Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Vazquez Cabello | + 2387 word(s) | 2387 | 2022-03-03 04:23:45 | | | |
| 2 | Catherine Yang | Meta information modification | 2387 | 2022-03-14 02:53:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cabello, J. Dental Implantology. Encyclopedia. Available online: https://encyclopedia.pub/entry/20493 (accessed on 08 February 2026).
Cabello J. Dental Implantology. Encyclopedia. Available at: https://encyclopedia.pub/entry/20493. Accessed February 08, 2026.
Cabello, Juan. "Dental Implantology" Encyclopedia, https://encyclopedia.pub/entry/20493 (accessed February 08, 2026).
Cabello, J. (2022, March 11). Dental Implantology. In Encyclopedia. https://encyclopedia.pub/entry/20493
Cabello, Juan. "Dental Implantology." Encyclopedia. Web. 11 March, 2022.
Copy Citation
Dental implants are most similar to natural teeth in their mastication and aesthetics; they are also biocompatible and require biocompatibility, masticatory feature, and aesthetic follow-up. The American Association of Oral and Maxillofacial Surgeons estimated that two million implants are placed per year worldwide. The longevity of the population and the demand for cosmetic dentistry have led to their increasing use.
dental implants
osseointegration
antimicrobial activity
biomaterials
therapeutics agents
1. Historical Overview of Implantology
A historical overview starting in Egypt is presented in Scheme 1. The need to replace natural teeth can be traced back to 2500 BC Egypt where seashells were anchored into human jawbone and stabilized with the use of gold wire. Famous archeological remains have shown that the civilizations in South and North America and regions of the Middle Asia and Mediterranean created artificial teeth using carved stone, shells, bones and gold more than 2000 years ago [1]. Moreover, ruins in Honduras had a fragment of a mandible with three shells carved into tooth shapes, confirming that the Mayan civilization had the earliest known examples of artificial substitutes, dating from about 600 AD [1]. Staple, subperiosteal, and blade vent implants represent the most successful designs of prosthodontic reconstruction that can be found in the early literature, made of noble or base metals. These were but affected by mechanical and biological failures [1][2].
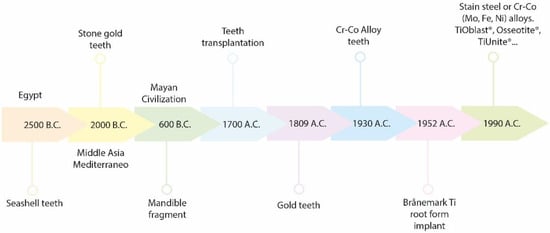
Scheme 1. Graphical historical overview.
In the 1700s, Dr. Hunter mentioned the possibility of relocating teeth from one human to another. During this time in Europe, teeth were extracted from the disadvantaged or from cadavers for allotransplantation [3]. In 1809, J. Maggiolo placed a gold implant into a tooth socket after extraction, but unfortunately an inflammation lead to failure [2][3]. Silver capsules, corrugated porcelain, and iridium tubes were some of the materials employed for dental implants [4].
In the 1930s, Dr. Alvin Strock became known for the successful treatment of shipboard periodontal issues with antibiotics, by provided anchorage and support for restoring teeth. He and his brother used Vitallium (a chromium-cobalt alloy) which at the time was considered a biocompatible material. Implant discovery continually increased during the 1900s and particularly during World War II [2][3]. However, the most important discovery occurred in 1952, when Dr. Per-Ingvar Brånemark, an orthopedic surgeon studying the bone-remodeling process in rabbit femurs, noticed that osseous matter could regenerate and attach to titanium [5]. He defined this phenomenon as osseointegration and used this concept for implant dentistry. In 1978, Dr. Brånemark presented a two-stage threaded titanium root-form implant that was fixed in his patients in 1965 and lasted for 40 years [6][7]. Brånemark Implants® have had a great impact on today’s dentistry [5][8][9]. Many brand devices have been developed (e.g., TiOblast®, Osseotite®, Steri-Oss Etched®, TiUnite®, ITI-TPS®, Laser-Lok®, SLActive®) [10], in order to improve the effectiveness of the dental implants and their rate of success. Besides, recent developments including a variety of surface modifications for dental implantology have been made to cope with the fact of being biologically inert, such as acid etching/grit blasting, hydrogen peroxide or acidic treatment, alternative nitride, hydroxyapatite or metal-based coatings among others [11][12][13][14][15][16].
