Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Biagio Todaro | + 343 word(s) | 343 | 2022-03-04 08:53:13 | | | |
| 2 | Rita Xu | + 4353 word(s) | 4696 | 2022-03-14 02:48:01 | | | | |
| 3 | Rita Xu | + 4353 word(s) | 4696 | 2022-03-14 02:48:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Todaro, B. Pioglitazone-Loaded PLGA Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/20492 (accessed on 08 February 2026).
Todaro B. Pioglitazone-Loaded PLGA Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/20492. Accessed February 08, 2026.
Todaro, Biagio. "Pioglitazone-Loaded PLGA Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/20492 (accessed February 08, 2026).
Todaro, B. (2022, March 11). Pioglitazone-Loaded PLGA Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/20492
Todaro, Biagio. "Pioglitazone-Loaded PLGA Nanoparticles." Encyclopedia. Web. 11 March, 2022.
Copy Citation
Albeit, the relationship between diabetes drugs and the progression of atherosclerosis is still elusive, Pioglitazone (PGZ; 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]–1,3- thiazolidine-2,4-diona), one of the most frequently prescribed anti-diabetic medication in the United States, slows the progression of atherosclerosis.
pioglitazone
PLGA
polymeric nanoparticles synthesis
nanoprecipitation
single emulsification-solvent evaporation
encapsulation efficiency
drug loading
drug release kinetics
1. Introduction
Atherosclerosis is a heavy condition characterized by progressive inflammation and slowly calcifying lesions in the intima and inner media of the arterial wall due to plaque formation [1]. The incidence of this disease is rising worldwide. Several millions of people were affected with atherosclerosis in 2021, and its burden is continuously rising, which may lead to an enormous effect on both the world economy and manpower [2]. Furthermore, atherosclerotic lesions are worsened in type-2 diabetes. Recent studies have reported that people with diabetes are more likely to have a carotid plaque with calcification and lipid-rich necrotic cores than people without diabetes [3][4].
Albeit, the relationship between diabetes drugs and the progression of atherosclerosis is still elusive, Pioglitazone (PGZ; 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]–1,3- thiazolidine-2,4-diona), one of the most frequently prescribed anti-diabetic medication in the United States, slows the progression of atherosclerosis [5]. PGZ is a slightly hydrophobic small molecule (logP = 2.3; experimental value from Human Metabolome Database) commonly used in treatment, or progression control, of type 2 diabetes. This drug acts by principally stimulating the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ), increasing, therefore, the sensitivity of peripheral tissues to insulin, reducing gluconeogenesis resistance in the liver and finally inhibiting macrophage activation and atherosclerotic plaque ruptures [6][7]. However, the application of PGZ is seriously limited due to its low and pH-dependent solubility, low half-life in plasma due to rapid liver metabolism, dose-dependent systemic side effects and nonspecific drug delivery [8]. To overcome this problem, suitable nanocarriers, such as nanoparticles (NPs), can improve PGZ therapeutic efficacy on PPAR-γ and reduce its side effects [9][10].
Nanoparticles are nanosystems in which a synthetic or natural polymeric membrane delimits a cavity that incorporates the active substance (nanocapsules), or in which the active substance is uniformly dispersed (nanospheres) [11]. In the broad sense of the term, NPs drug delivery approach relies upon the possibility of carrying active molecules mostly to the diseased tissue of interest and with a higher intracellular uptake than free drugs [12]. This elicits not only a significant drug protection from systemic degradations, but also a greater safeguard of healthy tissues [13]. In nanomedicine, several types of NPs have been widely investigated [14][15]. Various nanoscale colloidal carriers, such as carbon nanotubes, dendrimers, inorganic nanoparticles, lipid solid nanoparticles, liposomes, hydrogel nanoparticles and polymeric micelles, were developed and addressed to different therapeutic targets. In polymeric NPs, the polymer is often composed of natural or synthetic monomers; examples of polymers are polyamides, polyanhydrides, polycaprolactone (PLC), polyorthoesters and polyesters, including polylactic acid (PLA), polyglycolic acid (PGA), and poly(lactic-co-glycolic acid) (PLGA) [16][17]. Recent research on PLGA-based NPs for drug delivery is based on the field’s increasing understanding of PLGA properties and procedures of chemical modification, which are applied to the optimization of nanoparticle drug loading and release features. The polymeric NPs degradation’s kinetics are regulated by several factors: (i) the polymer–water interaction, since the more hydrophilic the material, the faster the rate of decomposition; (ii) the polymer crystallinity: the lower the material crystallinity, the faster the degradation; (iii) temperature, since it drives the kinetics of the reaction; (iv) the presence of heteroatoms and/or hydrophilic groups, which makes degradation easier; (v) polymer chain branching and use of initiators in synthesis; (vi) polymer concentration, pH, and salt concentration; (vii) in the case of PLGA, GA/LA ratio: it has been shown that optimal stability of the polymer in biofluids is obtained with a ratio of 50/50 LA/GA [18]. PLGA is generally decomposed by hydrolysis, through which oligomers are decomposed into their biocompatible monomers; then, lactic acid (LA) is disposed through the kidneys, like the one produced during intense physical activity in the muscles, whilst glycolic acid (GA) is transformed into pyruvate, which enters into the Krebs cycle [19]. Thus, PLGA is a Food and Drugs Administration (FDA) and European Medicine Agency (EMA)-approved material and is particularly appropriate for drug delivery, with its low toxicity, biocompatibility, biodegradability, and tunable physical properties [20][21].
Based on these advantages, some research was focused on the preparation of PGZ-loaded PLGA nanoparticles [22][23][24][25]. The influence of formulation parameters using different techniques, such as emulsion solvent evaporation/extraction, salting-out, nanoprecipitation, membrane emulsification, microfluidic technology, and flow focusing, was reported [26][27].
In this regard, the single emulsification-solvent evaporation (A) or nanoprecipitation (B) methods were suggested as the best formulation methods for encapsulating hydrophobic drugs. In the single emulsification-solvent evaporation technique, first described by Vanderhoff et al. in 1979 [28], the nanoparticles are formed in two steps. A polymer solution is first prepared in a water-immiscible volatile organic solvent. A colloidal suspension of micelles is then formed by adding an emulsifier aqueous solution and next vigorously mixing the two phases through an ultrasonic homogenizer; the nanoparticle suspension is finally obtained by evaporation of the solvent. Lipid stabilizer, like 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine (DSPE), are usually included in this method (Figure 1A) [29]. Recently, Shi et al. reported that the functionalization of a part of these lipids with Poly(ethylene glycol) (PEG) plays an important role in the biophysical and chemical properties (e.g., final size and stability) of nanoparticles [30]. Moreover, Takayama et al. reported that the phosphate group of DSPE-PEG is involved in the intermolecular interaction of the nanoparticles, notably affecting the physical properties and structure of the particles along with improving the bioavailability by prolonging the circulation time in the body [31]. On the other hand, the nanoprecipitation technique, firstly described by Fessi et al. in 1989 [32], is based on dropping a volatile water-miscible organic solvent containing the polymer in an aqueous solution. A colloidal nanoparticles suspension is formed under slow-stirring (Figure 1B). Therefore, unlike the first technique, in which a strong surfactant, such as DSPE-PEG, is necessary to stabilize the emulsion, a strong surfactant is not necessary in the case of nanoprecipitation, and the formed suspension can be stabilized by a mild amphiphilic compound, such as PVA. This method is usually simple, rapid, and produces particles with low PDI values [33]. Once the colloidal nanosuspension is synthetized, both synthetic strategies require a purification step (Figure 1C), usually followed by characterization, e.g., by Dynamic Light Scattering (DLS) and/or High-performance liquid chromatography (HPLC) (Figure 1D).
