Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hamaseh Tayari | + 2699 word(s) | 2699 | 2022-03-09 08:57:31 | | | |
| 2 | Rita Xu | Meta information modification | 2699 | 2022-03-17 02:46:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tayari, H. Ultrasound-Guided Saphenous Nerve Block in Rabbits. Encyclopedia. Available online: https://encyclopedia.pub/entry/20655 (accessed on 07 February 2026).
Tayari H. Ultrasound-Guided Saphenous Nerve Block in Rabbits. Encyclopedia. Available at: https://encyclopedia.pub/entry/20655. Accessed February 07, 2026.
Tayari, Hamaseh. "Ultrasound-Guided Saphenous Nerve Block in Rabbits" Encyclopedia, https://encyclopedia.pub/entry/20655 (accessed February 07, 2026).
Tayari, H. (2022, March 16). Ultrasound-Guided Saphenous Nerve Block in Rabbits. In Encyclopedia. https://encyclopedia.pub/entry/20655
Tayari, Hamaseh. "Ultrasound-Guided Saphenous Nerve Block in Rabbits." Encyclopedia. Web. 16 March, 2022.
Copy Citation
Ultrasound-guided (US-guided) loco-regional anesthesia techniques allow direct visualization and blockade of sensory nerves. The saphenous nerve (SN), a terminal branch of the femoral nerve (FN), is strictly a sensory nerve for which electrical locator devices are ineffective for localization as no effector muscle contractions can be evoked. US-guided SN block in species other than rabbits produces hind-limb analgesia without affecting FN motor function.
loco-regional anesthesia
analgesia
rabbit
ultrasound-guided
saphenous nerve
sensory nerve
1. Introduction
Unlike dogs and cats, rabbits have been domesticated for only a relatively short time; however, as their popularity as companion animals increases, there is greater demand for the provision of anesthesia in this species [1]. Unfortunately, possibly related to their peculiar anatomical, physiological, and behavioral features, there is a higher risk of peri-anesthetic death in rabbits (between 1.39% [2] to 4.8% [1]), compared to cats (0.24%) and dogs (0.17%) [2]. In most cases, the primary etiology of peri-anesthetic death is unknown but is often ascribed to either cardiovascular or respiratory causes [1][2]. Additionally, a high incidence of non-fatal gastrointestinal complications is also common in rabbits (38%) [1]. No specific risk factor has been associated with the higher peri-operative mortality rate recorded in rabbits, and this is most likely multifactorial (e.g., dietary changes, pain, disease, and medication side effects) [3].
Prey animals such as rabbits are reluctant to show signs of discomfort, making them particularly challenging for early detection of pain as well as the evaluation of the efficacy of an analgesic treatment if this is based on behavioral changes [1][4][5][6][7]. Therefore, adequate peri-operative analgesia plays a vital role in the outcome [4][5][6]. While no single technique or drug regimen has been shown to eliminate peri-anesthetic morbidity and mortality in any species, a pre-emptive analgesia strategy and a multimodal approach to pain management should ensure optimal peri-operative analgesia in rabbits, reducing the risk of under-detection of pain and the occurrence of possible related complications in the peri-anesthetic period, such as ileus [1][4][5][8].
Loco-regional anesthesia techniques, such as peripheral nerve blocks (PNB) and interfascial blocks, are fundamental components of a multimodal approach to pain management and are effective in reducing the need for systemically-administered analgesic and anesthetic drugs, potentially limiting the latters’ side effects [9][10]. However, in addition to preventing the transmission of nociceptive signals to the cortex, many loco-regional techniques will also result in a degree of motor block that may affect the rabbit’s normal behavior pattern. Temporary absence of physiological prey behaviors may increase hospitalization-associated stress, reduce food intake, and potentially increase the risk of stress-related complications, such as ileus [1]. Furthermore, prolonged immobility during the post-operative period secondary to impaired motor function may limit efficient ventilation, as reported in humans [11]; this may be particularly significant in rabbits, where there is a high incidence of pre-existing pulmonary disease, even in apparently healthy individuals [1].
Hind-limb innervation in dogs derives from the sciatic nerve and femoral nerve (FN); the latter runs inside the iliopsoas muscle and receives contributions from the fourth, fifth, and sixth lumbar spinal cord segments (L4–L6) [12]. Before leaving the iliopsoas muscle, the FN gives origin to the saphenous nerve (SN) and is located cranially to the femoral artery (FA) in a different interfascial plane [9]. The SN runs distally, cranial to FA, and within the same fascia (medial femoral fascia), forming a so-called ‘neurovascular bundle’ [9][13][14]. The SN supplies branches to the stifle joint and ends in the skin over the first digit (when present) [14][15]. Overall, in dogs, the SN is responsible for the sensory innervation of the medial aspect of the distal thigh, stifle joint, tibia, tarsus, and metatarsus, and the cranial aspect of the stifle [13].
Similarly, hind-limb sensory and motor innervation in rabbits is provided by the FN and sciatic nerve, which originate from the lumbosacral plexus (L4–L7), and the first and second sacral cord segments (S1–S2) [16][17][18].