2. Biomaterials Composition
The most important properties of an implant biomaterial are: the modulus of elasticity (e.g., 18 GPa for cortical bone); tensile, compressive and shear strength; yield and fatigue strength; ductility (e.g., 8% is needed for manufacturing requirements); hardness and toughness; surface tension and surface energy, and finally, surface roughness [17].
Metals, ceramics and polymers have represented the materials of choice for dental products. Polymeric materials have only been used for fabricating shock-absorbing components because of their low strength. Among ceramics, hydroxyapatite (HA) is the most used because of the great biocompatibility and capability to help the osteoblast activity due to its similar composition to the mineral structure of the bone. However, its low mechanical strength makes it only suitable as a coating material (i.e., plasma-sprayed coatings) [1][18], as discussed later. Something similar happens with bioactive glasses (BGs), a special type of glasses that induce the formation of HA when get in contact with body fluids [19].
Among metals, titanium and its alloys, Ti6Al4V and Ti-β, have been the most commonly used materials because they can osseointegrate. However, all these metals have and materials still have higher modulus compared to the cortical bone (20–25 GPa for cortical bone and 110 GPa for commercially pure titanium and Ti-β). Commercially pure (c.p.) Ti was used in the first Ti implant ever applied, the Brånemark Implants®, but they often fail due to high stiffness. Current dental implants have been manufactured to reduce their Young’s modulus. Trueba et al. [20] evaluated the mechanical behavior of superficially modified porous c.p. titanium dental implants fabricated by conventional powder-metallurgy and space-holder techniques. A novel, feasible and repetitive protocol of micro-milling of the implant thread (before powder metallurgy sintering), as well as surface modification treatments (after sintering), have also been implemented. These techniques add porosity and surface roughness to the stiffness and yield strength of implants. Macro-pores concentrate stress locally, and may act as a barrier to the propagation of micro-cracks. Higher rugosity was observed for virgin implants obtained with spacer particles. Concerning the superficial modification of implants, while BG 1393 was the most effective coating due to its greater infiltration and adhesion capacity, chemical etching could improve osteoblast adhesion because it modifies the roughness of the implant surface. Therefore, a new reliable protocol was developed and evaluated to fabricate titanium implants with improved biomechanical and biofunctional response of the interface with the host.
Lascano et al. [21] investigated the use of a Ti-β alloys, composed of Ti-Nb-Ta-xMn (x: 2, 4, and 6 wt%) and with a lower elastic modulus compared to the c.p. titanium. They included controlled porosity in the implants to reduce more their Young’s modulus, since it was still high (ranging from 50 to 60 GPa). In addition, they include a graphene layer onto the substrates’ surface to enhance their biocompatibility and cell adhesion. The alloy containing 4 wt% Mn favors the presence of the Ti-β phase, and Young’s modulus (8–9.3 MPa) closer to the trabecular bone. Furthermore, Ti6Al4V was developed by the aerospace industry but was applied to biomedical systems for its strength, corrosion resistance, and biocompatibility [22]. These implants have been fabricated in a variety of shapes, such as cylindrical, cone, hollow and screw shapes, and various diameters. Additionally, Ti6Al4V has also been used in orthodontic implants [23], which are temporary anchors. However, some authors reported its long-term toxicity with human osteoblastic and fibroblastic cells [24]. New research approaches tend to avoid the use of elements in the titanium alloys with the potential to cause tissue damage, such as vanadium [25].
Alternatively, zirconium and gold have also been used for the same purpose but, unfortunately, they have demonstrated poor bone-to-implant adhesion [1]. Other materials that have been used for the applications of titanium-based materials in dental implants are based on stainless steel, Co-Cr, Co-Cr-Mo-Ni-Fe or other metal combinations alloys where issues such as cytotoxicity, mechanical, chemical, electrochemical, or biological properties were addressed [26][27][28][29].