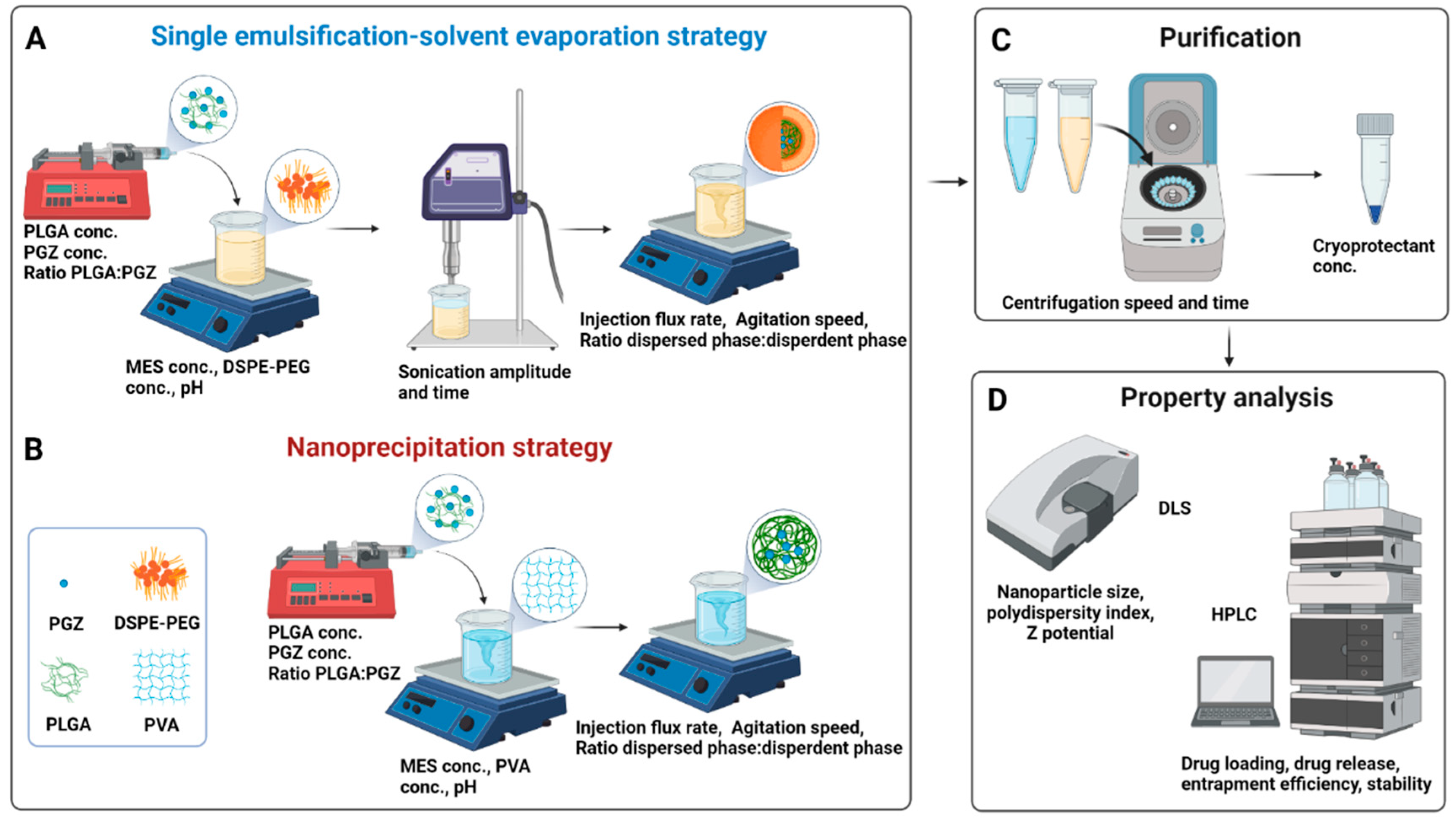
Figure 1. Synthetic workflow for the Pioglitazone-loaded PLGA nanoparticles preparation via single emulsification-solvent evaporation (A) or nanoprecipitation (B) techniques. A bubble-free non-water miscible (for A) or water-miscible (for B) organic solution, containing the drug and the polymer, was loaded into a syringe and injected within an aqueous solution (buffer). Nanoparticles formation needs a high shear force in the emulsification approach, while it is spontaneous in the nanoprecipitation approach thanks to phase separation. The organic solvent is then evaporated, and the nanoparticles are washed by centrifugation cycles and resuspended in a cryoprotectant solution for storage at low temperatures (C). Parameters that can be tuned are indicated within the panels (A–C). Physicochemical properties of nanoparticles are finally investigated through DLS and HPLC (D). Abbreviation: conc., concentration; PGZ, Pioglitazone; PLGA, Poly(D,L-lactic-co-glycolic acid); MES, 4-Morpholineethanesulfonic acid; PVA, polyvinyl alcohol; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] Carboxylic Acid; DLS, Dynamic Light Scattering; HPLC, High-performance liquid chromatography. Created with BioRender.com.
Over the last few decades, both single emulsification-solvent evaporation (A) or nanoprecipitation (B) methods were employed to create water-stable PLGA nanospheres for pioglitazone delivery against different disorders. For instance, Kanemaru M. et al. synthetized PLGA nanoparticles via the single emulsion-solvent evaporation method for local delivery of pioglitazone to attenuate skin fibrosis in model mice; with the same synthetic methodology, Woo et al. encapsulated pioglitazone within PLGA nanospheres for the treatment of Type 2 diabetes and, similarly, Laddha U. D. et al. prepared PGZ-PLGA nanoparticles for the treatment of diabetic retinopathy [34][35][36]. Conversely, other groups selected the nanoprecipitation method as ideal to develop PGZ-NPs. Lewis D. R. et al. developed pioglitazone-loaded nanoparticles via nanoprecipitation to inhibit macrophage activation and decreasing atherosclerotic plaque rupture; Silva-Abreu M. et al. showed that PGZ-NPs obtained by the solvent displacement (or nanoprecipitation) technique have in-vivo anti-inflammatory efficacy for preventing ocular inflammation and that such PLGA nanocarriers functionalized by PEG moieties can potentially cross the brain endothelium and have positive effects in the treatment of a mouse model of Alzheimer’s disease [37][38][39]. As demonstrated also in these works, these approaches offer nanoparticles with particularly enhanced biocompatibility, tissue permeability, and drug release control, along with facilitated targeted drug delivery and a prolonged circulation time thanks to avoiding rapid renal clearance. Thus, both methods are suitable for pioglitazone encapsulation, but which one is the most promising method is still not clear; furthermore, a reliable synthetic formulation for adapting the reported synthetic methodologies for the encapsulation of many compounds, like PGZ, is still lacking and there are still many obstacles to overcome in order to realize their clinical potential.
