Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Upendar Rao Golla | + 4511 word(s) | 4511 | 2022-03-08 09:21:06 | | | |
| 2 | Camila Xu | Meta information modification | 4511 | 2022-03-14 07:53:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Golla, U. ABHD11 Antisense RNA 1. Encyclopedia. Available online: https://encyclopedia.pub/entry/20520 (accessed on 07 February 2026).
Golla U. ABHD11 Antisense RNA 1. Encyclopedia. Available at: https://encyclopedia.pub/entry/20520. Accessed February 07, 2026.
Golla, Upendar. "ABHD11 Antisense RNA 1" Encyclopedia, https://encyclopedia.pub/entry/20520 (accessed February 07, 2026).
Golla, U. (2022, March 13). ABHD11 Antisense RNA 1. In Encyclopedia. https://encyclopedia.pub/entry/20520
Golla, Upendar. "ABHD11 Antisense RNA 1." Encyclopedia. Web. 13 March, 2022.
Copy Citation
ABHD11-AS1 is an RNA gene known as long intergenic non-protein coding RNA 35 (LINC00035) and Williams–Beuren syndrome chromosome region 26 (WBSCR26), located at 7q11.23.
lncRNA
ABHD11-AS1
cancer
signaling pathways
1. Introduction
Cancer is a dangerous disease characterized by the uncontrollable proliferation of abnormal cells with high morbidity and mortality [1]. As per WHO statistics, “cancer is the second leading cause of death globally, accounting for an estimated 9.6 million deaths, or one in six deaths, in 2018. Lung, prostate, colorectal, stomach and liver cancer are the most common types of cancer in men, while breast, colorectal, lung, cervical, and thyroid cancer are the most common among women” (https://www.who.int/ accessed on 3 January 2022). Despite enormous progress in cancer research, most cancers are diagnosed at advanced stages resulting in high mortality due to a lack of methods for early diagnosis, effective treatment, and disease relapse. The currently available cancer biomarkers for diagnosis and treatment in clinical use are proteins. Since 2% of the genome only codes for proteins, the research has been focused more on the non-coding genome. Over the past decade, elucidation of the functional importance of non-coding DNA in the human genome has gained the attention of researchers. As a result, several non-coding RNAs (ncRNAs) and long non-coding RNAs (lncRNAs) have been identified that play a critical regulatory role in various cellular processes and biological functions [2]. The timeline of significant discoveries in the field of ncRNAs is depicted in Figure 1.
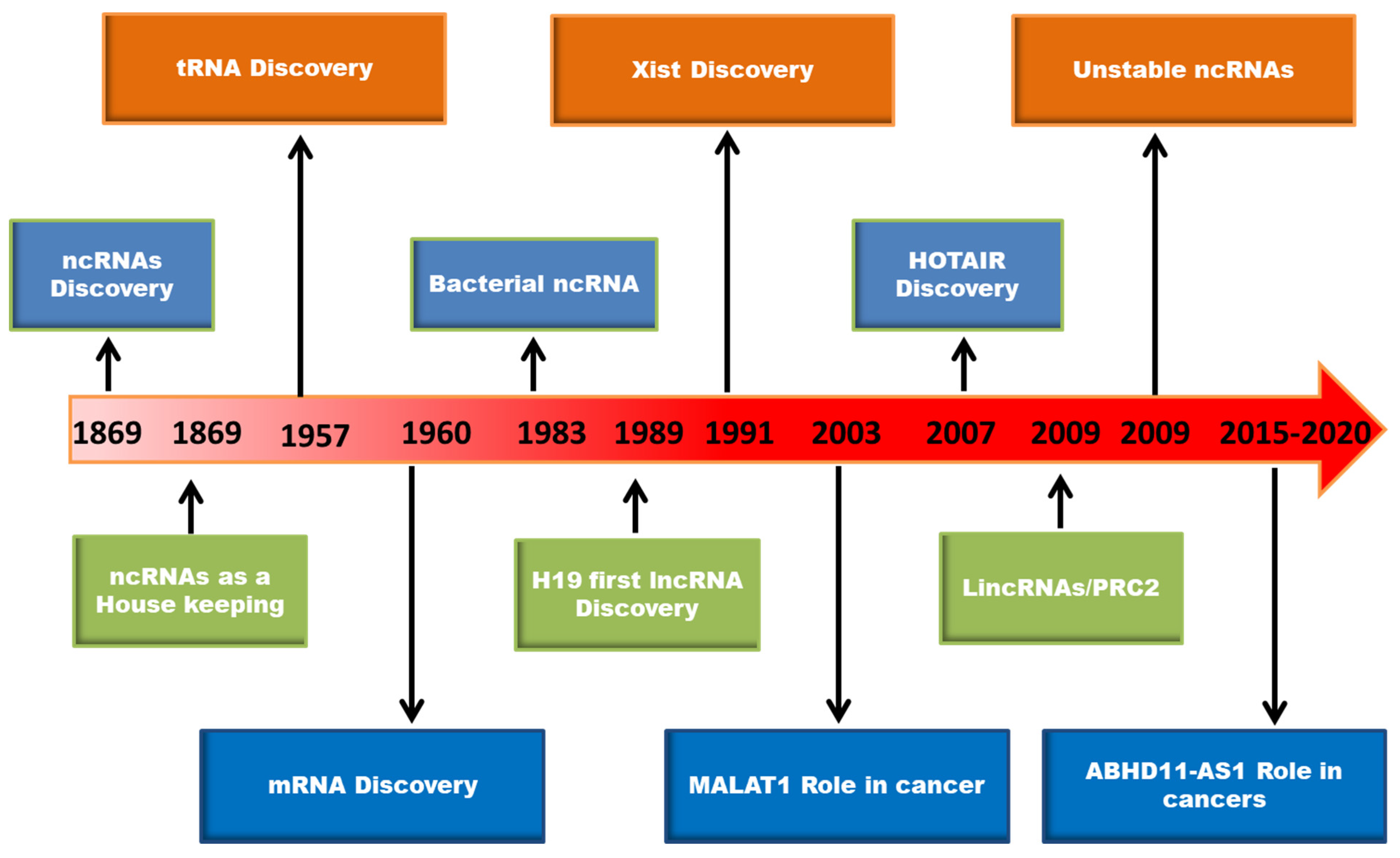 Figure 1. The timeline of major discoveries in the area of RNA biology.
Figure 1. The timeline of major discoveries in the area of RNA biology.LncRNAs are defined as non-protein coding RNA transcripts with over 200 nucleotides in length [3]. LncRNAs account for 80% of ncRNAs, and play important roles in various cellular functions through participating at multiple regulatory levels (transcriptional, post-transcriptional, translational, post-translational, and epigenetic) [4]. LncRNAs can interact with chromatin, RNA, and proteins to modulate the transcription of target genes. The potential mechanisms through which lncRNAs mediate their biological effects were extensively reviewed earlier, and are summarized in Figure 2 [5][6][7]. LncRNAs are categorized based on their cellular localization as nuclear lncRNAs and cytoplasmic lncRNAs. Nuclear lncRNAs are functionally enriched for chromatin interactions, transcriptional alterations, and RNA processing, whereas cytoplasmic lncRNAs modulate signaling pathways, translation, and stability of mRNA transcripts. In another way, lncRNAs can be classified based on their genomic location relative to coding genes as intronic lncRNAs, overlapping lncRNAs, bidirectional lncRNAs, sense lncRNAs, antisense lncRNAs, and long intergenic RNAs (lincRNAs), which include enhancer RNAs (eRNAs) which are transcribed from distal regulatory enhancers [8]. The aberrant regulation of lncRNAs has been associated with different diseases, including cancer [8][9]. One such lncRNA with oncogenic potential is ABHD11 antisense RNA 1 (ABHD11-AS1; ENSG00000225969), which has gained attention over the years for its critical role in cancer progression, diagnosis, and treatment.