3. OMICS in Dental Implantology
Mass spectrometry has been considered the most comprehensive strategy in “OMICS” [30], which refers to scientific fields ending with –omics such as genomics, transcriptomics, proteomics, or metabolomics [31]. Besides, the development of analytical methods to characterize polymer sequences has represented a challenge in current polymer science, due to efforts to tailor the properties of biomaterials for medical purposes, among others [30]. In addition, OMICS technologies have gained momentum in uncovering molecules and signaling pathways related to bone formation and osseointegration, in order to provide personalized treatment in dentistry and implantology [32]. The bioconjugate BMP2-(PEO-HA)2, composed of a dendron with two monodisperse poly(ethylene oxide) branches functionalized with a HA binding peptide, and a focal point substituted with a bone-growth stimulating peptide (BMP2), was successfully characterized by MS methods. With this aim, the authors took advantages of various techniques including matrix-assisted laser desorption ionization (MALDI), electrospray ionization (ESI), tandem mass spectrometry (MS/MS), and ion mobility mass spectrometry (IM-MS) [33].
4. Detemination of Biomarkers for the Evaluation of Biomaterial Effect and Clinic Outcomes
The National Institutes of Health Biomarkers Definitions Working Group defined biomarkers as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [34]. The proper evaluation of biomarkers in an oral cavity can represent a useful tool for evaluating the activity-therapeutic effect of new biomaterial used for dental-implant coating.
It was reported that various biomarkers were explored for the assessment of bone regeneration and healing around biomaterials. Many biomaterials were tested (e.g., titanium dental implants with surface modifications, and scaffolds loaded with drugs, osteogenic cells, or biological factors, etc.) in different trials. Bone regeneration, resulting in a biomarkers increase was reported in all these studies [35]. In particular, the rate of success of titanium dental implants can be monitored by the assessment of markers of osseointegration such as OCN (osteocalcin) and COL-1 (collagen type I) in the post-implant placement period [36][37][38]. In another study where the titanium dental implant was coated with a fluoride-material, the expression of OCN, RUNX-2 (Runt-related transcription factor 2), and COL-1 was associated with the positive effect of fluoride upon bone formation [39]. Likewise, a simvastatin coating proposed for in vivo trial, demonstrated the promotion of angiogenesis and osseointegration with the increased expression of VEGF and ALP markers [35][38].
With a similar aim, Kumar et al. evaluated the response of peri-implant connective tissue to titanium and zirconia abutments via the evaluation of MMP8 assayed by ELISA [40]. In fact, during the last decade the number of studies examining biomarkers in oral fluids as diagnostic tool for periodontal disease has represented a new trend. Since the 1920s, there have been many changes in the classification of periodontal diseases in order to obtain a proper diagnosis for the further treatment [41]. Saliva, gingival crevicular fluid, peri-implant sulcular fluid, and mouth rinse remnant have been considered non-invasive and biomarkers and high-content sources to reflect periodontal health. Furthermore, MMP8 (matrix metalloproteinase), MMP9, MMP13, MMP14, IL1β (interleukin), IL10, TIMP1 (metallopeptidase inhibitor), elastase, cathepsin G, cathepsin B, trypsin-like enzyme, sialidase, VEGF, RANKL (receptor activator of nuclear factor kappa beta), OPG (osteoprotegerin), TGF-β1 (tumor growth factor), nicotine, cotinine, cystatin C, MPO (myeloperoxidase), PAF (platelet-activating factor), and lactoferrin represent the common markers that researchers assayed over the last decade. On the other hand, ELISA, immunofluorometric assay (IFMA), colorimetric assay and chromatography-tandem MS have been selected for their determination, as previously reported by Gul et al. [42].
5. Analytical Methods for the Characterization of Micro- and Nanosized Implant-Related Particles and Metals in Inflamed Peri-Implants Ttissues
An emerging concept about etiopathogenesis of peri-implantitis has explored the degradation of the implant surface with the consequent particle release as an inflammation catalyst mechanism [43]. This phenomenon has been deeply studied for orthopedic implants, whereby the deterioration of metal biomaterials was demonstrated to accumulate exogenous particles in the peri-implant milieu [44]. Likewise, some authors reported that in dental devices, damage to the surface can be caused by both surface instrumentation and dynamic interactions in the implant–abutment interface; however, for titanium devices this can happen, independently from wear corrosion, following a no pre-clinical studied pathway [43][45]. In addition, differences among cell compositions were noticed in case of inflammation in peri-implantitis and periodontitis, with higher macrophage polarization in the first case [46][47]. However, other histological studies, conducted in presence of inflammation around titanium and ceramic implants, demonstrated that implantitis issues depend on a patient-level rather than a material level [48].