In dogs, the peripheral block of the SN has been shown to provide desensitization of the medial and cranial aspects of the distal limb (including the stifle) without affecting the motor function of the quadriceps femoris muscle [9][19]. Due to the neuro-anatomical similarities between dogs and rabbits [16][17][19][20], it is likely that this also applies to rabbits. Two previous studies reported successful blockade of FN (and sciatic nerve) using electrical stimulation in rabbits undergoing pelvic limb orthopedic procedures [17][18]. However, by blocking the FN (instead of SN), the motor function of the quadriceps, gastrocnemius, and cranial tibial muscles [16] will potentially be impaired during the post-operative period, affecting voluntary movements and possibly impacting negatively on the animal’s welfare.
Electrical locator devices are ineffective in localizing sensory nerves since motor fibers are lacking in these types of nerves and muscle contractions cannot be evoked [9][19]. To perform a SN block, as well as other sensory nerve blocks, ultrasound-guided (US-guided) techniques should be applied, as direct visualization of the targeted nerve and landmarks is possible. Besides these advantages, the US-guided techniques also allow real-time visualization of the local anesthetic spread around the nerves while reducing the risk of damage to other vital structures (e.g., arteries or veins) [9][19].
In dogs, the US-guided SN block technique has been designed as an interfascial block injection, as the local anesthetic is injected into the medial femoral fascia that contains the SN and both the FA and FN [9][19].
Recently, a case report describing the use of a combined US-guided and nerve stimulation-guided saphenous and sciatic nerves blocks in a pet rabbit undergoing calcaneal fracture repair demonstrated the effectiveness in peri-operative pain management with no impairment of motor function in the affected limb [20].
2. Gross Anatomical Investigation
In both hind-limbs, it was possible to observe the FN giving origin to the SN and to the FMB after leaving the psoas compartment at the proximal inguinal area. At this level, the FMB ran to the anterior aspect of the thigh, where it gave origin to other branches. The SN was identified within the medial femoral fascia as part of the neurovascular bundle, cranially to the FA and FV. The SN was localized below the sartorius muscle caudally to the rectus femoris and vastus medialis muscles and cranial to the pectineus and adductor muscles. In the middle compartment of the thigh, the pectineus muscle was identified as a triangle-shaped muscle located below the neuromuscular bundle. The distal insertion