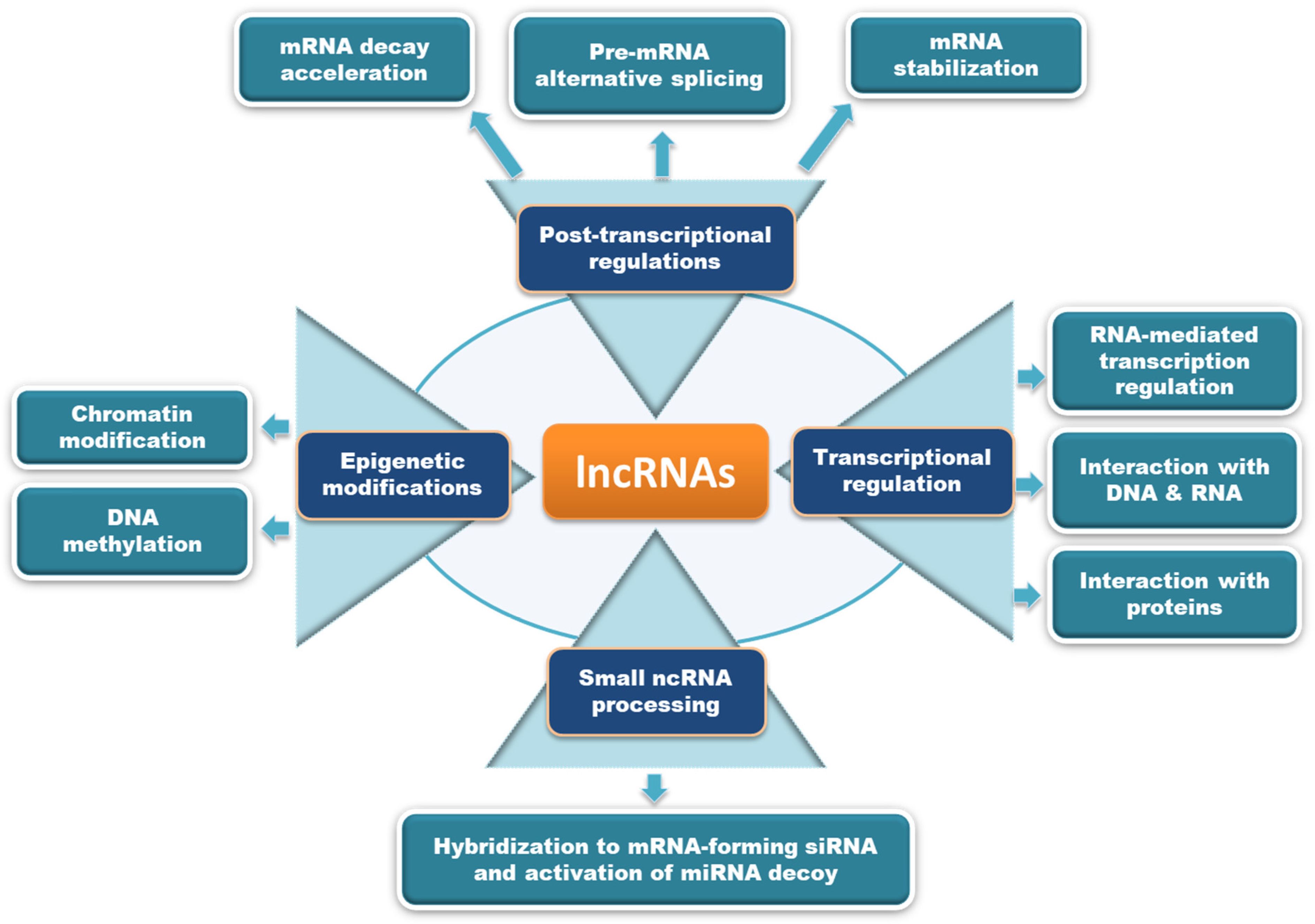 Figure 2. The potential mechanisms of action through which lncRNAs modulate biological functions are depicted here. LncRNAs may act either by processing small ncRNAs, epigenetic modifications, transcriptional regulation, or post-transcriptional modifications to mediate different biological effects.
Figure 2. The potential mechanisms of action through which lncRNAs modulate biological functions are depicted here. LncRNAs may act either by processing small ncRNAs, epigenetic modifications, transcriptional regulation, or post-transcriptional modifications to mediate different biological effects.ABHD11-AS1 is an RNA gene known as long intergenic non-protein coding RNA 35 (LINC00035) and Williams–Beuren syndrome chromosome region 26 (WBSCR26), located at 7q11.23. Firstly, ABHD11-AS1 was reported to upregulate and correlate with clinicopathological features in gastric cancer progression [10]. After that, ABHD11-AS1 gained researchers’ attention to exploit its role in the development, progression, and prognosis of breast, colorectal, lung, thyroid, pancreatic, bladder, endometrial, cervical, and ovarian cancers. In some studies, ABHD11-AS1 was found as circulatory lncRNA that serves as a molecular marker for the early diagnosis of cancers. Several mutations in ABHD11-AS1 lncRNA were reported, and their impact on its overall structure and function needs to be investigated. Here, the secondary structure of ABHD11-AS1 lncRNA predicted using RNAfold web server is depicted in Figure 3 [11].
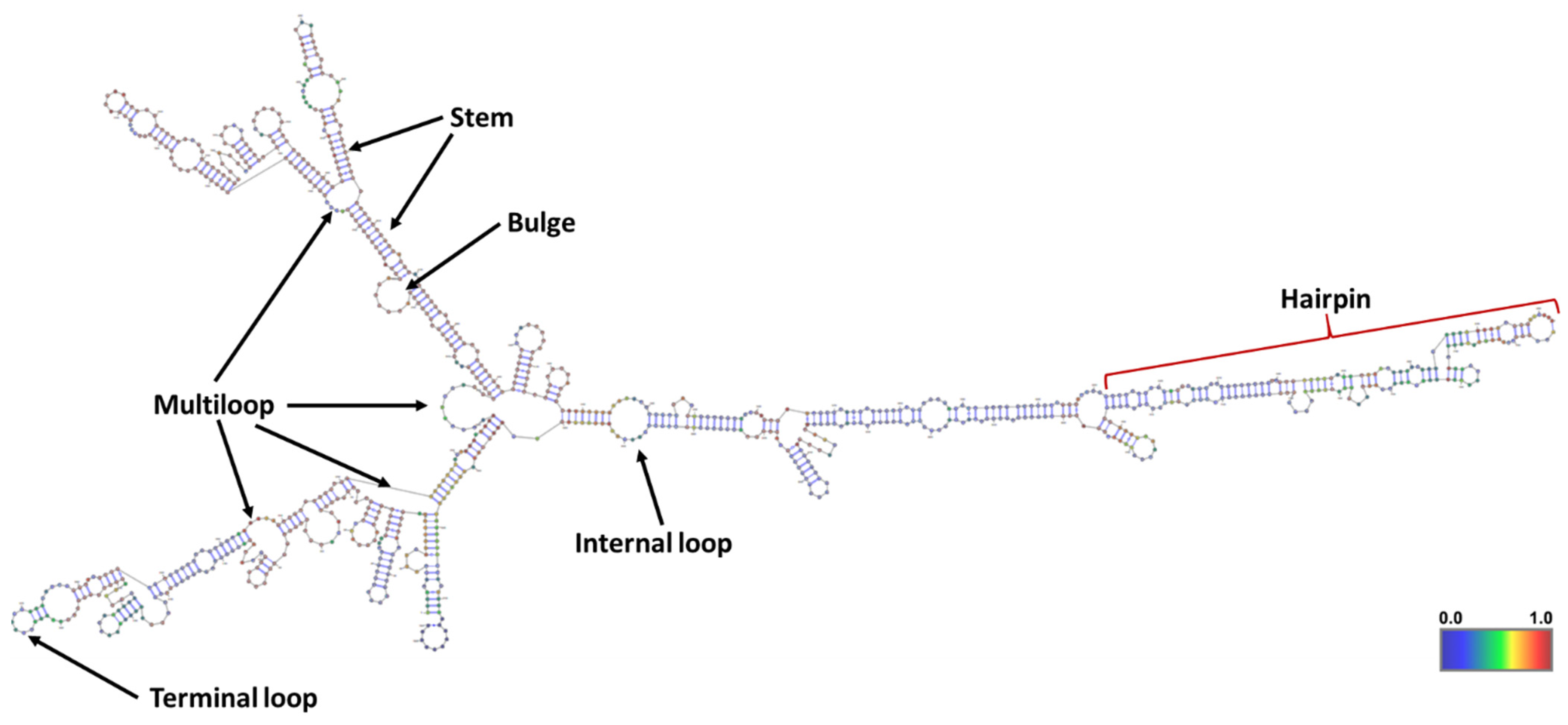 Figure 3. The minimum free energy (MFE) secondary structure of ABHD11-AS1 lncRNA predicted using RNAfold web server. Different components in the secondary structure of lncRNA corresponding to the topology (or shape) of base pairings were defined.
