli, e.g., by secretion of dentin [17]. They also possess sensory functions by transducing pH changes and pressure as well as other pain-related stimuli [18]. Thus, it is of great interest for dental pulp tissue engineering to identify cell sources that are capable of differentiating into odontoblasts.
Recent in vivo studies have shown that pulp regeneration is possible after stem cell transplantation [23,24]. Histology revealed newly differentiated odontoblast-like mineralizing cells in contact with dentin. This approach is based on the transplantation of previously isolated and expanded autologous dental pulp stem cells (hDPSCs) and has proven successful in clinical trials [14,25]. In vitro studies also display odontogenic differentiation and the mineralization potential of hDPSCs when cultured with dentin matrix proteins (eDMPs) [26]. Dental pulp stem cells express dentin sialoprotein and differentiate into odontoblast-like cells with cellular processes extending into the dentinal tubules when seeded into EDTA-conditioned dentin cylinders and transplanted subcutaneously into immunocompromised mice [27]. However, the cell transplantation approach using hDPSCs and other tooth-derived cell types is challenging due to the necessity of cell expansion to obtain a sufficient number of cells, the need for a donor tooth and the limited differentiation potential of the multipotent stem cells compared to pluripotent stem cells [28].
A potential cell source to overcome those obstacles might be the amnion [29], the innermost layer of the human placenta. It contains amniotic epithelial cells (hAECs), which are formed by day 8 after fertilization and therefore maintain the plasticity of pre-gastrulation cells. Thus, hAECs are able to differentiate into cells of all three embryological layers [30], whereas multipotent stem cells, such as hDPSCs, are only capable of differentiating into cell types of one germ layer [31]. Human AECs showed the expression of human embryonic and pluripotent stem cell markers [30], such as stage-specific embryonic antigen-4 (SSEA4), octamer-binding transcription factor 4 (OCT4) and nanog homebox (NANOG) [32]. Moreover, hAECs reportedly have antimicrobial properties [33], immunomodulating potential [34] and can induce angiogenesis [35], which makes a useful cell type for regenerative therapies [29]. Up to 300 million hAECs can be obtained from one human placenta by a simple isolation protocol [29] which may be either expanded, directly applied or cryopreserved, e.g., in cell banks [29], which would ease the provision of these pluripotent stem cells. They are already used to treat several medical conditions, e.g., liver diseases and Parkinson’s disease [36,37]. Moreover, amnion epithelial cells are not tumorigenic [32,38] and do not elicit an immune response upon heterologous transplantation, since they express very low levels of leukocyte antigens [30,32].
2. Isolation and Characterization of hAECs
As shown by the histological analysis, the first digestion of the amnion only partially detached hAECs (Figure 1A,B); however, the second digestion released nearly all cells (Figure 1C). Interestingly, the flow cytometric analysis of hAECs in culture revealed both epithelial (CD49f, CD326) and mesenchymal (CD105, CD44) surface antigens (Figure 1D,E).
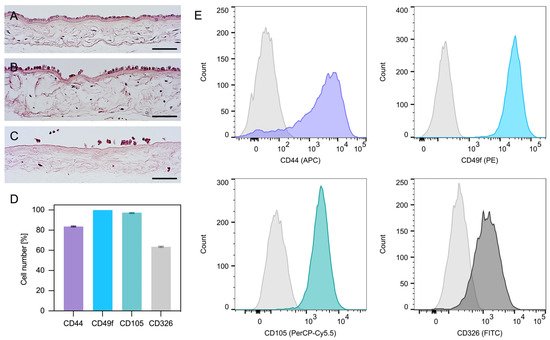
Figure 1. Amnion staining and expression profile of hAECs. Amnion before digestion (A) and after the first (B) and second digestion (C). The hAECs were attached to a collagen membrane forming a monolayer of columnar/cuboidal cells (hematoxylin and eosin; scale bars: 100 µm). Expression profile of hAECs determined by flow cytometry analysis (D). The hAECs in culture expressed both mesenchymal markers (CD44 and CD105) as well as epithelial markers (CD49f and CD326) (D,E).
3. Cell Viability
Human amnion epithelial cells showed a reduced viability compared to hDPSCs in all groups and at all time points (Figure 2A). Neither eDMPs nor StemPro had a significant impact on the viability of hAECs and hDPSCs at days 2 and 4; however, eDMP revealed a reduction at day 8 (Figure 2A).
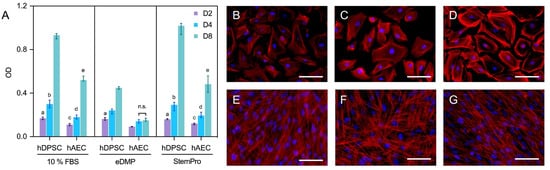
Figure 2. Viability and morphology of hAECs and hDPSCs. Cell viability of hAECs and hDPSCs cultured with eDMP and StemPro after 2, 4 and 8 days (A). Median values and 25–75% percentiles were calculated from three independent experiments performed in triplicate (n = 9). Fluorescence microscopy of hAECs and hDPSCs cultured with different media after 7 days and stained with DAPI and phalloidin (B–G). Cells were cultured in DMEM with 10% FBS (B,E), with eDMP (C,F) and with StemPro (D,G). hAECs exhibit a cobblestone-like morphology (B–D) while hDPSCs exhibit a mesenchymal stem cell phenotype (E–G). (Scale bars: 50 µm).