of the pectineus muscle was located at the end of the first proximal third of the femur on its posteromedial surface. The femur was located cranially to the neurovascular bundle, adductor, semimembranosus, and pectineus muscle, caudally to the FMB, rectus femoris, tensor fascia latae muscles, and laterally to the vastus medialis muscle. Distally, at the proximal aspect of the stifle joint, the SN ran deeper to innervate the articular capsule (Figure 1).
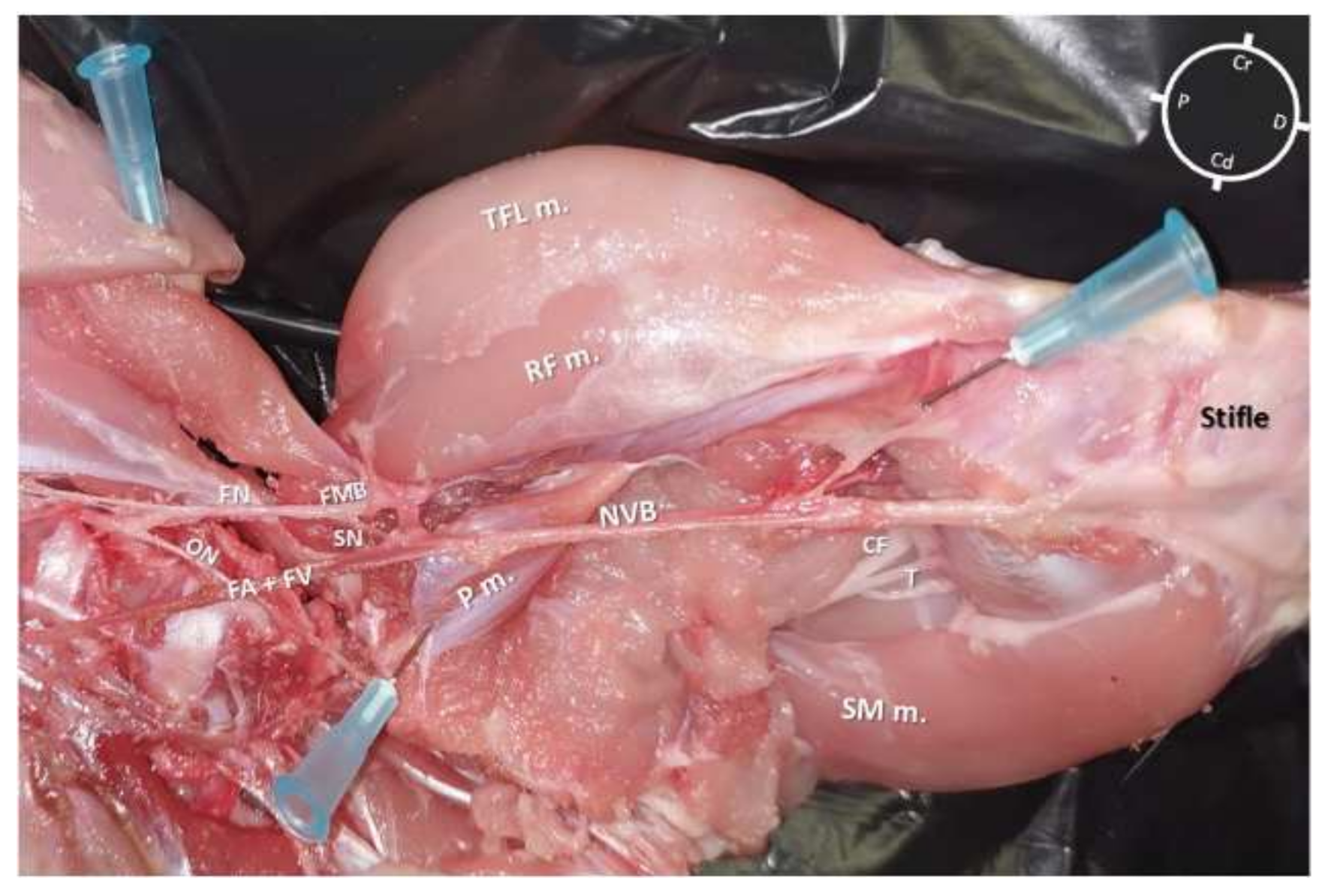
Figure 1. Skinned rabbit hind-limb positioned in dorsal recumbency with the hind-limb extended in a natural position after blunt dissection of the medial aspect of the left thigh and removal of the vastus medialis, sartorius, gracilis, and adductor muscles. The femoral nerve is observed leaving the psoas compartment and, at the level of the inguinal crease, giving origin to a motor branch that runs into the muscles located in the anterior aspect of the thigh and to the saphenous nerve, which joins into the same fascia of the femoral artery and vein forming the neurovascular bundle (NVB). This bundle runs medial and caudal to the femur, caudal to the vastus medialis, rectus femoris (RF m.), and tensor fascia latae (TFL m.) muscles, cranial to the adductor, semimembranosus (SM m.), and gracilis muscles and below the sartorius muscle. P: Proximal; D: Distal; Cr: Cranial; Cd: Caudal; FN: Femoral nerve; ON: Obturator nerve; SN: Saphenous nerve; FA + FV: Femoral artery + Femoral vein; FMB: Femoral motor branch; P m.: Pectineus muscle; CF: Common fibular nerve; T: Tibial nerve.
3. Sono-Anatomy Study and US-Guided Saphenous Nerve Block
Despite all cadavers being considered correctly thawed for the anatomical study (based on palpation), in one of the hind-limbs, a mild degree of ice crystallization was identified in the middle thigh muscles during the sonography. However, as this finding did not interfere with the identification process of the acoustic target window and the neurovascular bundle, this hind-limb was also included.
In all hind-limbs, it was possible to identify the targeted acoustic windows at the level of the middle thigh using the landmarks as described in the anatomical study. The landmark structures (muscle, bone, and neurovascular bundle) were all visualized after mild probe movements within 1.5 cm depth for all cadavers.
According to the anatomical findings, the US-guided SN block technique was designed, placing the ultrasound probe approximately at the central portion of the middle thigh transversally to the long axis of the hind-limb. At this level, the visualization of the neurovascular bundle was at the center of the acoustic window (Figure 2).
