| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Benedetta Ciuffi | + 1220 word(s) | 1220 | 2020-09-15 08:37:02 | | | |
| 2 | Dean Liu | Meta information modification | 1220 | 2020-09-18 07:39:04 | | |
Video Upload Options
Gasification with supercritical water (SCWG) is a thermochemical process which, exploiting the properties of supercritical water (374.1 °C and 22.1 MPa ), allows to obtain a syngas rich in hydrogen. Both biomass and waste plastic can be used as feedstock.
1. Supercritical Water Gasification: main notions
1.1. Properties of supercritical water
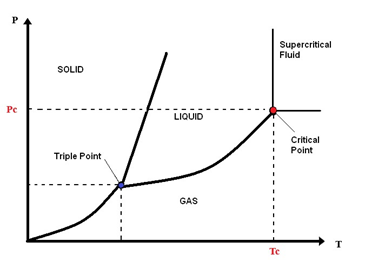
Supercritical water is water above its critical temperature, 374.1 °C, and critical pressure, 22.1 MPa[1]. Subcritical water (SbCW) occurs at temperatures below or near the critical temperature and at a pressure above the saturation pressure [2]. These concepts are clarified in Figure 1.
Figure 1. Schematic diagram phase of water.
The supercritical water (SCW) is a single phase that possesses the characteristics of both gas and liquid without surface tension and liquid/gas phase boundary. Supercritical water has sufficient density to give appreciable dissolving power, diffusivity higher than that in liquids, and lower viscosity to enhance mass transport [3]. The dielectric constant of water changes near the critical point, for example it decreases from 78.5 F·m−1 at 25 °C and 0.1 MPa, to 5.9 F·m−1 at 400 °C and 25 MPa [4]. Therefore, water is a highly polar solvent at room temperature and a non-polar solvent in supercritical conditions. SCW is, therefore, an excellent solvent for non-polar organic compounds [4][5] such as lignin. Thanks to these properties SCW provides a homogeneous and rapid reaction environment for gasification.
1.2. Biomass SCWG Process
Gasification with supercritical water, as well as other types of gasification, has been studied extensively for biomass These studies allowed laying the foundations for the understanding of the SCWG of plastic material.
Supercritical water gasification of biomass is typically performed at temperatures between 600 and 650 °C and at a pressure of about 30 MPa. Above 600 °C, water acts as a strong oxidant. Carbon atoms are oxidized and CO2 is preferably formed. Moreover, the use of water results in a high hydrogen yield, in fact hydrogen atoms not only from biomass but also from water are released to form hydrogen [6]. We can write an overall chemical reaction that describes the biomass SCWG process [4]:
|
CHxOy + (2y)H2O → CO2 + (2-y +x/2) H2 |
(1) |
where x and y are the elemental molar ratios of H/C and O/C of the biomass, respectively. The product of the reaction is the synthesis gas, whose quality depends on x and y.
During the process, however, three other competitive reactions can occur:
1. Steam reformation
|
CHxOy + (1-y)H2O → CO + (1-y + x/2) H2 |
(2) |
2. Methanation for CO
|
CO + 3H2 ↔ CH4 + H2O |
(3) |
3. Methanation for CO2
|
CO2 + 3H2 ↔ CH4 + H2O |
(4) |
4. Water–gas shift reaction
|
CO + H2O ↔ CO2 + H2 |
(5) |
As described by Okolie et. al. [7], the effects of pressure on the SCWG process are still not very clear. With an increase in pressure, the methanation reactions are shifted to the right (towards a smaller number of molecules) with an increase in the formation of methane at the expense of CO, CO2, and H2. Moreover, the increase in pressure causes a change in the density of the water. In particular, ionic reactions are favored at high pressure due to the ion stabilization effect given by the high density water. On the contrary, the radical reactions are disadvantaged.
A typical SCWG plant is described in detail by Basu P. et al. [4]. Below, we summarize the main features of the plant:
-
Water and feedstock must be pressurized to the supercritical pressure required for the process. The biomass is then ground and mixed with water using an emulsifying agent, in order to obtain a pumpable liquid.
-
The resulting slurry is pumped into the reactor. The pressurized water is preheated by passing it through a heat exchanger, which exploits the heat of the product leaving the reactor. The pressurized water also passes through an externally heated preheater before entering the reactor.
-
The syngas obtained in the process is cooled and passed through a gas-liquid separator. The gas mixture passes through further purification equipment such as the scrubber or pressure-swing adsorption unit.
1.3. Advantages and Disadvantages in The Use of SCW
Supercritical water gasification has numerous advantages over conventional gasification:
-
Supercritical water is an active reactant which results in a high hydrogen yield [6].
-
In the SCWG, the drying step of feedstock is not required. This leads to greater energy efficiency especially for biomass with a high moisture content. Therefore, there is a considerable economic saving, since drying and pre-treatment of biomass add extra cost to the process economics [8].