2. A Rational Optimization of the Single Emulsification-Solvent Evaporation Formulation Parameters
In the single emulsification-solvent evaporation method, NPs are not formed instantaneously: some hours are necessary, also because the organic solvent has to evaporate. To minimize the loss of PGZ due to a diffusion from the dispersed phase (organic solution) to the dispersing phase (aqueous solution), researchers carried out preliminary tests to optimize the pH of the aqueous solution using a buffered system. Since the PGZ is a weak acid (pKa 5.19), the solubility can be reduced working with an acid dispersing phase [40]. From these first experiments, an optimal pH of 6.2 was found, whilst NPs were not obtained at a lower pH. Accordingly, an MES buffer (pKa 6.15) was chosen as buffer system at pH 6.2.
Following the protocols found in the literature and preliminary works in their laboratory, the initial PLGA* and DSPE-PEG concentrations were set at 14.3 mg/mL and 5.2 mg/mL, respectively [41][42][43]. Moreover, they observed that using the maximum sonication amplitude did not produce suitable NPs: using 100% sonication power with all the other standard parameters as in Table 1 (or even changing the DSPE-PEG concentration to 7.8 mg/mL), DLS experiments showed the presence of NPs of diameters around 300 (260) nm, but the PDI was not so good, around 0.88 (0.40), and most importantly, the ζp was very low in absolute value, being equal to −0.13 ± 0.17 mV (−13 ± 8 mV; compare all these data with the ones reported in Figure 2 for NPs obtained at a sonication power of 80%). Although there are reports that an increase in the power of sonication and injection flux rate reduces the size of the emulsion droplets, an excessive shear stress could destroy the particulate system or forms several particle populations [44]. Hence, for their experiments, the sonication amplitude was decreased at 80% and the injection flux was initially set at 30 mL/h.
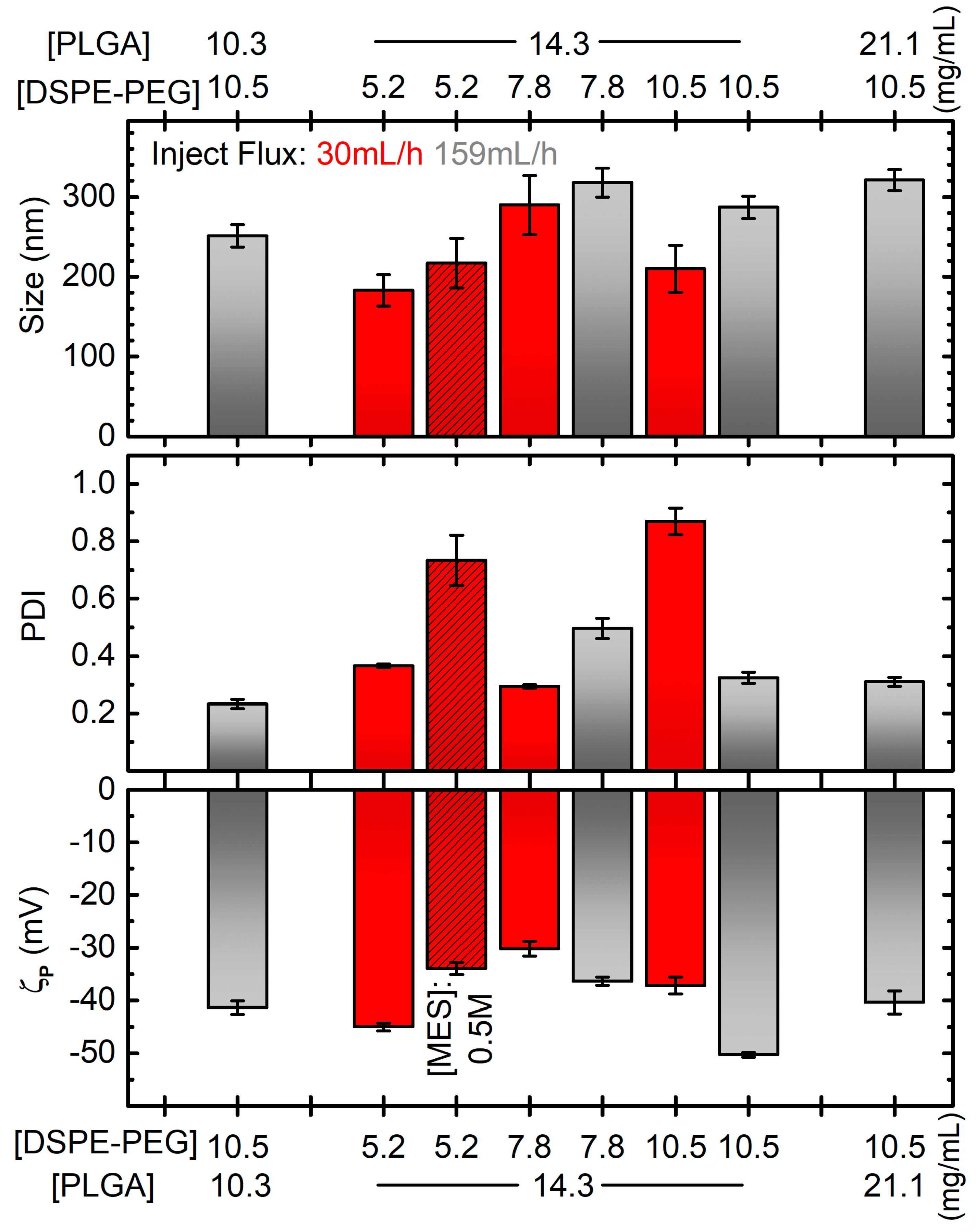
Figure 2. Diameter (top), PDI (middle) and zeta potential (ζp, bottom) of PGZ-NPs developed by single emulsification-solvent evaporation method as a function of DSPE-PEG and PLGA concentrations and different parameters: inject flux 159 mL/h for grey bars, 30 mL/h for red bars; MES concentration of 0.5 M for dashed bar, 0.1 M in all the other cases. All the other parameters are like the bold ones in Table 1, and in particular the “reference” formulation corresponds to the grey bar at [PLGA] = 14.3 mg/mL and [DSPE-PEG] = 10.5 mg/mL. Error bars are standard errors. Horizontal grey dotted lines correspond to the values for the formulation considered the best compromise in terms of the plotted quantities.