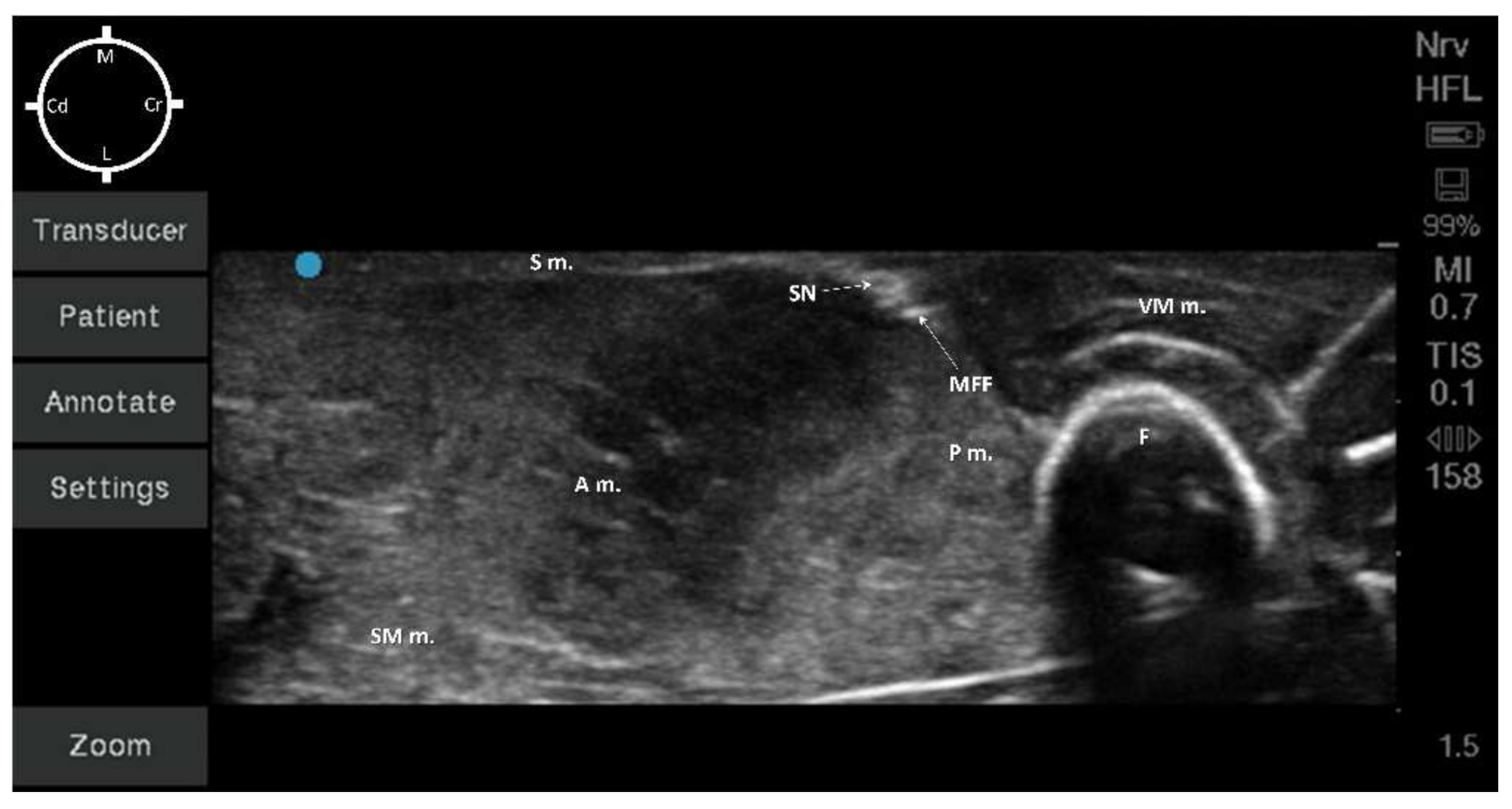
Figure 2. Sono-anatomy of the pelvic limb. The saphenous nerve (SN) can be visualized as a hyperechoic round structure, within the same fascial plane (medial femoral fascia) of other hyperechoic round structures (collapsed femoral artery and vein due to absence of blood flow). These structures were caudally to the vastus medialis muscle (VM m.), medially and caudally to the femur (F), cranially and medially to the adductor (A m.) and semimembranosus (SM m.) muscles, medially to the pectineus muscle (P m.) and immediately below the sartorius muscle (S m.). MFF: Medial Femoral Fascia; M: Medial; L: Lateral; Cr: Cranial; Cd: Caudal.
All the muscles (sartorius, adductor, semimembranosus, pectineus, and vastus medialis muscles) were displayed as structures with heterogeneous echogenicity. The femur was displayed as a hyperechoic structure with acoustic shadow. At the center of the targeted acoustic window, the terminal portion of the pectineus muscle was visualized as a triangular-shaped structure with heterogeneous echogenicity caudally to the femur. In addition, the neurovascular bundle was displayed as a piriform-shaped structure with a hyperechoic outer layer (medial femoral fascia) containing three hypoechoic round-shaped structures (SN, FA, and FV). It was not possible to confirm the position of the SN in relation to both the FA and FV due to the absence of blood flow (Figure 2). The neurovascular bundle was always located below the sartorius muscle and medially to the pectineus muscle within 0.5 cm depth for all cadavers. In addition, the vastus medialis muscle was located cranially, the adductor and the semimembranosus muscles were located caudally to the neurovascular bundle (Figure 2).
In all of the 10 hind-limbs, the US-guided SN block was feasible at the first attempt. The timing to successfully perform the block was around 5 min. During needling, the shaft of the needle was always visible, as was the tip when piercing the medial femoral fascia. The dye solution was injected in five hind-limbs with a low volume (0.05 mL/kg; resultant mean volume of 0.08 ± 0.007 mL) and in the other five with a high dose (0.1 mL/kg; resultant mean volume of 0.16 ± 0.011 mL). During injection, an anechoic area was formed within the medial femoral fascia, which improved the visualization of the SN for all cadavers (Figure 3a,b).