Taking into account that implant-surface degradation may result in the release of titanium ions, as well as particles, which leads to peri-implant inflammation and clinical failure, and it was hypothesized that release can occur in cases for which titanium implants are exposed to corrosion. This study demonstrated, for the first time, via synchrotron X-ray fluorescence mapping, a scattered and heterogeneous distribution of titanium in inflamed tissues [49]. Besides, the release of particles from Ti and ZrO2 implants has been observed in pigs after 12 weeks of implant placement [50]. Thus, to explore size, distribution, and the chemical speciation of substances that can be released from dental implants, Nelson et al. proposed a synchrotron-based characterization of micro- and nanosized implant-related particles arising from Ti and ZrO2 dental implants in patients with peri-implantitis. For this purpose, synchrotron μ-XRF, nano-XRF and μ-XANES were employed as an analytical tool. They also tried to explain the mechanism behind particle release from ceramic implants as a consequence of stress that induces the local transformation of zirconia from a tetragonal to a monoclinic crystalline phase, susceptible to microlesions. The study concluded by reporting on the presence of Ti particles, with variable speciation, in all tissue sections implanted with Ti devices, as well as ceramic products were identified in five out of eight tissue samples around ceramic implants [43].
Because of the possibility of degradation of the implant surface within metals release, high performance liquid chromatography coupled with inductively coupled plasma mass spectrometry (HPLC-ICP-MS) was used for the study of distribution and chemical speciation of metals [51][52]. Balcaen et al. developed a reliable method for the determination of trace levels (limit of detection down to 3 ng L−1) of titanium in human serum, based on the use of ICP-MS/MS. In fact, it has been reported that the presence of implants in the human body can result in the high presence of titanium in serum [53]. Other researchers provided HPLC-ICP-MS (Figure 1) as a method for the estimation of total chromium and Cr(III) and Cr(VI) species released from metal implants into whole blood and joint effusion. The results showed higher chromium levels in joint effusion samples obtained from implanted patients [51].
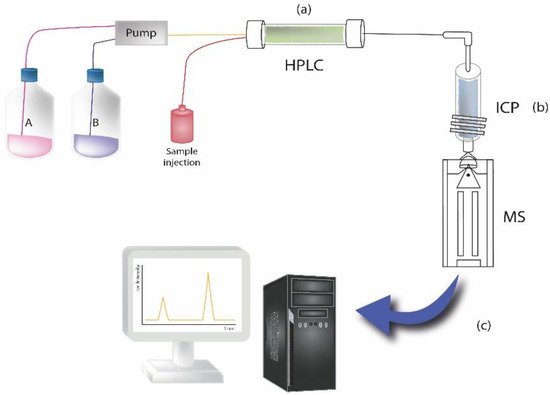
Figure 1. The illustration represents an example of sample analysis carried out by HPLC coupled to inductively coupled plasma mass spectrometry to measure elements at trace levels in samples. Briefly, the instrument consists of the following components: (a) HPLC system, where the chromatographic separation of the sample takes place; (b) ICP system, where plasma is ionized by inductively heating the gas (i.e., Argon) with an electromagnetic coil; it represents the ionization source. (c) MS detector system, for detecting the formed ions.
Laser ablation-inductively-coupled-plasma mass spectrometry (LA-ICP-MS) was employed as an analytical procedure for the determination of elements derived from titanium implants and physiological elements in soft tissues. Results showed a quantitative mapping of Ti and Al released from dental implant and Mg, Ca, Fe, Zn, Cu, Mn as physiological elements in oral mucosa. Moreover, authors were able to obtain two-dimensional maps of distribution of elements in tested samples which confirmed the release of Ti and Al derived from implants [54].
References
- Gaviria, L.; Salcido, J.P.; Guda, T.; Ong, J.L. Current Trends in Dental Implants. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 50–60.
- Abraham, C.M. A Brief Historical Perspective on Dental Implants, Their Surface Coatings and Treatments. Open Dent. J. 2014, 8, 50–55.