Table 1. Parameters used in pioglitazone-loaded PLGA nanoparticles synthesis and purification; in italic, the parameters found for the best synthesis; in bold the standard ones, while in different syntheses mostly only one of the other multi-valued parameters was changed. The concentration values refer to the concentration within the used stock solution, not to the one in the final solution.
| Single Emulsification-Solvent Evaporation Method (A) | Nanoprecipitation Method (B) | |
|---|---|---|
| PLGA concentration (mg/mL) | --- | 7.5, 10, 12.5, 15 |
| PLGA* concentration (mg/mL) | 10.3, 14.3, 21.1 | --- |
| PGZ concentration (mg/mL) | 10 | 5, 10, 20 |
| Volume ratio PLGA:PGZ | 7.7:1 | 8:1 |
| Dispersed phase volume (μL) | 228.7 | 225, 425, 525 |
| Dispersant phase volume (μL) | 2062.5 | 600, 800, 1200, 1400 |
| MES concentration (mg/mL) | 97.6, 19.5 | 195.2 |
| pH of dispersant phase | 6.2 | 6.0 |
| PVA concentration (% w/V) | --- | 1, 2, 4 |
| DSPE-PEG(2000) Carboxylic Acid concentration (mg/mL) | 5.2, 7.8, 10.5 | --- |
| Dispersed:dispersant phases Ratio | 1:9 | 1:1.5, 1:1.9, 1:2.66, 1:3.5, 1:5.3, 1:6.2 |
| Stirring rate (rpm) | 400 | 400 |
| Stirring time (min) | 10 | 10 |
| Injection flux rate (mL/h) | 30, 159 | 30 |
| Sonication amplitude (%) | 80, 100 | --- |
| Sonication time (min) | 4.8 | --- |
| Centrifugation speed [rpm (× g)] | 9100 (8000) | 9100 (8000) |
| Centrifugation time (min) | 5 | 5 |
| Cryoprotectant concentration (mg/mL) | 10 | 10 |
After these preliminary decisions, other parameters were evaluated, such as the effect of MES, DSPE-PEG and PLGA* concentration along with the influence of the injection flux rate on the diameter, PDI, and ζp of nanoparticles (Figure 2).
Firstly, two MES concentration values (0.1 M and 0.5 M) were studied. As reported in Figure 2, adequate size (around 200 nm) and satisfactory |ζp| (greater than 30 mV) values were found with both concentrations. On the contrary, a narrower polydisperse population was found with 0.1 M (PDI around 0.3) than 0.5 M (PDI around 0.7). The lower viscosity and the lower amounts of salts in the 0.1 M MES solution probably allow a better homogenization during sonication. Hence, a 0.1 M buffer concentration was employed for their following synthesis.
Then, the DSPE-PEG concentration effect was evaluated. As discussed in the introduction, PEGylated lipid coating has a pivotal role in improving the physicochemical properties of nanoparticles. For their single emulsification-solvent evaporation synthesis, 5.2 mg/mL, 7.8 mg/mL or 10.5 mg/mL of DSPE-PEG were employed. As researchers can observe from the red non-dashed bars in Figure 2, the average diameter, PDI and ζp were very variable without a clear correlation, and with the best formulation considering PDI being the worse considering ζp. Moreover, they observed precipitates during DLS measurements for every sample, indicating that most of the reagents had probably not reacted. Researchers hypothesized that the addition of the organic phase to the dispersant phase was not rapid enough to obtain stable particles. Thus, the syntheses were repeated with the injection flow of the dispersed (organic) phase increased to 159 mL/h. Except for the unexpected result for the formulation prepared with 5.2 mg/mL of DSPE-PEG, which showed macroscopic white aggregate (DLS measurements not evaluated), stable nanoparticles without precipitate were obtained. DLS size measurements showed no significant differences using DSPE-PEG of 7.8 mg/mL and 10.5 mg/mL, with diameters around 270 nm. Conversely, the PDI improved from 0.5 to 0.3 by increasing DSPE-PEG amount. A similar trend was found for ζp, with values from −36 to −50 mV. Hence, researchers demonstrated that the injection flux improvement could better stabilize the nanoparticle suspension and that the PEGylated lipid concentration plays a key role in the development of uniform NPs. From here on, 10.5 mg/mL of DSPE-PEG amount was employed as optimal for encapsulating their drug.
Next, the effect of PLGA* concentration over the diameter, PDI and ζp of PGZ-loaded nanoparticles were assessed, while the rest of the conditions and procedures were maintained constant. One lower (10.3 mg/mL) and one higher (21.1 mg/mL) PLGA* concentrations were assessed in addition to the one used in the syntheses discussed above (14.3 mg/mL). Observing the grey bars (DSPE-PEG concentration at 10.5 mg/mL) in Figure 2, there is a positive correlation between the PLGA* concentration and the size of the NPs, which increases from ~250 to ~320 nm for a polymer concentration increasing from 10.3 to 21.1 mg/mL. This is probably due to a greater quantity of PLGA* that had to be contained within the DPSE-PEG shell. The effect on PDI and ζp is much less pronounced and less clear, obtaining PDIs between 0.23 and 0.32 and and ζp between −50.3 mV and −40 mV. Despite the bigger ζp (in absolute values) for NPs obtained at the intermediate tested concentration of PLGA*, lower diameter size and PDI values were achieved with its lowest value.
In summary, researchers found that the lowest assessed PLGA* concentration and the highest assessed DSPE-PEG concentration represent the best compromise to prevent aggregative phenomena and to develop, via the single emulsification-solvent evaporation method, a homogenous and stable PGZ-loaded PLGA nanoparticulate.
3. A Rational Optimization of the Nanoprecipitation Formulation Parameters
The effect of PVA, PGZ and PLGA concentration along with the influence of the organic and the aqueous phase volumes over the diameter size, PDI, ζp and EE% of nanoparticles, are presented in Figure 3. Unlike the single emulsification-solvent evaporation, a slightly lower pH (6.0) could be used in the nanoprecipitation method with the aim of improving the PGZ encapsulation efficiency.