4. Fluorescence Microscopy
Morphologically, the primary culture of the hAECs appeared homogenous with cobblestone-like morphology (Figure 2B–D), whereas hDPSCs were spindle-shaped and considerably smaller (Figure 2E–G). Overall, no relevant medium-dependent changes in cellular morphology were displayed by either cell type.
5. Cell Adhesion to Dentin
Representative scanning electron microscopic images of hDPSCs and hAECs on dentin disks are shown in Figure 3. Scanning electron microscope images revealed that hDPSCs (Figure 3A,B) and hAECs (Figure 3C,D) were homogeneously distributed on the dentin. Moreover, both cell types showed adhesion to dentin and spread their processes over the surface in both EDTA-conditioned (Figure 3B,D) and unconditioned (Figure 3A,C) dentin. EDTA-conditioned dentin exhibited a clean dentin surface where dentin tubules were visible, while tubules were covered with a smear layer in unconditioned disks. Both hDPSCs and hAECs extended processes to form cellular contacts (Figure 3B,D). Whereas the hDPSCs adhered to dentin appeared spindle-shaped (Figure 3A,B), the hAECs retained their typical cubic morphology (Figure 3C,D).
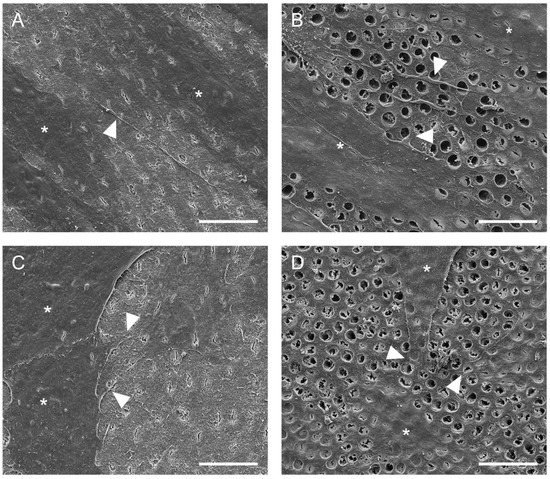
Figure 3. Adhesion of hDPSC and hAECs onto dentin surface. Representative SEM images of dentin surface with hDPSC (A,B) and hAECs (C,D) after 48 h (cells marked by asterisks). Cell adhesion and spreading on the surface of dentin was evident with (B,D) and without (A,C) EDTA conditioning. Some cytoplasmic processes (arrowheads) were evident in both cell types. (Scale bars: 20 µm).
6. Gene Expression
Genes associated with odontoblast differentiation and mineralization (collagen type I alpha 1 chain (COL1A1), bone morphogenetic protein 4 (BMP4), integrin binding sialoprotein (IBSP), nestin (NES) and bone gamma-carboxyglutamate protein or osteocalcin (BGLAP)) were either not expressed in hAECs or the expression was significantly downregulated in comparison to the hDPSCs (Figure 4A). However, genes associated with epithelial–mesenchymal transition were upregulated in hAECs compared to hDPSCs. Specifically, the insulin like growth factor binding protein 2 (IGFBP2) gene was significantly upregulated in hAECs cultured with StemPro or eDMPs at days 1 and 7, and S100 calcium binding protein A4 (S100A4) was considerably upregulated in hAECs at all time points (Figure 4A). Glutathione peroxidase 3 (GPX3), a gene associated with the reduction of hydrogen peroxide, which arises from oxidative stress, was significantly upregulated in hAECs in almost all groups and at all time points.
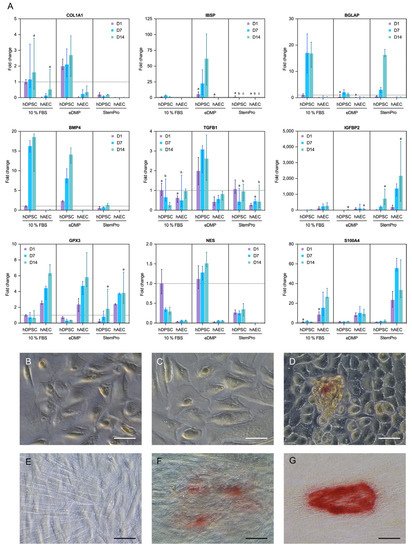
Figure 4. Expression of odontogenic and mineralization-associated genes. Effect of eDMPs and StemPro on expression of odontogenic and mineralization-associated marker genes (COL1A1, BMP4, IBSP, IGFBP-2, NES, TGFB1 and BGLAP) in hAECs and hDPSCs at days 1, 7 and 14 (A). Genes indicative of epithelial–mesenchymal transition (S100A4) and protection against oxidative damage (GPX3) are also depicted (A). Target gene expressions are depicted relative to the untreated control (hDPSCs with 10% FBS at day 1) and median values were calculated from two independent experiments in duplicated samples (n = 4). Non-significant differences between hAECs and hDPSCs for each medium and follow-up point are marked with lowercase letters (a, b, c). The effect of eDMPs and StemPro on mineralization of hAECs (B–D) and hDPSCs (E–G) using Alizarin Red staining assay. Calcium deposits were evident in hAECs cultured with StemPro (D) and hDPCS cultured with eDMPs (F) and StemPro (G). (Scale bars: 50 µm).
This entry is adapted from the peer-reviewed paper 10.3390/ijms23052830
This entry is offline, you can click here to edit this entry!