Figure 3. The minimum free energy (MFE) secondary structure of ABHD11-AS1 lncRNA predicted using RNAfold web server. Different components in the secondary structure of lncRNA corresponding to the topology (or shape) of base pairings were defined.2. Oncogenic Role of ABHD11-AS1 lncRNA in Human Cancers
Among numerous lncRNAs, a handful were characterized as they were very poorly expressed, poorly conserved, and more tissue specific. Existing literature indicates that some lncRNAs function in ‘trans’, far from their site of transcription, while others act in ‘cis’, modulating the expression of nearby genes. Some lncRNAs play functional roles in cellular homeostasis, growth, differentiation, and act as oncogenes or tumor suppressors [12]. Though the functions of ABHD11-AS1 lncRNA in healthy tissues are not characterized, a recent comprehensive analysis of genotype–tissue expression (GTEx) datasets revealed its expression in spleen and blood tissues [13]. The mouse homolog of ABHD11-AS1 (Abhd11os) was expressed in striatal neurons, and significantly downregulated in Huntington disease (HD) models. Overexpression of Abhd11os showed neuroprotection against an N-terminal fragment of mutant huntingtin, while knockdown was protoxic [14]. Recent pan-cancer comparison revealed that lncRNAs were deregulated more than tumor-specific than mRNAs [13]. Most of the deregulated lncRNAs were tumor-specific, while few of them act as “onco-lncRNAs”, which are dysregulated across several cancer types [15]. ABHD11-AS1 is one of such onco-lncRNAs that was overexpressed in different cancers such as gastric cancer (GC), papillary thyroid cancer (PTC), non-small cell lung cancer (NSCLC), pancreatic cancer (PC), colorectal cancer (CRC), ovarian cancer (OC), breast cancer (BC), endometrial cancer (EC), cervical cancer (CC), and bladder cancer, as summarized in Table 1 and discussed in detail as follows.
Table 1. Summary of studies describing oncogenic role of ABHD11-AS1 lncRNA in different cancers.
| Tumor Type | Sample Type | Patient/Control Size | Expression | Clinical Correlation | Function | Biomarker | Relevant to Prognosis | Overall Survival | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| GC | Tissue | 75/75 | Upregulated | Degree of differentiation, Lauren type | Diagnosis | 2014 | [10] | |||
| Tissue | 73/37 | Upregulated | 2016 | [16] | ||||||
| Gastric juice | 39/45 | Upregulated | Gender, tumor size, tumor stage, Lauren type, blood CEA levels | Diagnosis/ Early diagnosis |
||||||
| Plasma | 10/10 | No change | 2018 | [17] | ||||||
| Tissue | 41/41 | Upregulated | Proliferation, apoptosis | 2020 | [18] | |||||
| PTC | Tissue | 82/82 | Upregulated | TNM stage, lymph node metastasis, tumor infiltration | Proliferation, apoptosis, migration | Negative correlation | Poor | 2018 | [19] | |
| Tissue | 80/80 | Upregulated | Proliferation, colony formation, migration, invasion, apoptosis | Diagnosis | 2019 | [20] | ||||
| Serum | 64/50 | Upregulated | Tumor diameter, lymph node metastasis | Proliferation, apoptosis | Diagnosis | Negative correlation | Poor | 2021 | [21] | |
| Tissue | 98/98 | Upregulated | Lymph node metastasis | Proliferation, invasion, migration | Diagnosis | 2022 | [22] | |||
| NSCLC | Tissue | 40/40 | Upregulated | TNM stage | Proliferation, Warburg effect | Negative correlation | Poor | 2020 | [23] | |
| PC | Tissue | 147/147 | Upregulated | TNM stage, distant metastasis, and tumor differentiation |
Proliferation, colony formation, migration, invasion, apoptosis | Prognosis | Negative correlation | Poor | 2018 | [24] |
| Plasma | 114/46 | Upregulated | Diagnosis/ Early diagnosis |
Negative correlation | Poor | 2019 | [25] | |||
| Tissue/ TCGA |
179/171 | Upregulated | Proliferation, migration, invasion, apoptosis | Poor | 2020 | [26] | ||||
| CRC | Tissue | 132/132 | Upregulated | TNM stage, lymph node metastasis | Proliferation, colony formation, migration, invasion, apoptosis | Negative correlation | Poor | 2018 | [27] | |
| Tissue | 53/53 | Upregulated | Proliferation, colony formation, invasion | 2019 | [28] | |||||
| Tissue | 60/60 | Upregulated | Proliferation, migration, invasion, apoptosis | 2021 | [29] | |||||
| OC | Tissue | 51/13 | Upregulated | Tumor stage, Degree of differentiation | Proliferation, apoptosis, invasion, migration | 2017 | [30] | |||
| Tissue | 53/53 | Upregulated | Proliferation, invasion, migration, colony formation | 2019 | [31] | |||||
| Tissue | 50/50 | Upregulated | Tumor stage, lymph node metastasis | Proliferation, apoptosis, invasion, migration | Poor | 2021 | [32] | |||
| Bladder Cancer | Tissue | 66/66 | Upregulated | TNM stage, Histological grade, tumor invasion depth | Proliferation, apoptosis, migration, | 2017 | [33] | |||
| BC | Tissue | 79/79 | Upregulated | No association | Same | 2021 | [34] | |||
| EC | Tissue | 89/27 | Upregulated | Proliferation, apoptosis, invasion, migration | 2018 | [35] | ||||
| CC | Cell lines | Upregulated | Proliferation, apoptosis, colony formation, invasion, migration | 2021 | [36] | |||||
| Tissue/ Serum |
72/78 | Upregulated | Proliferation, apoptosis, invasion, migration | Diagnosis/ Prognosis |
Negative correlation | Poor | 2022 | [37] |
2.1. Gastric Cancer
Accumulating evidence highlights the role of lncRNAs in the development and progression of GC [38][39][40]. ABHD11-AS1 is one of such lncRNAs studied explicitly and projected as a potential biomarker for early diagnosis and treatment of GC [10][16][18]. In 2014, the expression analysis showed that ABHD11-AS1 was upregulated in GC tissues from 75 patients compared to normal tissues. The ABHD11-AS1 expression levels in GC tissues were significantly related to the degree of differentiation, Lauren histologic classification, and carbohydrate antigen 19-9 (CA199) of GC patients [10]. Later, the same research group revealed that the high expression level of ABHD11-AS1 in gastric juice from GC patients could serve as a potential biomarker for the early diagnosis of GC [16]. ABHD11-AS1 levels in gastric juice from GC patients were associated with gender, tumor size and stage, Lauren classification, and blood carcinoembryonic antigen (CEA). The early GC detection rate is approximately 72% when gastric juice ABHD11-AS1 levels are used as a marker [16]. The expression levels of ABHD11-AS1 lncRNA in the plasma of GC patients of different stages were not high enough to differentiate from that of healthy control subjects [17]. Very recently, Xin et al. have exploited the function and mechanism of ABHD11-AS1 in the development and progression of GC [18]. Similar to previous reports, ABHD11-AS1 was upregulated in GC tissues and GC cell lines (SGC-7901, MKN28, AGS, MGC-803, and BGC-823), relative to the matched normal tissues and normal gastric cell lines (GES-1 and RGM-1), respectively. The siRNA-mediated knockdown of ABHD11-AS1 in gastric cell lines (MGC-803 and BGC-823) hampered the proliferation, and enhanced the apoptosis in vitro, and inhibited tumor progression in GC xenograft models in vivo [18]. Thus, ABHD11-AS1 was established as a potential therapeutic target and biomarker for the early diagnosis and treatment of GC.