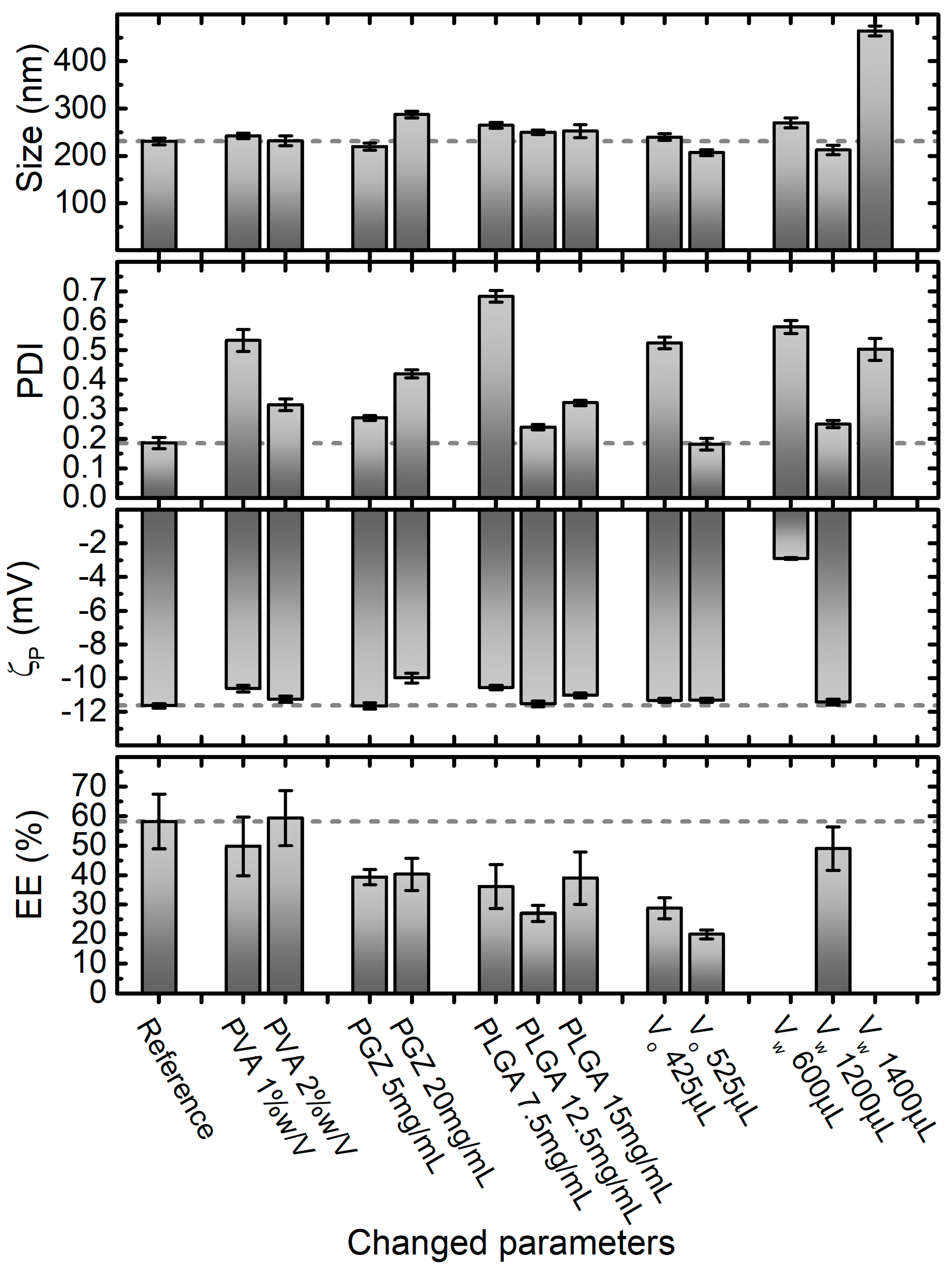
Figure 3. Effect of varying nanoprecipitation formulation parameters on PGZ-loaded NPs size, PDI, ζp and entrapment efficiency (EE%). Parameters in the Reference formulation are the bold ones in Table 1 (in particular, PVA 4% w/V, PGZ 10 mg/mL, PLGA 10 mg/mL, dispersant phase volume VW 800 μL, and dispersed phase volume VO 225 μL equal to VW/3.5). Error bars are standard errors. Dashed lines are at the values of size, PDI, ζp and EE% of the formulation that researchers identify as the best compromise in terms of these parameters, which in this case corresponds to the reference one.
Stock aqueous solutions of PVA at 1%, 2%, and 4% were used for the preparation of NPs with the nanoprecipitation technique, obtaining final PVA concentrations within the aqueous phase of 0.5%, 1%, and 2%, respectively. In this range of PVA concentrations, no significant effect on the diameter size and ζp of PGZ-loaded NPs was observed, with diameters and ζp values around 235 nm and −11 mV, respectively, in all formulations. Inversely, the PDI value decreases approximately from 0.5 to 0.2 with the increase of PVA. This could be explained considering that, at low concentrations, PVA is not well adsorbed on the nanoparticle surface and is not able to stabilize the NPs. On the other hand, higher PVA concentrations are covering better the nanoparticle surface and, interacting by hydrogen and Van der Waals bonds with PLGA, can stabilize it. This is also supported by the ζp values slightly decreasing (increasing in absolute value) with the increase of PVA.
Moreover, the PLGA concentration showed a noteworthy effect, especially on PDI. Four concentrations of PLGA were used in the nanoparticle preparations by nanoprecipitation: 7.5 mg/mL, 10.0 mg/mL, 12.5 mg/mL and 15.0 mg/mL. In this range, nanoparticles with average diameters of 230–260 nm were obtained, indicating the 10 mg/mL formulation as optimal (231 ± 7 nm). The PLGA concentration plays a pivotal role in the development of uniform NPs. As reported in Figure 3, a PLGA concentration lower than a certain threshold (10 mg/mL in their case) is not satisfactory to form reasonable nanoparticles (PDI around 0.7). Instead, with PLGA concentrations higher than 10 mg/mL, NPs are formed but they have less uniform size, probably because all the polymer is not completely stabilized by PVA. On the contrary, ζp did not show big variations in the evaluated range of PLGA concentrations, obtaining ζp values around −11 mV, with the best value obtained again at the PLGA concentration of 10 mg/mL.
Next, three different organic phase volumes (VO) were employed to assess the four characteristics shown in Figure 3 for the nanoparticles obtained via the nanoprecipitation technique. 10.0 mg/mL, 18.9 mg/mL and 23.3 mg/mL of PGZ dissolved in 25 μL of DMF were mixed respectively with 200.0, 400.0 and 500 μL of acetone solution, containing PLGA at 10 mg/mL. In this way, researchers changed the volume but not the composition of the organic phase. Except for the unexpected result for the formulation prepared with 425.0 μL of Vo, which showed a dramatically higher PDI value (0.525 ± 0.0198), no significant effect on the diameter size and PDI of PGZ-loaded NPs was observed using a Vo of 225.0 and 525 μL, obtaining diameters around 230 nm and PDI values around 0.18 respectively. Similarly, ζp did not show variations in the evaluated range of Vo (average ζp = −11.4 mV). However, they noticed that the EE% decreased using higher Vo.
To further reduce the ratio between organic phase and aqueous phase and to avoid problems due to the solubility of the reagents (for the organic phase), researchers did not further reduce the organic phase, but they increased the aqueous phase. Four VW were used in the nanoparticle preparations by nanoprecipitation, while keeping the MES buffer and PVA solution volumes ratio constant at 1:1. As depicted in Figure 3, the average diameter, PDI and ζp values were highly variable and only 800 μL and 1200 μL of Vw allowed to fabricate stable and homogeneous nanoparticles. The EE% of the 600 μL and 1400 μL formulation were not evaluated, because of the too-high PDI values. Changing the Vo and Vw, researchers evaluated the NPs changes with the fraction volume of the organic phase. This parameter is important for the size optimization of the NPs: standard ratio between 1:3 to 1:10 are usually used [45]. With the reduction of the ratio between the organic phase and the aqueous phase, an increase in diffusion of the water-miscible organic solvent is obtained. The optimal ratio allows a fast diffusion, but is slow enough for the formation of the NPs. The best results in terms of EE% and PDI were achieved using 225 μL for the organic phase and 800 μL for the aqueous phase.