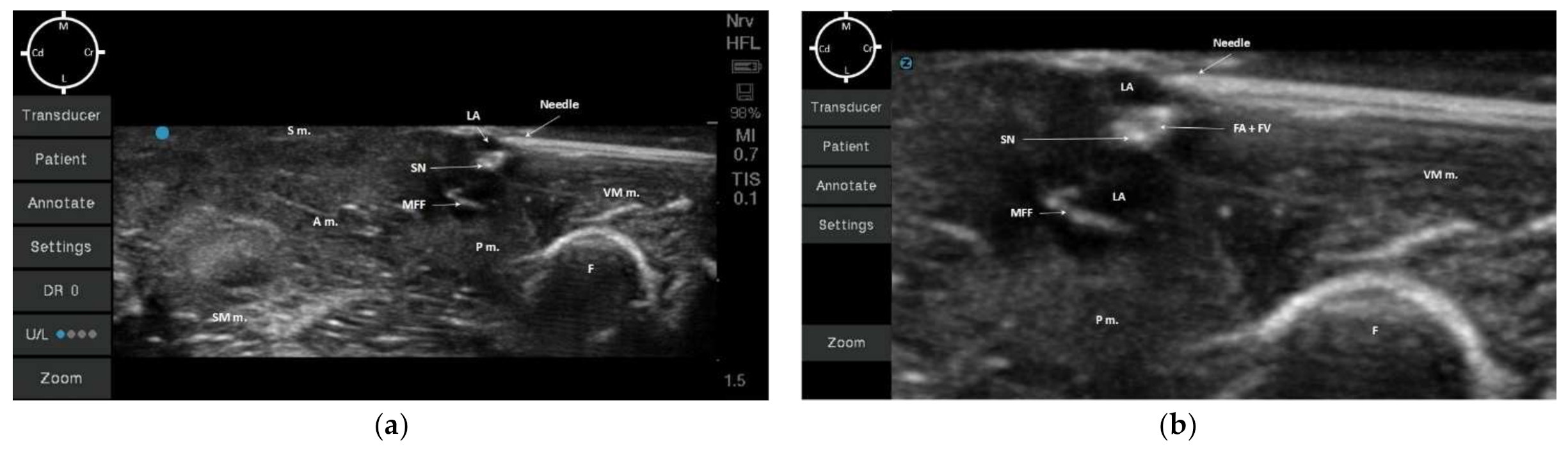
Figure 3. (a) Ultrasonographic image of the medial hind-limb in a rabbit cadaver at the level of medial femoral fascia (MFF). The saphenous nerve (SN) can be visualized as a hyperechoic round structure, next to other hyperechoic round structures (collapsed femoral artery and vein due to absence of blood flow), which are highlighted due to the presence of the anechoic dye solution (LA: permanent tissue dye solution and lidocaine 2% (1:50)); Once the tip of the needle pierced the medial femoral fascia, the dye solution was injected. (b) Highlighted ultrasound image of the injected solution within the fascia containing the saphenous nerve (using zoom option on the ultrasound device). S m.: Sartorius muscle; A m.: Adductor magnus muscle; SM m.: Semimembranosus muscle; P m.: Pectineus muscle; F: Femur; VM m.: Vastus medialis muscle; MFF: Medial Femoral Fascia; FA + FV: Femoral artery + Femoral vein; M: Medial; L: Lateral; Cr: Cranial; Cd: Caudal.
4. Anatomical Dissection following US-Guided Saphenous Nerve Block
In all cadavers, the dye solution was injected within the target area (medial femoral fascia) without involving the surrounding structures (Figure 4a and Figure 5a). It was possible to separate all the surrounding anatomical structures maintaining the neurovascular bundle integrity. The dye solution was visible within the medial femoral fascia containing the SN and both FA and FV in all of the 10 pelvic limbs (Figure 4b and Figure 5b). All SNs, regardless of the dye solution volume injected, were stained around their entire diameter and for more than 1 cm of length (Figure 4c,d and Figure 5c,d). In addition, none of the FNs nor FMB were stained with either high or low volumes of dye solution.