2.2. Papillary Thyroid Cancer
Recently, the lncRNAs were found to play critical roles in the initiation and progression of cancers, including thyroid cancer [41][42]. Several studies have reported that ABHD11-AS1 lncRNA is vital for PTC progression, and could be a potential therapeutic target [19][20][21][22]. In these studies, ABHD11-AS1 is highly expressed in PTC patient samples and cell lines (BCPAP, BHP2-7, BHP5-16, BHT-101, GLAG-66, IHH-4, K-1, KTC-1, and TPC-1), compared to normal tissues and the control cell line (Nthy-ori 3-1) respectively. The high levels of ABHD11-AS1 significantly correlated with PTC clinicopathological features, such as extrathyroidal extension, lymph node metastasis, tumor infiltration, tumor size, and advanced TNM (Tumor, Node, Metastasis) stage [19][20][22]. Kaplan–Meier analysis revealed the predictive value of ABHD11-AS1 in PTC, as the patients with higher ABHD11-AS1 had poorer overall survival [19]. Moreover, Hou et al. reported that serum ABHD11-AS1 levels were elevated in PTC patients than healthy controls, and closely correlated only with tumor diameter and lymph node metastasis [21]. The higher expression of serum ABHD11-AS1 remarkably related to poor prognosis group (patients suffered from recurrence or progression of PTC or died) that exhibited a lower five-year overall survival rate. The Cox analysis revealed TNM staging, lymph node metastasis, and ABHD11-AS1 were independent prognostic factors for PTC [21]. Furthermore, the reduction of ABHD11-AS1 levels exhibited slower proliferation, invasion, metastasis with higher apoptosis in PTC cell lines in vitro, and decreased in vivo tumor progression in PTC xenograft models [19][20]. The PTC cell lines BCPAP [19] and TPC-1 [20], transfected with ABHD11-AS1 shRNA and then injected subcutaneously into nude mice, had exhibited a drastic reduction in tumor growth rate, weights, and sizes, compared to that of empty vector control xenografts. Interestingly, xenografts with ABHD11-AS1 silencing had suppressed the liver and lung metastasis in vivo [19]. Therefore, ABHD11-AS1 exerts malignant properties in PTC progression, and its serum levels could serve as a potential biomarker for the diagnosis and treatment of PTC.
2.3. Ovarian Cancer
Ovarian cancer (OC) represents the most lethal gynecological cancer of the female reproductive organs. Epithelial ovarian cancer (EOC) is prevalent, and accounts for more than 90% of OC cases worldwide [43]. EOC is the leading cause of OC deaths due to extensive peritoneal metastasis, high relapse rate, a lack of specific symptoms, and a lack of reliable methods for early diagnosis. In spite of current developments in chemotherapy and cytoreductive surgery, the five-year overall survival is approximately 30%, with the prognosis of OC remaining very poor [44]. In search of potential biomarkers, lncRNAs have emerged as critical players in the development and progression of various cancers, including OC/EOC [45][46]. ABHD11-AS1 lncRNA was proven to be a potential candidate biomarker for OC/EOC by several studies [30][31][32]. The lncRNA ABHD11-AS1 was significantly overexpressed in expression analysis of EOC [30][31] and OC [32] patient samples, compared to the normal tissue controls. A similar trend of ABHD11-AS1 expression was observed in EOC (HO8910, OVCA429) and OC cell lines (A2780, HEY, SKOV-3, and OVCAR-3), relative to the normal ovarian epithelial cell line (IOSE80) and normal ovarian cells (HOSEpiC). The expression of ABHD11-AS1 was positively correlated with the lymph node metastasis and tumor stage, while negatively affecting the overall survival rate in OC patients. The knockdown of ABHD11-AS1 in EOC/OC cell lines exhibited decreased proliferation, invasiveness, and migration with more apoptosis. In comparison, the overexpression of ABHD11-AS1 resulted in opposite effects in vitro [30][31][32]. The oncogenic potential of ABHD11-AS1 lncRNA was evaluated in different xenograft models of OC in vivo [30][31]. The OC xenograft was constructed in nude mice by intraperitoneal injection of the OC cell line. Intraperitoneal delivery of ABHD11-AS1 shRNA (shABHD11-AS1) into OC xenografts suppressed in vivo tumor growth, as evidenced by reduced tumor weights and volumes, compared to mock controls [31]. In another study, OC xenografts were generated in BALB/c nude mice by injecting 10 million A2780 cells transfected with lncRNA ABHD11-AS1 (or mock control) subcutaneously or intraperitoneally [30]. Remarkably, tumor xenografts showed increased tumor growth rate and tumor volumes upon lncRNA ABHD11-AS1 overexpression, compared to mock controls. The xenografts exhibited significantly widespread metastatic lesions among mesentery in the ABHD11-AS1 overexpression group, relative to the control [30]. Thus, the current evidence strongly indicates that ABHD11-AS1 could be a potential prognostic factor for OC.
2.4. Colorectal Cancer
Colorectal cancer (CRC) is the most frequently diagnosed digestive system malignant tumor, and the leading cause of cancer deaths worldwide [47]. CRC is developed by the contribution of several genetic, epigenetic, and environmental factors. Although there has been tremendous progress in screening and treatment, the prognosis and five-year overall survival rate are still poor due to recurrence, distant metastasis, and limitations in the methods for early diagnosis [48]. Over the past decade, lncRNAs have gained a lot of attention due to their potential as a reliable and valid biomarker for early diagnosis, treatment, and drug resistance in different cancers, including CRC [49][50]. Several studies have reported the significance of ABHD11-AS1 lncRNA in the development, progression, and invasion of CRC [27][28][29]. Consistently, ABHD11-AS1 was highly expressed in CRC patient samples and various cell lines (SW-480, HT-29, LoVo, HCT-116, HCT-8, SW-620, Caco-2) relative to the normal controls. Kaplan–Meier survival analysis with log-rank tests revealed that CRC patients with higher ABHD11-AS1 lncRNA expression had significantly poorer progression-free survival and overall survival than those with lower levels. Moreover, the expression levels of ABHD11-AS1 are clinically associated with TNM stage and lymph node metastasis in CRC patients. The CRC cell lines (SW-480, HCT-116) exhibited a considerable reduction in the proliferation, migration, and invasion with an increase in the apoptosis upon si-RNA [27][28] or shRNA [29] targeted knockdown of ABHD11-AS1 in vitro. Accordingly, the knockdown of ABHD11-AS1 impaired the in vivo growth of SW-480 [28] and HCT-116 [29] cell lines in the xenograft model. The CRC xenografts had shown a significant reduction in tumor growth and size upon ABHD11-AS1 knockdown, relative to the control [28][29]. The findings from multiple reports highlighted the oncogenic role of ABHD11-AS1 lncRNA in CRC progression and prognosis.