The final considered parameter was the concentration of PGZ in the organic phase (VO). The effects of 5 mg/mL, 10 mg/mL (Reference) and 20 mg/mL PGZ concentrations were evaluated, obtaining nanoparticles with increasing average diameters of 219 ± 7 nm, 231 ± 7 nm, and 287 ± 7 nm, respectively. In agreement with Hernández-Giottonini et al., the average diameter values of PGZ-loaded nanoparticles developed via nanoprecipitation method tend to increase as the PGZ concentration increase [33]. In an analogous way, the effects of PGZ concentration over the PDI and ζp of nanoparticles are reported. As reported in Figure 2D, the PDI at the lowest and highest PGZ concentration are higher than at 10 mg/mL, indicating a larger size distribution; the ζp values ranged between −10 and −12 mV in the studied range of concentrations, denoting low but uniform stability. The EE% shows a maximum at 10 mg/mL and lower values at 5 mg/mL and 20 mg/mL of PGZ. A lower EE% for the 5 mg/mL concentration is anomalous; researchers could hypothesize that with the 10 mg/mL concentration, the diffusion of PGZ between the two phases is lower due to intermolecular interaction between different PGZ molecules. With a lower concentration, these interactions are less marked, causing faster diffusion and a lower EE%. On the other hand, for the 20 mg/mL formulation, the quantity of PGZ is too high to be encapsulated in the nanoparticles.
4. Comparison between the Two Syntheses
The best synthetic formulation regarding size, PDI, ζp and EE% of nanoparticles were: (A) for single emulsification-solvent evaporation, 200.0 μL PLGA* (10.3 mg/mL) + 28.7 μL Pio (10.0 mg/mL) + 62.5 μL DSPE-PEG (10.5 mg/mL) + 2.0 mL MES (0.1 M, pH: 6.2), 159.0 mL/h injection flux rate, sonication time of 4.8 min with 80.0% of amplitude; and (B) for nanoprecipitation method, 200.0 μL PLGA (10.0 mg/mL) + 25.0 μL Pio (10.0 mg/mL) + 400.0 μL PVA (4.0%) + 400.0 μL MES (1.0 M, pH: 6.0), 30.0 mL/h injection flux rate. As depicted in Figure 4, these synthetic procedures were compared to establish the best PGZ-loaded PLGA NPs over the size, PDI, ζp, EE%, DL%, and stability.
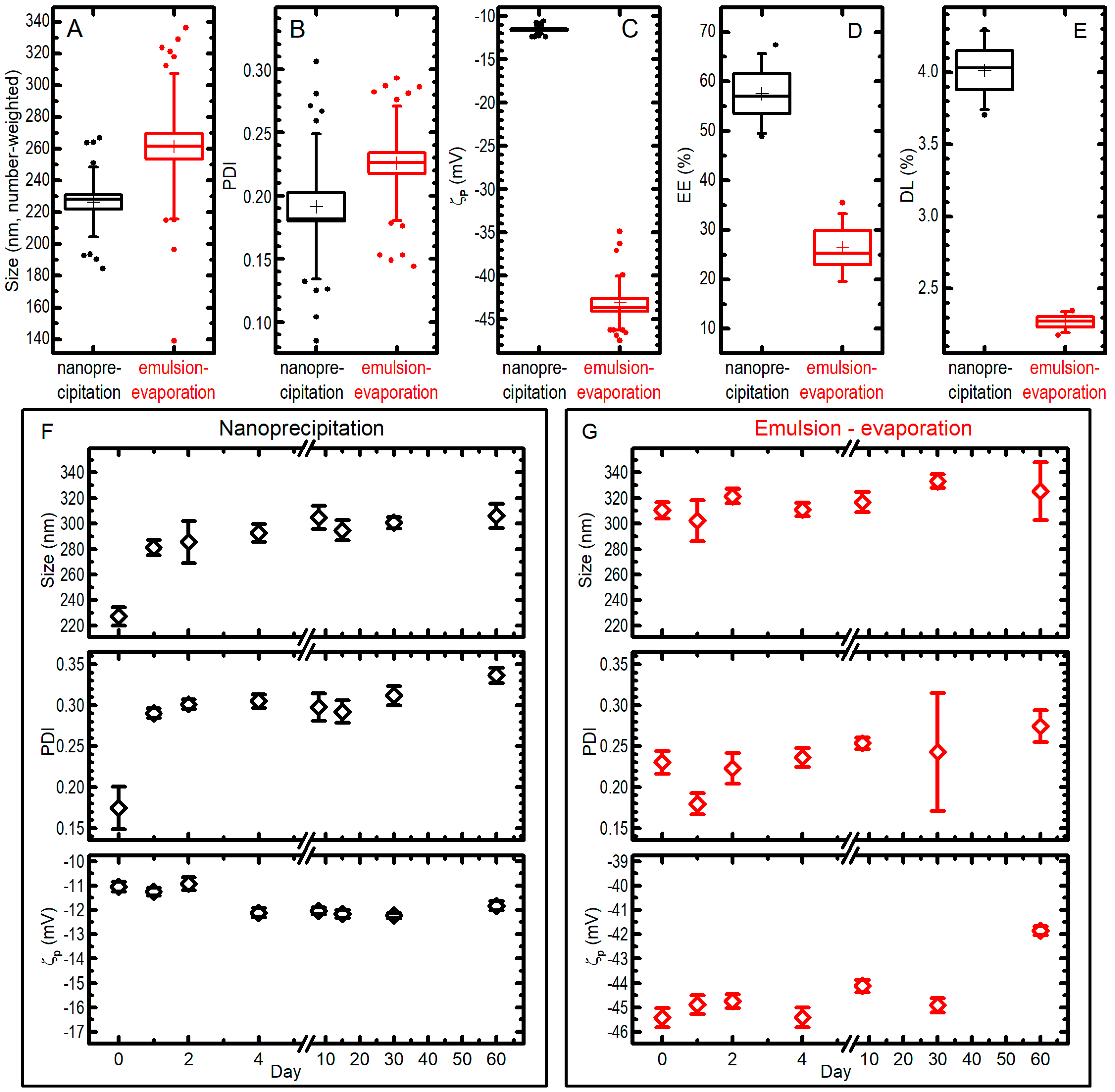
Figure 4. The best synthetic formulations for nanoprecipitation (black data) and single emulsion–solvent evaporation (red data) techniques were compared for size (A), PDI (B), ζp (C), entrapment efficiency EE% (D), drug loading DL% (E). In the box plots, boxes are standard errors (SEs), whiskers are standard deviations, lines are medians, crosses are averages, dots are outliers; all the data from different measurements (25 for nanoprecipitation, 31 for emulsion-evaporation for panels (A–C); 4 for panels (D,E)) on different syntheses (4 for nanoprecipitation and 5 for emulsion-evaporation in panels (A–C); 4 in panel (D) and 2 in panel (E) for both methods) are pooled together. Moreover, the behaviour of size, PDI, and ζp for a single preparation of PGZ-NPs via nanoprecipitation (F) or single emulsification-solvent evaporation (G) when kept at −20 °C for a different number of days is presented and compared with the ones of an aliquot of the fresh-prepared colloid (data at day 0); bars are SEs over 7–10 measurements.