-
High pressure of the gaseous product enables the transportation, usage, carbon capture, and further purification of the product gas through steam reforming or PSA (pressure swing adsorption).
-
The reaction temperature is much lower than that used in conventional gasification and pyrolysis processes.
-
Tar and coke formation is inhibited by a fast solution of the formed gas components in the supercritical water [6].
-
The gaseous product is very clean, as NOx and SOx are generated in very small quantities and the CO concentration is very low, especially in the presence of a catalyst to improve the water-gas shift reaction [9].
At the same time, this technique has some disadvantages that must be taken into consideration. First of all, the reactor must be made of materials resistant not only to corrosion but also to high temperatures and pressures. Furthermore, there is a considerable energy expenditure to heat up the water to the reaction temperature. This entails high investment costs.
1.4. SCWG of Plastic
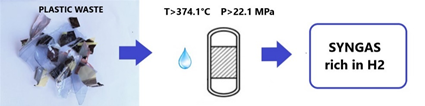
As said previously, in supercritical conditions, water acts as a non-polar solvent which can solve the problems of poor heat transfer and high viscosity of plastic material by dissolving the plastic fragments [10]. Moreover, water also acts as a hydrogen donor which helps plastic cracking and gasification. For these reasons, this technology can be used for various types of plastic material, for example cross-linked polyethylene, plastic with flame retardants or contaminants. Figure 2 shows a graphic scheme of the SCWG of waste plastics.
Figure 2. Graphical scheme of the supercritical water gasification (SCWG) of waste plastics.
An interesting application is that related to the removal of microplastics from the marine environment. Other types of processes such as pyrolysis and gasification are not a suitable choice because, the high water content of plastics recovered from the ocean would require a drying step, which would significantly increase the cost of recycling [11]. At present, there are few studies on the characteristics of plastic supercritical water gasification and how plastics react with supercritical water. A very active research group on this technology is that of Bai Bin, at Xi’an Jiaotong University (China). This group between 2018 and 2020 had published five articles on this topic.
2. Final considerations
Gasification in supercritical water is a poorly studied technology, which has generated great interest in recent years thanks to its numerous advantages, for example, the inhibition of tar and coke formation and the fact that the gaseous product is very clean (no NOx and SOx are generated in SCWG) and the CO concentration is very low, especially with the catalyst to enhance the water-gas shift reaction. This technology can be used for microplastic removal as described by the recent works of Bai B., but also for the recycling of particular types of plastics (strongly cross-linked polymers). The high costs and complexity of the process made it difficult to implement gasification plants with supercritical water on a large scale. Therefore, it will be important to understand which operating parameters can be optimized for greater energy savings.
References
- Wagner, W.; Pruß, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. 2002, 31, 387–535, doi:10.1063/1.1461829.
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical water as reaction environment: fundamentals of hydrothermal biomass transformation. ChemSusChem. 2011, 23, 566-79
- Guo, L.; Jin, H.; Lu, Y. Supercritical water gasification research and development in China. J. Supercrit. Fluids 2015, 96, 144–150, doi:10.1016/j.supflu.2014.09.023.
- Basu, P.; Mettanant, V. Biomass gasification in supercritical water—A review. Int. J. Chem. React. Eng. 2009, 7, doi:10.2202/1542-6580.1919.
- Lachos-Perez, D.; Prado, J.M.; Mayanga, T.P.; Forster-Carneiro, T.; Angela, M.; Meireles, A. Supercritical water gasification of biomass for hydrogen production: Variable of the process. Food Public Heal. 2015, 6, 92–101, doi:10.5923/j.fph.20150503.05.
- Heidenreich, S.; Müller, M.; Foscolo, P.U. New and improved gasification concepts. In Advanced Biomass Gasification; Elsevier: Amsterdam, The Netherlands , 2016; pp. 98–114
- Okolie, J.A.; Ajay, S.N.; Dalai, K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109–546
- Okolie, J.A.; Rana, R.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass: A state-of-the-art review of process parameters, reaction mechanisms and catalysis. Sustain. Energy Fuels 2019, 3, 578–598, doi:10.1039/c8se00565f.
- Guo, L.; Cao, C.; Lu, Y. Supercritical water gasification of biomass and organic wastes. Biomass 2010, doi:10.5772/9774
- Li, K.; Xu, Z. A review of current progress of supercritical fluid technologies for e-waste treatment. J. Clean. Prod. 2019, 227, 794–809, doi:10.1016/j.jclepro.2019.04.104.
- Purkarová, E.; Ciahotný, K.; Šváb, M.; Skoblia, S.; Beňo, Z. Supercritical water gasification of wastes from the paper industry. J. Supercrit. Fluids 2018, 135, 130–136.