2.5. Pancreatic Cancer
Pancreatic cancer (PC) is the seventh most common malignant tumor worldwide, with high mortality due to lack of specific symptoms, high invasiveness, and metastasis. PC can develop in either exocrine cells or neuroendocrine cells (islet cells) of the pancreas. The exocrine cancers are most common in PC, and diagnosed at an advanced stage, whereas pancreatic neuroendocrine tumors (NETs) or islet cell tumors are less familiar with a better prognosis. Despite the current research and therapeutic development progress, the median survival time is only 3–6 months, with a five-year survival rate remaining under 5% [51][52]. The lack of effective methods for early PC detection is a prime reason for the poor survival rate. As of today, lncRNAs have been exploited as the biomarkers for the clinical diagnosis and treatment of various cancers, including PC [53][54]. One such circulatory lncRNA is ABHD11-AS1, which was recently studied for its significance in PC progression, diagnosis, and treatment [24][25][26]. In 2018, Qiao X. et al. reported that ABHD11-AS1 was significantly upregulated in different PC cell lines (CFPAC-1, BXPC-3, L3.6pl, and PANC-1) and 147 PC patient tissues in comparison to pancreatic cell line (HPDE6-C7) and matched normal pancreatic tissues, respectively [24]. Interestingly, the high expression of ABHD11-AS1 correlated clinically with distant metastasis, TNM stage, tumor differentiation, and a five-year reduction in overall survival of PC patients. Similarly, the analysis of TCGA gene expression data for PC also revealed that ABHD11-AS1 was highly expressed and negatively correlated with survival rate in patients with PC [26].
Moreover, the knockdown of ABHD11-AS1 in three PC cell lines (L3.6pl, MiaPaCa-1, and PANC-1) resulted in decreased cell proliferation, colony formation, and metastasis (migration and invasion) with increased apoptosis [24][26]. Targeted knockdown of ABHD11-AS1 significantly repressed the in vivo growth of PANC-1 cells injected subcutaneously into a nude mice xenograft model [26]. Liu Y. et al. identified that the expression levels of circulatory ABHD11-AS1 lncRNA alone or combined with carbohydrate antigen 19-9 (CA199) in the plasma are potential biomarkers for early detection of PC [25]. Thus, ABHD11-AS1 plays a critical role in the diagnosis, progression, metastasis, and treatment of PC. Further studies to test the part of ABHD11-AS1 in large-scale PC patient cohorts are necessary to validate its clinical significance.
2.6. Luminal Breast Cancer
Breast cancer (BC) is a common cancer among women, accounting for 30% of all female cancer cases reported worldwide. BC is the second leading cause of cancer-related deaths [55]. The luminal subtype of BC is characterized by estrogen and progesterone receptors’ expression, and accounts for more than 70% of BC. The luminal subtype of BC is further classified as luminal A or luminal B, based on the levels of human epidermal growth factor receptor 2 (HER2) and ki-67 [56]. The emerging reports indicate that lncRNAs are dysregulated in BC, and the differential expression of different lncRNAs in BC subtypes has been explored [57]. One such lncRNA is ABHD11-AS1, which has been studied for its expression levels and clinical correlation in BC, specifically luminal subtype, using bioinformatics and systems biology analyses [34]. The authors have tested the expression of ABHD11-AS1 lncRNA by qPCR, and discovered that it is significantly upregulated in 79 luminal BC tissues and cell lines (T47D and MCF7), compared to the normal controls. However, the authors failed to find a considerable correlation between ABHD11-AS1 expression levels and luminal BC clinicopathological features. Accordingly, no significant association was observed with ABHD11-AS1 lncRNA expression with the overall survival of the luminal BC patients in Kaplan–Meier analysis. Thus, ABHD11-AS1 levels were not suggested as a promising biomarker for diagnosing luminal subtypes of BC. The International Cancer Genome Consortium (ICGC) portal results indicated 17 mutations in ABHD11-AS1 across luminal BC, of which one is insertion, and 16 are substitutions, with only one donor out of 19,729 (0.01%) affected. Out of 17 reported mutations, 10 of them occurred upstream, and 6 of them were located downstream of ABHD11-AS1, with only one substitution in the exon region (chr7:g.73149393A>T) [34]. Since the substitution mutations affect the secondary structure of lncRNAs and contribute to the development of cancers, further detailed studies are necessary for understanding the functional consequences of ABHD11-AS1 mutations [58]. Bioinformatic analysis of genes co-expressed with ABHD11-AS1 lncRNA are involved in signal transducer activity, transmembrane transport, reproductive process, Wnt ligand biogenesis, mesodermal commitment pathway, FGFR1 mutant receptor activation, and Wnt signaling. The protein–protein interactions (PPI) network analysis of the ABHD11-AS1 co-expressed genes identified four (LACTB2, SPANXA1, SPANXA2, and SPANXC) hub genes [34]. Recently, Wang X. et al. integrated gene co-expression network analysis in BC with clinical data, and revealed ABHD11-AS1 as a potential biomarker or BC–related risk target in several prognostic modules [59]. ABHD11-AS1 lncRNA was identified as a critical gene with significantly higher expression status in the BC survival–related modules. The prognostic module consists of ABHD11-AS1 in combination with other clinical indicators, such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), and TNM, had shown high accuracy and sensitivity for BC risk-stratification [59]. Although the bioinformatics analysis identified the possible role of lncRNA, further studies are warranted to prove the clinical significance of ABHD11-AS1 in the prognosis and treatment of luminal BC.
2.7. Non-Small Cell Lung Cancer
Lung cancer incidence and mortality have increased over the past decade globally. Non-small cell lung cancer (NSCLC) is the most common histological type, accounting for 85% of reported lung cancer cases and deaths. Despite the tremendous progress in NSCLC diagnosis and treatment regimen over recent years, the five-year survival rate of NSCLC patients remains around 15% [60]. Therefore, the development of early diagnosis methods and new therapeutic targets would be the most effective approach for reducing NSCLC deaths. The emerging reports have been highlighted that lncRNAs play a critical role in the occurrence, progression, metastasis, and chemotherapy resistance of several cancers, including NSCLC [4]. Incredibly, several lncRNAs with important biological functions aberrantly expressed and detected in the peripheral body fluids would be ideal biomarkers for the early detection of NSCLC [61]. One such circulating ABHD11-AS1 lncRNA with prognostic value in different cancers was recently studied in NSCLC by Xue L. et al. [23]. The authors have found that ABHD11-AS1 is upregulated in NSCLC cell lines (NCI-H1299, A549, HCC827, and NCI-H1650), compared to normal lung epithelial cells (BEAS-2B). The qPCR expression analysis using 40 NSCLC tissue specimens showed significant overexpression of ABHD11-AS1 compared with the adjacent normal lung tissue. Notably, ABHD11-AS1 expression was much higher in the advanced tumor stage (TNM grading) than in the primary tumor stage NSCLC tissue specimens. Accordingly, the overall survival rate was significantly lower in the NSCLC patient group with higher ABHD11-AS1 levels. Furthermore, the shRNA targeted silencing of ABHD11-AS1 decreased the proliferation of the H1299 cell line by reducing glucose uptake, lactate production, and ATP accumulation. The extracellular acidification rate (ECAR) analysis showed that ABHD11-AS1 knockdown reduced the glycolytic capacity of cells. The overexpression of ABHD11-AS1 in the H1650 cell line exhibited contrary effects to knockdown, as expected. Altogether, the authors established the oncogenic role of ABHD11-AS1 lncRNA that promotes the Warburg effect in NSCLC [23]. Further mechanistic studies are warranted to detect ABHD11-AS11 in the peripheral circulation of NSCLC patients to highlight its diagnostic value.