- Ring, M.E. Dentistry: An Illustrated History; Abrams: New York, NY, USA, 1985; ISBN 978-0-8109-1100-0.
- Lee, J.-H.; Frias, V.; Lee, K.-W.; Wright, R.F. Effect of Implant Size and Shape on Implant Success Rates: A Literature Review. J. Prosthet. Dent. 2005, 94, 377–381.
- Brånemark, P.I. Osseointegration and Its Experimental Background. J. Prosthet. Dent. 1983, 50, 399–410.
- Linkow, L.I.; Dorfman, J.D. Implantology in Dentistry. A Brief Historical Perspective. N. Y. State Dent. J. 1991, 57, 31–35.
- Goldberg, N.I.; Gershkoff, A. The Implant Lower Denture. Dent. Dig. 1949, 55, 490–494.
- Brånemark, P.-I. (Ed.) Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Quintessence Books; 1. Reprinting; Quintessence: Chicago, IL, USA, 1986; ISBN 978-0-86715-129-9.
- Donati, M.; Ekestubbe, A.; Lindhe, J.; Wennström, J.L. Marginal Bone Loss at Implants with Different Surface Characteristics—A 20-Year Follow-up of a Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2018, 29, 480–487.
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982.
- Shao, L.; Du, Y.; Dai, K.; Wu, H.; Wang, Q.; Liu, J.; Tang, Y.; Wang, L. β-Ti Alloys for Orthopedic and Dental Applications: A Review of Progress on Improvement of Properties through Surface Modification. Coatings 2021, 11, 1446.
- Rahimi, S.; Tengku Mohd Ariff, T.; Affendi, N.; Ahmad, R. Surface Modifications of Dental Implant and Its Clinical Performance: A Review. Compend. Oral Sci. 2022, 9, 52.
- Zhu, G.; Wang, G.; Li, J.J. Advances in Implant Surface Modifications to Improve Osseointegration. Mater. Adv. 2021, 2, 6901–6927.
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the Impact of Titanium Surface Modifications on Osteogenic Processes in Vitro and in Vivo. Bioeng. Transl. Med. 2022, 7, e10239.
- Kandavalli, S.R.; Wang, Q.; Ebrahimi, M.; Gode, C.; Djavanroodi, F.; Attarilar, S.; Liu, S. A Brief Review on the Evolution of Metallic Dental Implants: History, Design, and Application. Front. Mater. 2021, 8, 646383.
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface Modification Techniques of Titanium and Titanium Alloys for Biomedical Dental Applications: A Review. Mater. Today Proc. 2021, 39, 84–90.
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338.
- Triplett, R.G.; Frohberg, U.; Sykaras, N.; Woody, R.D. Implant Materials, Design, and Surface Topographies: Their Influence on Osseointegration of Dental Implants. J. Long. Term Eff. Med. Implant. 2003, 13, 18.
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978.
- Trueba, P.; Navarro, C.; Rodríguez-Ortiz, J.A.; Beltrán, A.M.; García-García, F.J.; Torres, Y. Fabrication and Characterization of Superficially Modified Porous Dental Implants. Surf. Coat. Technol. 2021, 408, 126796.
- Lascano, S.; Chávez-Vásconez, R.; Muñoz-Rojas, D.; Aristizabal, J.; Arce, B.; Parra, C.; Acevedo, C.; Orellana, N.; Reyes-Valenzuela, M.; Gotor, F.J.; et al. Graphene-Coated Ti-Nb-Ta-Mn Foams: A Promising Approach towards a Suitable Biomaterial for Bone Replacement. Surf. Coat. Technol. 2020, 401, 126250.
- Toledano-Serrabona, J.; Sánchez-Garcés, M.Á.; Gay-Escoda, C.; Valmaseda-Castellón, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical Properties and Corrosion Behavior of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part II. Materials 2021, 14, 6519.
- Bammidi, R.; Prasad, K.S. Ti-6AL-4V as Dental Implant. EAS J. Dent. Oral. Med. 2020, 2, 14–18.
- Toledano-Serrabona, J.; Gil, F.J.; Camps-Font, O.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Physicochemical and Biological Characterization of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part I. Materials 2021, 14, 6507.
- W Nicholson, J. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116.