The nanoparticles developed via the nanoprecipitation method (B) had a mean diameter of 226 ± 22 nm (note: in this paragraph the uncertainties are standard deviations from all measurements pooled together; see Figure 4) and a very narrow size distribution (PDI = 0.19 ± 0.06), while the nanoparticles developed via the single emulsification-solvent evaporation method (A) had a higher mean diameter (262 ± 46 nm) and a significantly (at 0.05 level) larger size distribution with a PDI of 0.23 ± 0.05 (Figure 4A,B). The ζp measured for the nanoprecipitation technique showed an average value of −11.6 ± 0.5 mV, whilst ζp values for the single emulsification-solvent evaporation technique showed a more negative average value of −43.2 ± 3.1 mV (Figure 4C); researchers attribute this to the DSPE-PEG that is localized on the nanoparticle surface, which donates a higher charge (in absolute value) with respect to a simple PLGA nanoparticle. The EE% values of PGZ-loaded NPs were 58 ± 8% and 26 ± 7% for the nanoprecipitation and single emulsification-solvent evaporation techniques, respectively (Figure 4D). Instead, DL% were 4.01 ± 0.27% and 2.27 ± 0.07% (results from two independent syntheses) for the nanoprecipitation and single emulsification-solvent evaporation techniques, respectively (Figure 4E). These values strongly depend on the type of polymeric matrix, in particular on its molecular weight. The nanoprecipitation method presents a higher EE%, most likely because a higher velocity of nanoparticle formation causes a minor loss in PGZ due to diffusion in the aqueous phase, as described above.
Concerning the storage stability study, PGZ-loaded NP samples were stored at −20 °C and the nanoparticles characteristics were monitored over 60 days by DLS (Figure 4F,G). For the nanoprecipitation technique, the stability assay showed a certain propensity towards aggregation during the freezing process: NP diameters (number-weighted) went from 227 ± 22 nm to 306 ± 28 nm in 60 days (uncertainties in this paragraph are SDs from 7–10 repeated measures on a single batch of NPs). It must be noted, however, that the biggest change happens already at the first freezing–thawing cycle, while the dimensions increases only slightly in the following 59 days of observation for frozen samples. The PDI showed a trend similar to the one observed for the size, i.e., it was possible to notice an initial increase, passing from 0.17 ± 0.08 in day 0 to 0.29 ± 0.02 in day 1, due to the aggregation during the freezing and thawing process, and only a slow trend towards higher values after that. As far as ζp is concerned, no dramatic differences were highlighted over the 60 days. On the other hand, PGZ-loaded NPs obtained via the single emulsification–solvent evaporation method did not show important changes over freezing and time in 60 days: in the batch used in this experiments, mean (number-weighted) diameters went from 310 ± 20 to 325 ± 71 nm after staying frozen for 60 days; the size distribution and the ζp remained similar, reaching a PDI of 0.27 ± 0.06 and a ζp of −41.9 ± 0.6 mV after 60 days. Researchers could expect the enhanced stability of the DSPE-PEGylated nanoparticles (single emulsification-solvent evaporation method), because of the more negative ζp due to the negatively charged DSPE-PEG (both from the phosphate group and the carboxylic acid). However, size and PDI of the two formulations remain comparable also after storage, and the significantly better EE% and DL% of NPs obtained by nanoprecipitation make them more suitable for a PGZ formulation.
Finally, the costs of both syntheses were calculated, taking into account the EE%. In order to encapsulate 0.145 mg of PGZ, reagents’ cost for the NPs obtained by nanoprecipitation and by the emulsion–solvent evaporation technique were 0.40 Euro and 7.22 Euro for a total NPs weight of 3.6 mg and 6.3 mg, respectively (Table 2). In addition, the emulsion–solvent evaporation method is more time-consuming, which in turn causes a further increase in the production costs. This showed the clear convenience of an encapsulation carried out by nanoprecipitation.
Table 2. Cost estimation summary for encapsulation of PGZ (0.145 mg) within PLGA nanoparticles via nanoprecipitation or emulsion–solvent evaporation methods. Costs reference period: 2021.
| Nanoprecipitation | Emulsion–Solvent Evaporation | |||||||
|---|---|---|---|---|---|---|---|---|
| Reagent | Cost/Unit | Quantities Used for the Synthesis | Costs (€) | Quantities Used for the Synthesis | Costs (€) | |||
| DMF | 155.- | €/1 L | 25.0 | µL | 0.004 | 55.1 | µL | 0.0085 |
| MilliQ water | 13.6 | €/1 L | 2.60 | mL | 0.0354 | 11.9 | mL | 0.0054 |
| PVA (4%) | 211.- | €/500 g | 48.0 | mg | 0.020 | |||
| MES (1 M) | 501.- | €/500 g | 234 | mg | 0.235 | |||
| MES (0.1 M) | 501.- | €/500 g | 224 | mg | 0.225 | |||
| PGZ | 34.1 | €/50 g | 0.250 | mg | 0.0002 | 0.551 | mg | 0.00038 |
| Trehalose (10 mg/mL) | 472.- | €/250 g | 2.00 | mg | 0.004 | 3.84 | mg | 0.0072 |
| PLGA | 238.- | €/5 g | 2.00 | mg | 0.095 | |||
| PLGA* | 225.- | €/5 g | 3.95 | mg | 0.178 | |||
| Acetone | 47.4 | €/1 L | 200 | µL | 0.0095 | |||
| Chloroform | 107.- | €/1 L | 384 | µL | 0.041 | |||
| DSPE-PEG | 268.- | €/50 mg | 1.26 | mg | 6.75 | |||
| Ethanol | 39.6 | €/500 mL | 120 | µL | 0.0095 | |||
| Sum (in Euro) | 0.403 | Sum (in Euro) | 7.22 | |||||
The best synthetic formulations and corresponding parameters are summarized in Table 3.
Table 3. Summarized results on Pioglitazone-loaded PLGA nanoparticles synthesis; the best compromise formulations are considered.
| Method | Dispersed Phase Conditions | Dispersant Phase Conditions | Other Synthetic Parameters | Size (nm) | PDI | ζp (mV) | EE (%) | DL (%) | Cost for Encapsulating 0.145 mg PGZ (€) |
|---|---|---|---|---|---|---|---|---|---|
| Single emulsification-solvent evaporation (A) | 200.0 μL PLGA* (10.3 mg/mL) + 28.7 μL Pio (10.0 mg/mL) | 62.5 μL DSPE-PEG (10.5 mg/mL) + 2.0 mL MES (0.1 M, pH: 6.2) | 159.0 mL/h injection flux rate, sonication time of 4.8 min with 80.0% of amplitude | 262 ± 46 | 0.23 ± 0.05 | −43.2 ± 3.1 | 26 ± 7 | 2.27 ± 0.07 | 7.22 |
| Nanoprecipitation (B) | 200.0 μL PLGA (10.0 mg/mL) + 25.0 μL Pio (10.0 mg/mL) | 400.0 μL PVA (4.0%) + 400.0 μL MES (1.0 M, pH: 6.0) | 30.0 mL/h injection flux rate | 226 ± 22 | 0.19 ± 0.06 | −11.6 ± 0.5 | 58 ± 8 | 4.01 ± 0.27 | 0.403 |
References
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-Targeted Nanomedicine for the Diagnosis and Treatment of Atherosclerosis. Nat. Rev. Cardiol. 2021, 1–22.
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743.
- Nambi, V.; Chambless, L.; Folsom, A.R.; He, M.; Hu, Y.; Mosley, T.; Volcik, K.; Boerwinkle, E.; Ballantyne, C.M. Carotid Intima-Media Thickness and Presence or Absence of Plaque Improves Prediction of Coronary Heart Disease Risk. J. Am. Coll. Cardiol. 2010, 55, 1600–1607.