2.8. Bladder Cancer
Bladder cancer is the ninth most commonly diagnosed cancer globally, with an estimate of 500,000 cases annually. Since 2017, several studies have reported aberrant expression of lncRNAs that play a functional role in the prognosis, migration, and invasion of bladder cancer [62][63][64]. Recently, Su G. et al. performed a systematic meta-analysis of lncRNAs aberrantly regulated in bladder cancer, and discovered that UCA1 lncRNA could serve as a potential marker for bladder cancer diagnosis [65]. Chen M. et al. studied the functional impact of emerging ABHD11-AS1 lncRNA in bladder cancer tissues and cell lines in vitro [33]. The authors first established that ABHD11-AS1 was upregulated in 66 bladder cancer patient tissues and cell lines relative to normal healthy controls. Interestingly, the upregulation of ABHD11-AS1 had a significant association with clinical pathologic grading (p < 0.001), tumor invasion depth (p = 0.001), and TNM stage. The authors have implicated different bladder cancer cell lines (T24, 5637, and SW780), which had higher ABHD11-AS1 expression than SV-HUC-1, a normal control cell line established from healthy ureter tissue. The siRNA-mediated knockdown of ABHD11-AS1 resulted in decreased cell proliferation (in MTT and EdU assays) and cell migration (scratch assay), along with enhanced apoptosis (Caspase-3 activity and flow cytometry) in bladder cancer cell lines. The overexpression of ABHD11-AS1 in bladder cancer cell lines exhibited contrary effects to its silencing. Considering these in vitro results, authors have concluded that ABHD11-AS1 lncRNA serves as an oncogene, and might be a potential therapeutic target in bladder cancer [33]. Further in vivo studies are necessary with more patient samples and mechanistic insights to explore the prognostic value of ABHD11-AS1 lncRNA in bladder cancer.
2.9. Endometrial Cancer
Endometrial cancer (EC) is also called uterine cancer, in which malignant cells form in the endometrium tissue lining of the uterus. The incidence of EC is increasing every year, and leads to the second highest rates of death from gynecological cancers in women worldwide. EC is diagnosed at an average age of 60, and primarily affects post-menopausal women [66]. In recent years, the contribution of lncRNAs to cancer progression and their potential as biomarkers and therapeutic targets in EC has been explored [67][68]. One such lncRNA is ABHD11-AS1, which was reported to promote EC development and progression by Liu Y. et al. in 2018 [35]. In this study, the authors have tested ABHD11-AS1 expression in 89 EC tissues collected from patients undergoing surgical resection and 27 normal endometrial specimens. Interestingly, the real-time PCR analysis revealed that ABHD11-AS1 expression was significantly higher in EC tissues than in normal endometrial tissues. The knockdown of ABHD11-AS1 using siRNA in EC cell line HEC-1B (with high ABHD11-AS1 levels) resulted in decreased cell proliferation, migration, invasion, and G1 phase arrest in the cell cycle, with increased apoptosis. Overexpression of ABHD11-AS1 in the EC cell line Ishikawa (with low ABHD11-AS1 levels) resulted in increased cell proliferation, invasion, and migration, with G1-S progression in cell cycle and decreased apoptosis in vitro. Moreover, the authors have established the role of ABHD11-AS1 in EC progression in vivo using a xenograft model. The EC xenograft mice injected with Ishikawa cells overexpressing ABHD11-AS1 showed higher tumor volumes compared to mock injected control mice. The authors have established that ABHD11-AS1 promotes cell proliferation and invasion in EC [35]. Further studies are encouraged to elucidate the molecular mechanisms, through which ABHD11-AS1 contributes to EC development and progression.
2.10. Cervical Cancer
Cervical cancer (CC) is the primary gynecological malignant tumor that develops in a woman’s cervix. As per WHO statistics of 2018, 570,000 were diagnosed, and 300,000 women have died from CC globally. Mostly, the infection with high-risk human papillomaviruses (HR-HPV) has been linked to CC cases. In addition to HR-HPV infection, an individual’s genetic and epigenetic alterations significantly contribute to the development of CC [69][70]. The recent literature demonstrated the critical role of lncRNAs as biomarkers for CC development, invasion, metastasis, treatment response, and drug resistance [71][72]. Hou S. et al. have established the role of ABHD11-AS1 lncRNA in the progression of CC [36]. The authors found that ABHD11-AS1 was highly expressed in four different CC cell lines (HCC94, HeLa, C-33A, and CaSki) compared to the normal endocervical End1/E6E7 cell line initially. Later, the knockdown of ABHD11-AS1 using its specific shRNAs in CC cell lines C-33A and CaSki exhibited decreased cell proliferation by EdU assay and increased apoptosis by TUNEL assay. The results from the transwell assay depicted that the migration and invasion of CC cell lines decreased upon ABHD11-AS1 knockdown. Accordingly, the depletion of ABHD11-AS1 in the CaSki CC cell line significantly suppressed tumor growth and metastasis in vivo using the CC xenograft model [36]. Lately, Zhu D. et al. had established that ABHD11-AS1 levels were higher in the serum and tissues of CC patients than healthy controls, and significantly associated with the poorer prognosis and three-year overall survival of CC patients [37]. ABHD11-AS1 knockdown in HeLa and CaSki CC cell lines resulted in slower growth, reduced invasion, and metastasis with higher apoptosis, while overexpression of ABHD11-AS1 showed contrary effects in vitro [37]. Altogether, these studies had established that ABHD11-AS1 lncRNA levels could serve as a biomarker for CC diagnosis and correlate with prognosis, metastasis, and treatment in CC patients.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Chandra Gupta, S.; Nandan Tripathi, Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer 2017, 140, 1955–1967.
- Kung, J.T.; Colognori, D.; Lee, J.T. Long noncoding RNAs: Past, present, and future. Genetics 2013, 193, 651–669.
- Ginn, L.; Shi, L.; Montagna, M.; Garofalo, M. LncRNAs in Non-Small-Cell Lung Cancer. Noncoding RNA 2020, 6, 25.
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206.
- Sebastian-delaCruz, M.; Gonzalez-Moro, I.; Olazagoitia-Garmendia, A.; Castellanos-Rubio, A.; Santin, I. The Role of lncRNAs in Gene Expression Regulation through mRNA Stabilization. Noncoding RNA 2021, 7, 3.
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118.
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463.
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54.
- Lin, X.; Yang, M.; Xia, T.; Guo, J. Increased expression of long noncoding RNA ABHD11-AS1 in gastric cancer and its clinical significance. Med. Oncol. 2014, 31, 42.
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neubock, R.; Hofacker, I.L. The Vienna RNA websuite. Nucleic Acids Res. 2008, 36, W70–W74.
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208.