- Cordeiro, J.M.; Barão, V.A.R. Is There Scientific Evidence Favoring the Substitution of Commercially Pure Titanium with Titanium Alloys for the Manufacture of Dental Implants? Mater. Sci. Eng. C 2017, 71, 1201–1215.
- Oshida, Y.; Tominaga, T. Chapter 11. Properties in Biological Environment. In Nickel-Titanium Materials; De Gruyter: Berlin, Germany, 2020; pp. 480–572. ISBN 978-3-11-066611-3.
- Kassapidou, M.; Hjalmarsson, L.; Johansson, C.B.; Hammarström Johansson, P.; Morisbak, E.; Wennerberg, A.; Franke Stenport, V. Cobalt–Chromium Alloys Fabricated with Four Different Techniques: Ion Release, Toxicity of Released Elements and Surface Roughness. Dent. Mater. 2020, 36, e352–e363.
- Fathi, M.H.; Salehi, M.; Saatchi, A.; Mortazavi, V.; Moosavi, S.B. In Vitro Corrosion Behavior of Bioceramic, Metallic, and Bioceramic–Metallic Coated Stainless Steel Dental Implants. Dent. Mater. 2003, 19, 188–198.
- Altuntaş, E.; Schubert, U.S. “Polymeromics”: Mass Spectrometry Based Strategies in Polymer Science toward Complete Sequencing Approaches: A Review. Anal. Chim. Acta 2014, 808, 56–69.
- Vailati-Riboni, M.; Palombo, V.; Loor, J.J. What Are Omics Sciences? In Periparturient Diseases of Dairy Cows; Springer: Cham, Switzerland, 2017; pp. 1–7.
- Calciolari, E.; Donos, N. The Use of Omics Profiling to Improve Outcomes of Bone Regeneration and Osseointegration. How Far Are We from Personalized Medicine in Dentistry? J. Proteom. 2018, 188, 85–96.
- Alalwiat, A.; Tang, W.; Gerişlioğlu, S.; Becker, M.L.; Wesdemiotis, C. Mass Spectrometry and Ion Mobility Characterization of Bioactive Peptide–Synthetic Polymer Conjugates. Anal. Chem. 2017, 89, 1170–1177.
- Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95.
- Albeshri, S.; Alblaihess, A.; Niazy, A.A.; Ramalingam, S.; Sundar, C.; Alghamdi, H.S. Biomarkers as Independent Predictors of Bone Regeneration around Biomaterials: A Systematic Review of Literature. J. Contemp. Dent. Pract. 2018, 19, 605–618.
- Colombo, J.S.; Balani, D.; Sloan, A.J.; Crean, S.J.; Okazaki, J.; Waddington, R.J. Delayed Osteoblast Differentiation and Altered Inflammatory Response around Implants Placed in Incisor Sockets of Type 2 Diabetic Rats: Delayed Osteoblast Differentiation in Diabetic Bone Healing. Clin. Oral Implant. Res. 2011, 22, 578–586.
- Monjo, M.; Ramis, J.M.; Rønold, H.J.; Taxt-Lamolle, S.F.; Ellingsen, J.E.; Lyngstadaas, S.P. Correlation between Molecular Signals and Bone Bonding to Titanium Implants. Clin. Oral Implant. Res. 2012, 24.
- Song, C. Single-Dose Local Simvastatin Injection Improves Implant Fixation via Increased Angiogenesis and Bone Formation in an Ovariectomized Rat Model. Med. Sci. Monit. 2015, 21, 1428–1439.
- Monjo, M.; Lamolle, S.F.; Lyngstadaas, S.P.; Rønold, H.J.; Ellingsen, J.E. In Vivo Expression of Osteogenic Markers and Bone Mineral Density at the Surface of Fluoride-Modified Titanium Implants. Biomaterials 2008, 29, 3771–3780.
- Kumar, Y.; Jain, V.; Chauhan, S.S.; Bharate, V.; Koli, D.; Kumar, M. Influence of Different Forms and Materials (Zirconia or Titanium) of Abutments in Peri-Implant Soft-Tissue Healing Using Matrix Metalloproteinase-8: A Randomized Pilot Study. J. Prosthet. Dent. 2017, 118, 475–480.