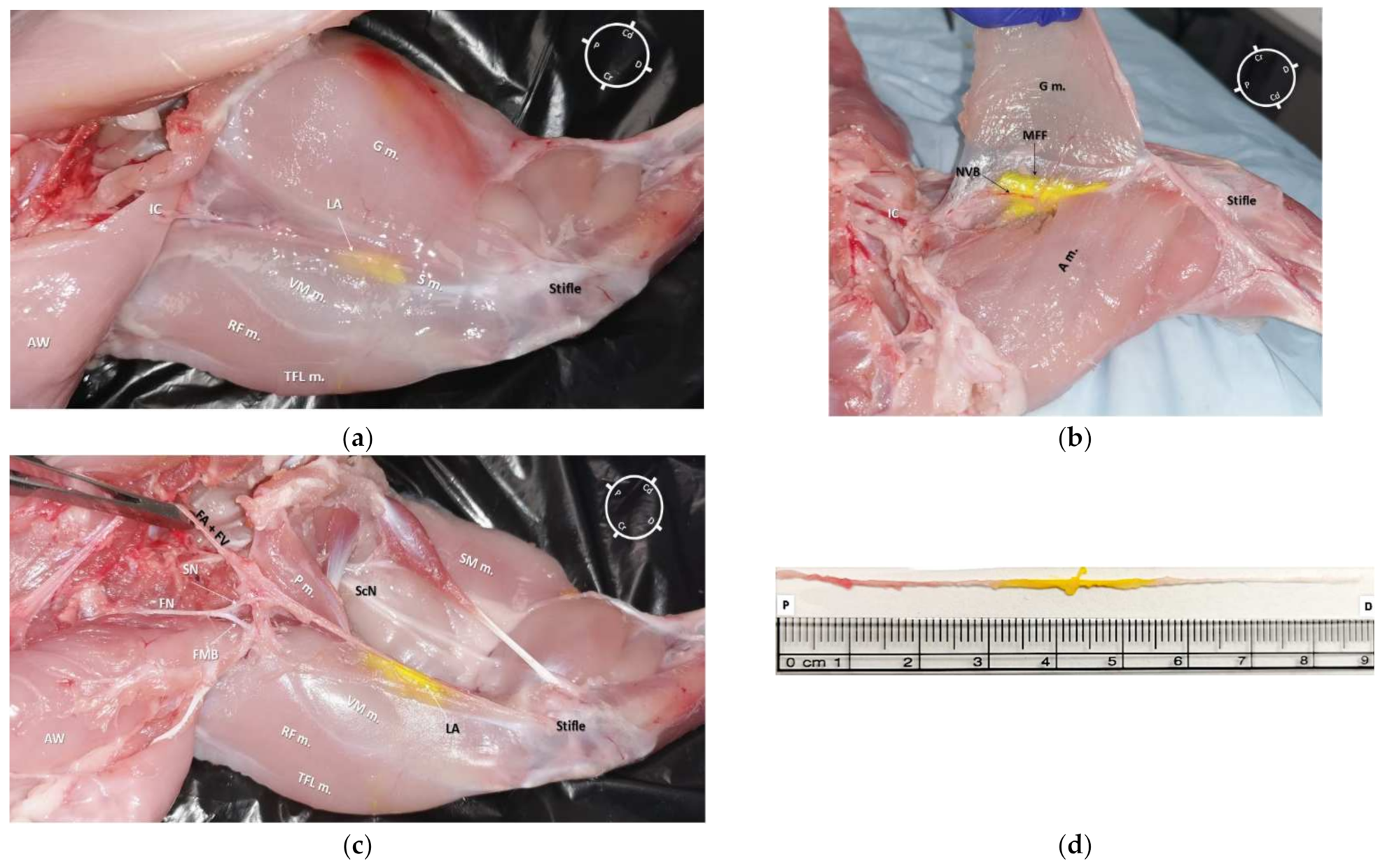
Figure 4. (a) Medial view of the right hind-limb of a rabbit cadaver in which a low volume dye solution (0.05 mL/kg, permanent yellow tissue dye solution and lidocaine 2% (1:50)) was injected. It is possible to observe the underlying tissues stained by dye solution in the central portion of the medial aspect of the thigh. (b) Medial view of the left hind-limb (different rabbit than (a)) of a rabbit cadaver after partial dissection of the gracilis muscle. It was possible to observe the distribution of dye solution inside the medial femoral fascia (MFF), which contains the saphenous nerve (SN) and both the femoral artery (FA) and vein (FV). (c) Anatomical dissection of the medial aspect of a left hind-limb. The gracilis, sartorius, and adductor magnus muscles were bluntly removed. It was possible to observe the dye solution within the medial femoral fascia staining the neurovascular bundle (SN, FA, and FV); the dye solution did not stain the femoral nerve (FN) and its motor nerve branches (FMB). It was possible to localize the pectineus muscle insertion onto the surface of the femur at the level of the first third of the thigh, which was caudal and lateral in relation to the SN. (d) The SN was completely removed from the cadaver (from where it branched off the FN to the proximal stifle, where it deepened to innervate the articular capsule) for the evaluation of its total length (8.8 cm) and extent of staining from the tissue dye solution (2.4 cm). S m.: Sartorius muscle; VM m.: vastus medialis muscle; G m.: gracilis muscle; TFL m.: tensor fascia latae muscle; RF m.: rectus femoris muscle IC: Inguinal crease; NVB: Neurovascular bundle; AW: Abdominal wall; LA: local anesthetic and tissue dye solution; P m.: pectineus muscle; ScN: sciatic nerve; SM m.: semimembranosus muscle; A m.: adductor magnus muscle; P: Proximal; D: Distal; Cr: Cranial; Cd: Caudal.