- Unfried, J.P.; Serrano, G.; Suarez, B.; Sangro, P.; Ferretti, V.; Prior, C.; Boix, L.; Bruix, J.; Sangro, B.; Segura, V.; et al. Identification of Coding and Long Noncoding RNAs Differentially Expressed in Tumors and Preferentially Expressed in Healthy Tissues. Cancer Res. 2019, 79, 5167–5180.
- Francelle, L.; Galvan, L.; Gaillard, M.C.; Petit, F.; Bernay, B.; Guillermier, M.; Bonvento, G.; Dufour, N.; Elalouf, J.M.; Hantraye, P.; et al. Striatal long noncoding RNA Abhd11os is neuroprotective against an N-terminal fragment of mutant huntingtin in vivo. Neurobiol. Aging 2015, 36, 1601.e7–1601.e16.
- Chiu, H.S.; Somvanshi, S.; Patel, E.; Chen, T.W.; Singh, V.P.; Zorman, B.; Patil, S.L.; Pan, Y.; Chatterjee, S.S.; Cancer Genome Atlas Research, N.; et al. Pan-Cancer Analysis of lncRNA Regulation Supports Their Targeting of Cancer Genes in Each Tumor Context. Cell Rep. 2018, 23, 297–312.e12.
- Yang, Y.; Shao, Y.; Zhu, M.; Li, Q.; Yang, F.; Lu, X.; Xu, C.; Xiao, B.; Sun, Y.; Guo, J. Using gastric juice lncRNA-ABHD11-AS1 as a novel type of biomarker in the screening of gastric cancer. Tumour Biol. 2016, 37, 1183–1188.
- Xian, H.P.; Zhuo, Z.L.; Sun, Y.J.; Liang, B.; Zhao, X.T. Circulating long non-coding RNAs HULC and ZNFX1-AS1 are potential biomarkers in patients with gastric cancer. Oncol. Lett. 2018, 16, 4689–4698.
- Xin, H.; Yan, Z.; Cao, J. Long non-coding RNA ABHD11-AS1 boosts gastric cancer development by regulating miR-361-3p/PDPK1 signalling. J. Biochem. 2020, 168, 465–476.
- Wen, J.; Wang, H.; Dong, T.; Gan, P.; Fang, H.; Wu, S.; Li, J.; Zhang, Y.; Du, R.; Zhu, Q. STAT3-induced upregulation of lncRNA ABHD11-AS1 promotes tumour progression in papillary thyroid carcinoma by regulating miR-1301-3p/STAT3 axis and PI3K/AKT signalling pathway. Cell Prolif. 2019, 52, e12569.
- Zhuang, X.; Tong, H.; Ding, Y.; Wu, L.; Cai, J.; Si, Y.; Zhang, H.; Shen, M. Long noncoding RNA ABHD11-AS1 functions as a competing endogenous RNA to regulate papillary thyroid cancer progression by miR-199a-5p/SLC1A5 axis. Cell Death Dis. 2019, 10, 620.
- Hou, S.; Zhuang, Y.Y.; Lin, Q.Y.; Chen, Z.; Zhao, H.G.; Zhang, L.; Lin, C.H. Overexpression of serum lncRNA-ABHD11-AS1 as poor prognosis of patients with papillary thyroid carcinoma. Exp. Mol. Pathol. 2021, 121, 104658.
- Lu, H.; Zhu, C.; Chen, Y.; Ruan, Y.; Fan, L.; Chen, Q.; Wei, Q. LncRNA ABHD11-AS1 promotes tumor progression in papillary thyroid carcinoma by regulating EPS15L1/EGFR signaling pathway. Clin. Transl. Oncol. 2022. Online ahead of print.
- Xue, L.; Li, J.; Lin, Y.; Liu, D.; Yang, Q.; Jian, J.; Peng, J. m(6) A transferase METTL3-induced lncRNA ABHD11-AS1 promotes the Warburg effect of non-small-cell lung cancer. J. Cell Physiol. 2021, 236, 2649–2658.
- Qiao, X.; Lv, S.X.; Qiao, Y.; Li, Q.P.; Ye, B.; Wang, C.C.; Miao, L. Long noncoding RNA ABHD11-AS1 predicts the prognosis of pancreatic cancer patients and serves as a promoter by activating the PI3K-AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8630–8639.
- Liu, Y.; Feng, W.; Liu, W.; Kong, X.; Li, L.; He, J.; Wang, D.; Zhang, M.; Zhou, G.; Xu, W.; et al. Circulating lncRNA ABHD11-AS1 serves as a biomarker for early pancreatic cancer diagnosis. J. Cancer 2019, 10, 3746–3756.
- Liu, B.; Wang, W.; Sun, S.; Ding, H.; Lan, L.; Li, X.; Han, S. Knockdown of lncRNA ABHD11-AS1 Suppresses the Tumorigenesis of Pancreatic Cancer via Sponging miR-1231. OncoTargets Ther. 2020, 13, 11347–11358.
- Lei, X.; Li, L.; Duan, X. Long non-coding RNA ABHD11-AS1 promotes colorectal cancer development through regulation of miR-133a/SOX4 axis. Biosci. Rep. 2018, 38, BSR20181386.
- He, D.; Yue, Z.; Liu, L.; Fang, X.; Chen, L.; Han, H. Long noncoding RNA ABHD11-AS1 promote cells proliferation and invasion of colorectal cancer via regulating the miR-1254-WNT11 pathway. J. Cell Physiol. 2019, 234, 12070–12079.
- Luo, J.; Jiang, Y.; Wu, L.; Zhuo, D.; Zhang, S.; Jiang, X.; Sun, Y.; Huang, Y. Long non-coding RNA ABHD11-AS1 promotes colorectal cancer progression and invasion through targeting the integrin subunit alpha 5/focal adhesion kinase/phosphoinositide 3 kinase/Akt signaling pathway. Aging 2021, 13, 20179–20191.
- Wu, D.D.; Chen, X.; Sun, K.X.; Wang, L.L.; Chen, S.; Zhao, Y. Role of the lncRNA ABHD11-AS1 in the tumorigenesis and progression of epithelial ovarian cancer through targeted regulation of RhoC. Mol. Cancer 2017, 16, 138.
- Zeng, X.Y.; Jiang, X.Y.; Yong, J.H.; Xie, H.; Yuan, J.; Zeng, D.; Dou, Y.Y.; Xiao, S.S. lncRNA ABHD11-AS1, regulated by the EGFR pathway, contributes to the ovarian cancer tumorigenesis by epigenetically suppressing TIMP2. Cancer Med. 2019, 8, 7074–7085.
- Zhang, W.; Huang, X.; Shi, J. EZH2-mediated lncRNA ABHD11-AS1 promoter regulates the progression of ovarian cancer by targeting miR-133a-3p. Anticancer Drugs 2021, 32, 269–277.
- Chen, M.; Li, J.; Zhuang, C.; Cai, Z. Increased lncRNA ABHD11-AS1 represses the malignant phenotypes of bladder cancer. Oncotarget 2017, 8, 28176–28186.
- Mehrpour Layeghi, S.; Arabpour, M.; Shakoori, A.; Naghizadeh, M.M.; Mansoori, Y.; Tavakkoly Bazzaz, J.; Esmaeili, R. Expression profiles and functional prediction of long non-coding RNAs LINC01133, ZEB1-AS1 and ABHD11-AS1 in the luminal subtype of breast cancer. J. Transl. Med. 2021, 19, 364.
- Liu, Y.; Wang, L.L.; Chen, S.; Zong, Z.H.; Guan, X.; Zhao, Y. LncRNA ABHD11-AS1 promotes the development of endometrial carcinoma by targeting cyclin D1. J. Cell Mol. Med. 2018.