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions–Introduction and Key Changes from the 1999 Classification; Wiley Online Library: Hoboken, NJ, USA, 2018; ISBN 0022-3492.
- Gul, S.S.; Abdulkareem, A.A.; Sha, A.M.; Rawlinson, A. Diagnostic Accuracy of Oral Fluids Biomarker Profile to Determine the Current and Future Status of Periodontal and Peri-Implant Diseases. Diagnostics 2020, 10, 838.
- Nelson, K.; Hesse, B.; Addison, O.; Morrell, A.P.; Gross, C.; Lagrange, A.; Suárez, V.I.; Kohal, R.; Fretwurst, T. Distribution and Chemical Speciation of Exogenous Micro- and Nanoparticles in Inflamed Soft Tissue Adjacent to Titanium and Ceramic Dental Implants. Anal. Chem. 2020, 92, 14432–14443.
- Schoon, J.; Hesse, B.; Rakow, A.; Ort, M.J.; Lagrange, A.; Jacobi, D.; Winter, A.; Huesker, K.; Reinke, S.; Cotte, M.; et al. Metal-Specific Biomaterial Accumulation in Human Peri-Implant Bone and Bone Marrow. Adv. Sci. 2020, 7, 2000412.
- Delgado-Ruiz, R.; Romanos, G. Potential Causes of Titanium Particle and Ion Release in Implant Dentistry: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 3585.
- Carcuac, O.; Berglundh, T. Composition of Human Peri-Implantitis and Periodontitis Lesions. J. Dent. Res. 2014, 93, 1083–1088.
- Garaicoa-Pazmino, C.; Fretwurst, T.; Squarize, C.H.; Berglundh, T.; Giannobile, W.V.; Larsson, L.; Castilho, R.M. Characterization of Macrophage Polarization in Periodontal Disease. J. Clin. Periodontol. 2019, 46, 830–839.
- Fretwurst, T.; Müller, J.; Larsson, L.; Bronsert, P.; Hazard, D.; Castilho, R.M.; Kohal, R.; Nelson, K.; Iglhaut, G. Immunohistological Composition of Peri-implantitis Affected Tissue around Ceramic Implants—A Pilot Study. J. Periodontol. 2021, 92, 571–579.
- Addison, O.; Davenport, A.J.; Newport, R.J.; Kalra, S.; Monir, M.; Mosselmans, J.F.W.; Proops, D.; Martin, R.A. Do ‘Passive’ Medical Titanium Surfaces Deteriorate in Service in the Absence of Wear? J. R. Soc. Interface 2012, 9, 3161–3164.
- He, X.; Reichl, F.-X.; Milz, S.; Michalke, B.; Wu, X.; Sprecher, C.M.; Yang, Y.; Gahlert, M.; Röhling, S.; Kniha, H.; et al. Titanium and Zirconium Release from Titanium- and Zirconia Implants in Mini Pig Maxillae and Their Toxicity In Vitro. Dent. Mater. 2020, 36, 402–412.
- Pechancová, R.; Pluháček, T.; Gallo, J.; Milde, D. Study of Chromium Species Release from Metal Implants in Blood and Joint Effusion: Utilization of HPLC-ICP-MS. Talanta 2018, 185, 370–377.
- Balcaen, L.; Bolea-Fernandez, E.; Resano, M.; Vanhaecke, F. Accurate Determination of Ultra-Trace Levels of Ti in Blood Serum Using ICP-MS/MS. Anal. Chim. Acta 2014, 809, 1–8.
- Sarmiento-González, A.; Encinar, J.R.; Marchante-Gayón, J.M.; Sanz-Medel, A. Titanium Levels in the Organs and Blood of Rats with a Titanium Implant, in the Absence of Wear, as Determined by Double-Focusing ICP-MS. Anal. Bioanal. Chem. 2009, 393, 335–343.
- Sajnóg, A.; Hanć, A.; Koczorowski, R.; Barałkiewicz, D. New Procedure of Quantitative Mapping of Ti and Al Released from Dental Implant and Mg, Ca, Fe, Zn, Cu, Mn as Physiological Elements in Oral Mucosa by LA-ICP-MS. Talanta 2017, 175, 370–381.
More
Information
Subjects:
Materials Science, Biomaterials
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