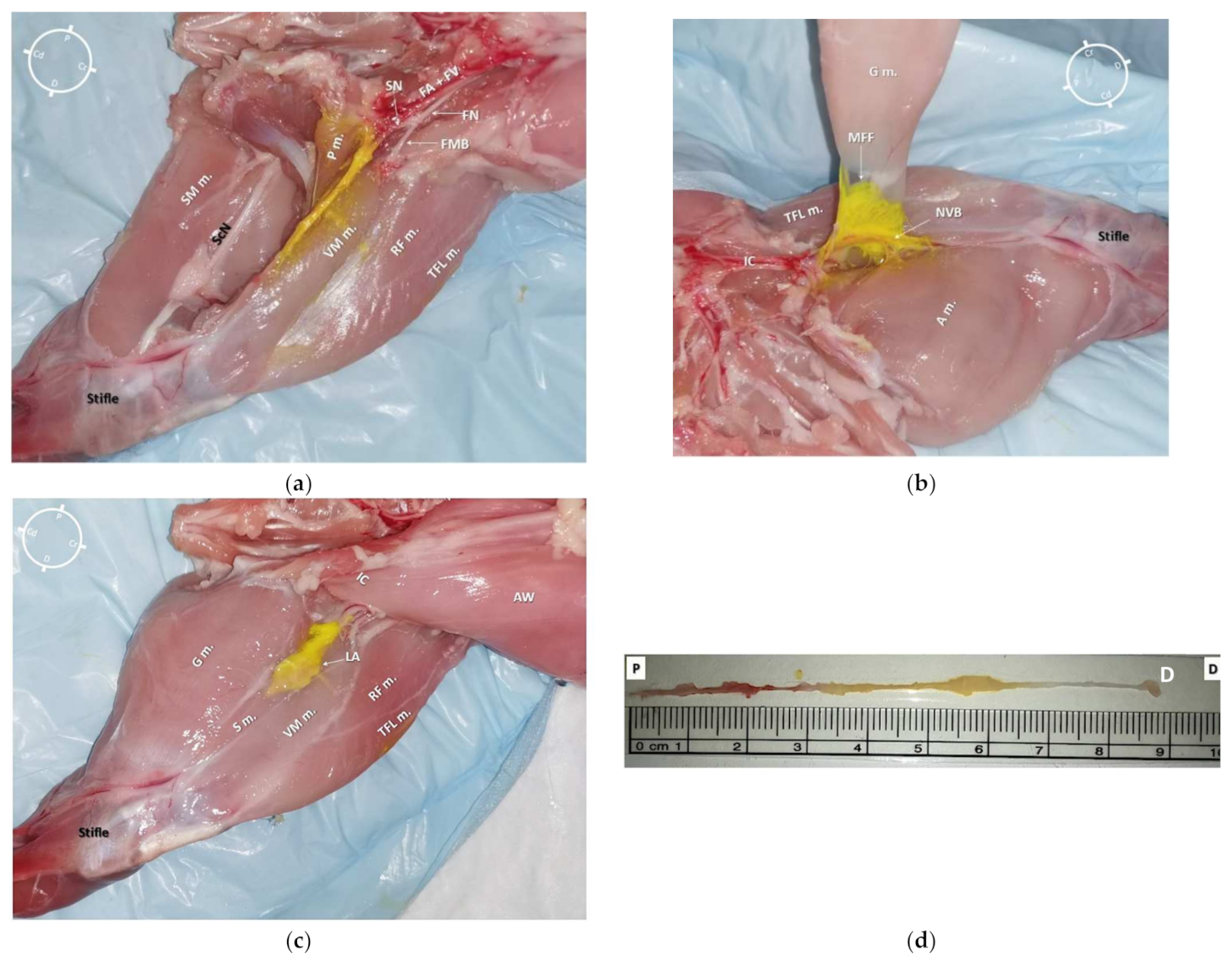
Figure 5. (a) Medial view of a left hind-limb of a rabbit cadaver in which a high volume of dye solution (0.1 mL/kg, of permanent yellow tissue dye solution and lidocaine 2% (1:50) was injected)). It was possible to observe the underlying tissues stained by the dye solution in the central portion of the medial aspect of the thigh. (b) Medial view of the same hind-limb after partial dissection of the gracilis muscle. It was possible to observe the distribution of the dye solution inside the medial femoral fascia (MFF), which contained the saphenous nerve (SN) and both the femoral artery (FA) and vein (FV). (c) Anatomical dissection of the medial aspect of the same hind-limb. The gracilis, sartorius, and adductor magnus muscles were bluntly removed. It was possible to observe the staining along the neurovascular structures (SN, FA, and FV) without staining both the femoral nerve (FN) and its motor nerve branches (the tissue dye stain on the vastus medialis and pectineus muscles resulted after opening of the medial femoral fascia during the dissection). It was possible to localize the pectineus muscle insertion onto the surface of the femur at the level of the first third of the thigh, which was caudal and lateral in relation to the SN. (d) The SN was completely removed from the cadaver (from where it branched off the FN to the proximal stifle, where it deepened to innervate the articular capsule) for the evaluation of its total length (7.8 cm) and extent of staining from the tissue dye solution (4.8 cm). S m.: sartorius muscle; VM m.: vastus medialis muscle; G m.: gracilis muscle; TFL m.: tensor fascia latae muscle; RF m.: rectus femoris muscle; P m.: pectineus muscle; IC: Inguinal crease; NVB: neurovascular bundle; AW: Abdominal wall; LA: local anesthetic and tissue dye solution; FMB: femoral motor branches; ScN: Sciatic nerve; SM m.: Semimembranosus muscle; A m.: adductor magnus muscle; P: Proximal; D: Distal; Cr: Cranial; Cd: Caudal.