- Hou, S.; Zhang, X.; Yang, J. Long non-coding RNA ABHD11-AS1 facilitates the progression of cervical cancer by competitively binding to miR-330-5p and upregulating MARK2. Exp. Cell Res. 2022, 410, 112929.
- Zhu, D.; Hao, Q.; Qian, M.; Hu, Y.; Wu, F. LncRNA ABHD11-AS1 Participates in the Progression of Cervical Carcinoma by Targeting miR-1254 and Is the Key to the Diagnosis and Treatment of Cervical Carcinoma in the Future. J. Healthc. Eng. 2022, 2022, 8387458.
- Wang, L.L.; Zhang, L.; Cui, X.F. Downregulation of long noncoding RNA LINC01419 inhibits cell migration, invasion, and tumor growth and promotes autophagy via inactivation of the PI3K/Akt1/mTOR pathway in gastric cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919874651.
- Xuan, Y.; Wang, Y. Long non-coding RNA SNHG3 promotes progression of gastric cancer by regulating neighboring MED18 gene methylation. Cell Death Dis. 2019, 10, 694.
- Gao, Y.; Wang, J.W.; Ren, J.Y.; Guo, M.; Guo, C.W.; Ning, S.W.; Yu, S. Long noncoding RNAs in gastric cancer: From molecular dissection to clinical application. World J. Gastroenterol. 2020, 26, 3401–3412.
- Peng, X.; Zhang, K.; Ma, L.; Xu, J.; Chang, W. The Role of Long Non-Coding RNAs in Thyroid Cancer. Front. Oncol. 2020, 10, 941.
- Javed, Z.; Ahmed Shah, F.; Rajabi, S.; Raza, Q.; Iqbal, Z.; Ullah, M.; Ahmad, T.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; et al. LncRNAs as Potential Therapeutic Targets in Thyroid Cancer. Asian Pac. J. Cancer Prev. 2020, 21, 281–287.
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388.
- Chen, Y.; Bi, F.; An, Y.; Yang, Q. Identification of pathological grade and prognosis-associated lncRNA for ovarian cancer. J. Cell Biochem. 2019, 120, 14444–14454.
- Zhan, L.; Li, J.; Wei, B. Long non-coding RNAs in ovarian cancer. J. Exp. Clin. Cancer Res. 2018, 37, 120.
- Salamini-Montemurri, M.; Lamas-Maceiras, M.; Barreiro-Alonso, A.; Vizoso-Vazquez, A.; Rodriguez-Belmonte, E.; Quindos-Varela, M.; Cerdan, M.E. The Challenges and Opportunities of LncRNAs in Ovarian Cancer Research and Clinical Use. Cancers 2020, 12, 1020.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30.
- Wang, W.; Kandimalla, R.; Huang, H.; Zhu, L.; Li, Y.; Gao, F.; Goel, A.; Wang, X. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin. Cancer Biol. 2019, 55, 37–52.
- He, Q.; Long, J.; Yin, Y.; Li, Y.; Lei, X.; Li, Z.; Zhu, W. Emerging Roles of lncRNAs in the Formation and Progression of Colorectal Cancer. Front. Oncol. 2019, 9, 1542.
- Poursheikhani, A.; Abbaszadegan, M.R.; Kerachian, M.A. Mechanisms of long non-coding RNA function in colorectal cancer tumorigenesis. Asia Pac. J. Clin. Oncol. 2021, 17, 7–23.
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27.
- Duguang, L.; Jin, H.; Xiaowei, Q.; Peng, X.; Xiaodong, W.; Zhennan, L.; Jianjun, Q.; Jie, Y. The involvement of lncRNAs in the development and progression of pancreatic cancer. Cancer Biol. Ther. 2017, 18, 927–936.
- Lv, Y.; Huang, S. Role of non-coding RNA in pancreatic cancer. Oncol. Lett. 2019, 18, 3963–3973.
- Zhou, W.; Chen, L.; Li, C.; Huang, R.; Guo, M.; Ning, S.; Ji, J.; Guo, X.; Lou, G.; Jia, X.; et al. The multifaceted roles of long noncoding RNAs in pancreatic cancer: An update on what we know. Cancer Cell Int. 2020, 20, 41.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31.
- Mathias, C.; Zambalde, E.P.; Rask, P.; Gradia, D.F.; de Oliveira, J.C. Long non-coding RNAs differential expression in breast cancer subtypes: What do we know? Clin. Genet. 2019, 95, 558–568.
- Minotti, L.; Agnoletto, C.; Baldassari, F.; Corra, F.; Volinia, S. SNPs and Somatic Mutation on Long Non-Coding RNA: New Frontier in the Cancer Studies? High Throughput 2018, 7, 34.
- Wang, X.; Zhao, Z.; Han, X.; Zhang, Y.; Zhang, Y.; Li, F.; Li, H. Single-Nucleotide Polymorphisms Promote Dysregulation Activation by Essential Gene Mediated Bio-Molecular Interaction in Breast Cancer. Front. Oncol. 2021, 11, 791943.
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441.
- Yuan, S.; Xiang, Y.; Guo, X.; Zhang, Y.; Li, C.; Xie, W.; Wu, N.; Wu, L.; Cai, T.; Ma, X.; et al. Circulating Long Noncoding RNAs Act as Diagnostic Biomarkers in Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 537120.
- Xue, M.; Pang, H.; Li, X.; Li, H.; Pan, J.; Chen, W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016, 107, 18–27.
- Wang, J.; Ma, W.; Liu, Y. Long non-coding RNA HULC promotes bladder cancer cells proliferation but inhibits apoptosis via regulation of ZIC2 and PI3K/AKT signaling pathway. Cancer Biomark. 2017, 20, 425–434.
- Cao, X.; Xu, J.; Yue, D. LncRNA-SNHG16 predicts poor prognosis and promotes tumor proliferation through epigenetically silencing p21 in bladder cancer. Cancer Gene Ther. 2018, 25, 10–17.
- Su, G.; He, Q.; Wang, J. Clinical Values of Long Non-coding RNAs in Bladder Cancer: A Systematic Review. Front. Physiol. 2018, 9, 652.
- Rizzo, S.; Femia, M.; Buscarino, V.; Franchi, D.; Garbi, A.; Zanagnolo, V.; Del Grande, M.; Manganaro, L.; Alessi, S.; Giannitto, C.; et al. Endometrial cancer: An overview of novelties in treatment and related imaging keypoints for local staging. Cancer Imaging 2018, 18, 45.
- Liu, H.; Wan, J.; Chu, J. Long non-coding RNAs and endometrial cancer. Biomed. PharmacoTher. 2019, 119, 109396.
- Li, B.L.; Wan, X.P. The role of lncRNAs in the development of endometrial carcinoma. Oncol. Lett. 2018, 16, 3424–3429.
- Aalijahan, H.; Ghorbian, S. Long non-coding RNAs and cervical cancer. Exp. Mol. Pathol 2019, 106, 7–16.
- Shi, D.; Zhang, C.; Liu, X. Long noncoding RNAs in cervical cancer. J. Cancer Res. Ther. 2018, 14, 745–753.
- He, J.; Huang, B.; Zhang, K.; Liu, M.; Xu, T. Long non-coding RNA in cervical cancer: From biology to therapeutic opportunity. Biomed. Pharmacother. 2020, 127, 110209.
- Zhong, Q.; Lu, M.; Yuan, W.; Cui, Y.; Ouyang, H.; Fan, Y.; Wang, Z.; Wu, C.; Qiao, J.; Hang, J. Eight-lncRNA signature of cervical cancer were identified by integrating DNA methylation, copy number variation and transcriptome data. J. Transl. Med. 2021, 19, 58.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
899
Revisions:
2 times
(View History)
Update Date:
14 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