References
- Lee, H.; Machin, H.; Adami, C. Peri-anaesthetic mortality and nonfatal gastrointestinal complications in pet rabbits: A retrospective study on 210 cases. Vet. Anaesth. Analg. 2018, 45, 520–528.
- Brodbelt, D.; Blissitt, K.; Hammond, R.; Neath, P.; Young, L.; Pfeiffer, D.; Wood, J. The risk of death: The Confidential Enquiry into Perioperative Small Animal Fatalities. Vet. Anaesth. Analg. 2008, 35, 365–373.
- Schnellbacher, R.W.; Divers, S.J.; Comolli, J.R.; Beaufrère, H.; Maglaras, C.H.; Andrade, N.; Barbur, L.A.; Rosselli, D.D.; Stejskal, M.; Barletta, M.; et al. Effects of intravenous administration of lidocaine and buprenorphine on gastrointestinal tract motility and signs of pain in New Zealand White rabbits after ovariohysterectomy. Am. J. Vet. Res. 2017, 78, 1359–1371.
- Benato, L.; Rooney, N.; Murrell, J. Pain and analgesia in pet rabbits within the veterinary environment: A review. Vet. Anaesth. Analg. 2019, 46, 151–162.
- Hampshire, V.; Robertson, S. Using the facial grimace scale to evaluate rabbit wellness in post-procedural monitoring. Lab. Anim. 2015, 44, 259–260.
- Banchi, P.; Quaranta, G.; Ricci, A.; von Degerfeld, M.M. Reliability and construct validity of a composite pain scale for rabbit (CANCRS) in a clinical environment. PLoS ONE 2020, 15, e0221377.
- Mayer, J. Use of behaviour analysis to recognize pain in small mammals. Lab. Anim 2007, 36, 43–48.
- Kehlet, H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br. J. Anaesth. 1997, 78, 606–617.
- Portela, D.A.; Verdier, N.; Otero, P.E. Regional anesthetic techniques for the pelvic limb and abdominal wall in small animals: A review of the literature and technique description. Vet. J. 2018, 238, 27–40.
- Viscasillas, J.; Sanchis-Mora, S.; Burillo, P.; Esteve, V.; Del Romero, A.; Lafuente, P.; Redondo, J.I. Evaluation of Quadratus Lumborum Block as Part of an Opioid-Free Anaesthesia for Canine Ovariohysterectomy. Animals 2021, 11, 3424.
- Watson, C.B. Respiratory complications associated with anesthesia. Anesthesiol. Clin. N. Am. 2002, 20, 513–537.
- Portela, D.A.; Otero, P.E.; Briganti, A.; Romano, M.; Corletto, F.; Breghi, G. Femoral nerve block: A novel psoas compartment lateral pre-iliac approach in dogs. Vet. Anaesth. Analg. 2013, 40, 194–204.
- Evans, H.E.; de Lahunta, A. Chapter 17: Spinal Nerves, Miller’s Anatomy of the Dog, 4th ed.; Saunders Elsevier: St. Louis, MO, USA, 2013; pp. 611–657.
- Rasmussen, L.M.; Lipowitz, A.J.; Graham, L.F. Development and verification of saphenous, tibial and common peroneal nerve block techniques for analgesia below the thigh in the nonchondrodystrophoid dog. Vet. Anaesth. Analg. 2006, 33, 36–48.
- O’Connor, B.L.; Woodbury, P. The primary articular nerves to the dog knee. J. Anat. 1982, 134, 563–572.
- Bensley, B.A.; Carigie, E.H. The Posterior Limb. In Bensley’s Practical Anatomy of the Rabbit: An Elementary Laboratory Textbook in Mammalian Anatomy, 8th ed.; Craigie, E.H., Ed.; The Blakiston Company: Philadelphia, PA, USA, 1948; pp. 272–291.
- d’Ovidio, D.; Rota, S.; Noviello, E.; Briganti, A.; Adami, C. Nerve Stimulator-guided sciatic and femoral block in pet rabbits (Oryctolagus cuniculus) undergoing hindlimb surgery: A case series. J. Exot. Pet. Med. 2014, 23, 91–95.
- Kluge, K.; Menzies, L.; Kloeppel, H.; Pearce, S.; Bettschart-Wolfensberger, R.; Kutter, A. Femoral and sciatic nerve blockades and incision site infiltration in rabbits undergoing stifle joint arthrotomy. Lab. Anim. 2017, 51, 54–64.
- Costa-Farré, C.; Blanch, X.S.; Cruz, J.I.; Franch, J. Ultrasound guidance for the performance of sciatic and saphenous nerve blocks in dogs. Vet. J. 2011, 187, 221–224.
- Felisberto, R.; Flaherty, D.; Tayari, H. Ultrasound- and nerve stimulation-guided sciatic and saphenous nerve blocks in a pet rabbit (Oryctolagus cuniculus) undergoing calcaneal fracture repair. Vet. Rec. Case Rep. 2022, e320.
More
Information
Subjects:
Anesthesiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
960
Revisions:
2 times
(View History)
Update Date:
17 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