- Gao, X.; Song, J.; Watase, H.; Hippe, D.S.; Zhao, X.; Canton, G.; Tian, F.; Du, R.; Ji, S.; Yuan, C.; et al. Differences in Carotid Plaques between Symptomatic Patients With and Without Diabetes Mellitus: A CARE-II Study. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1234–1239.
- Saremi, A.; Schwenke, D.C.; Buchanan, T.A.; Hodis, H.N.; Mack, W.J.; Banerji, M.; Bray, G.A.; Clement, S.C.; Henry, R.R.; Kitabchi, A.E.; et al. Pioglitazone Slows Progression of Atherosclerosis in Prediabetes Independent of Changes in Cardiovascular Risk Factors. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 393–399.
- Murakami-Nishida, S.; Matsumura, T.; Senokuchi, T.; Ishii, N.; Kinoshita, H.; Yamada, S.; Morita, Y.; Nishida, S.; Motoshima, H.; Kondo, T.; et al. Pioglitazone Suppresses Macrophage Proliferation in Apolipoprotein-E Deficient Mice by Activating PPARγ. Atherosclerosis 2019, 286, 30–39.
- Chang, K.; Francis, S.A.; Aikawa, E.; Kohler, R.H.; McCarthy, J.R.; Weissleder, R.; Plutzky, J.; Jaffer, F.A. Pioglitazone Suppresses Inflammation In Vivo In Murine Carotid Atherosclerosis: Novel Detection by Dual-Target Fluorescence Molecular Imaging. Arterioscler. Thromb. Vasc. Biol. 2011, 30, 1933–1939.
- Christensen, M.L.; Meibohm, B.; Capparelli, E.V.; Velasquez-Mieyer, P.; Burghen, G.A.; Tamborlane, W.V. Single- and Multiple-Dose Pharmacokinetics of Pioglitazone in Adolescents With Type 2 Diabetes. J. Clin. Pharmacol. 2005, 45, 1137–1144.
- Nakashiro, S.; Matoba, T.; Umezu, R.; Koga, J.; Tokutome, M.; Katsuki, S.; Nakano, K.; Sunagawa, K.; Egashira, K. Pioglitazone-Incorporated Nanoparticles Prevent Plaque Destabilization and Rupture by Regulating Monocyte/Macrophage Differentiation in ApoE−/− Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 491–500.
- Cervadoro, A.; Palomba, R.; Vergaro, G.; Cecchi, R.; Menichetti, L.; Decuzzi, P.; Emdin, M.; Luin, S. Targeting Inflammation With Nanosized Drug Delivery Platforms in Cardiovascular Diseases: Immune Cell Modulation in Atherosclerosis. Front. Bioeng. Biotechnol. 2018, 6, 177.
- Singh, R.; Lillard, J.W. Nanoparticle-Based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215–223.
- Santi, M.; Maccari, G.; Mereghetti, P.; Voliani, V.; Rocchiccioli, S.; Ucciferri, N.; Luin, S.; Signore, G. Rational Design of a Transferrin-Binding Peptide Sequence Tailored to Targeted Nanoparticle Internalization. Bioconjug. Chem. 2017, 28, 471–480.
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124.
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J. Control. Release 2017, 264, 306–332.
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine. Mater. Des. 2020, 193, 108805.
- Ahlawat, J.; Henriquez, G.; Narayan, M. Enhancing the Delivery of Chemotherapeutics: Role of Biodegradable Polymeric Nanoparticles. Molecules 2018, 23, 2157.
- Astete, C.E.; Sabliov, C.M. Synthesis and Characterization of PLGA Nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289.
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659.
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397.
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522.
- Silva-Abreu, M.; Miralles, E.; Kamma-Lorger, C.S.; Espina, M.; García, M.L.; Calpena, A.C. Stabilization by Nano Spray Dryer of Pioglitazone Polymeric Nanosystems: Development, In Vivo, Ex Vivo and Synchrotron Analysis. Pharmaceutics 2021, 13, 1751.
- Zhi, K.; Raji, B.; Nookala, A.R.; Khan, M.M.; Nguyen, X.H.; Sakshi, S.; Pourmotabbed, T.; Yallapu, M.M.; Kochat, H.; Tadrous, E.; et al. PLGA Nanoparticle-Based Formulations to Cross the Blood–Brain Barrier for Drug Delivery: From R&D to CGMP. Pharmaceutics 2021, 13, 500.
- Wang, H.; Zhou, Y.; Sun, Q.; Zhou, C.; Hu, S.; Lenahan, C.; Xu, W.; Deng, Y.; Li, G.; Tao, S. Update on Nanoparticle-Based Drug Delivery System for Anti-Inflammatory Treatment. Front. Bioeng. Biotechnol. 2021, 9, 630352.
- Matoba, T.; Koga, J.; Nakano, K.; Egashira, K.; Tsutsui, H. Nanoparticle-Mediated Drug Delivery System for Atherosclerotic Cardiovascular Disease. J. Cardiol. 2017, 70, 206–211.
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534.
- Sah, E.; Sah, H. Recent Trends in Preparation of Poly(Lactide-co-glycolide) Nanoparticles by Mixing Polymeric Organic Solution with Antisolvent. J. Nanomater. 2015, 2015, 794601.
- Vanderhoff, J.; El-Aasser, M.; Ugelstad, J. Polymer Emulsification Process. U.S. Patent 4177177A, 4 December 1979.
- Nava-Arzaluz, M.G.; Pinon-Segundo, E.; Ganem-Rondero, A.; Lechuga-Ballesteros, D. Single Emulsion-Solvent Evaporation Technique and Modifications for the Preparation of Pharmaceutical Polymeric Nanoparticles. Recent Pat. Drug Deliv. Formul. 2012, 6, 209–223.
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of Polyethylene Glycol on the Surface of Nanoparticles for Targeted Drug Delivery. Nanoscale 2021, 13, 10748–10764.
- Zeng, N.; Hu, Q.; Liu, Z.; Gao, X.; Hu, R.; Song, Q.; Gu, G.; Xia, H.; Yao, L.; Pang, Z.; et al. Preparation and Characterization of Paclitaxel-Loaded DSPE-PEG-Liquid Crystalline Nanoparticles (LCNPs) for Improved Bioavailability. Int. J. Pharm. 2012, 424, 58–66.
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int. J. Pharm. 1989, 55, R1–R4.
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA Nanoparticle Preparations by Emulsification and Nanoprecipitation Techniques: Effects of Formulation Parameters. RSC Adv. 2020, 10, 4218–4231.
- Kanemaru, M.; Asai, J.; Jo, J.-I.; Arita, T.; Kawai-Ohnishi, M.; Tsutsumi, M.; Wada, M.; Tabata, Y.; Katoh, N. Nanoparticle-Mediated Local Delivery of Pioglitazone Attenuates Bleomycin-Induced Skin Fibrosis. J. Dermatol. Sci. 2019, 93, 41–49.
- Woo, H.J.; Kim, J.S.; Huh, K.M.; Cho, K.J.; Lee, Y.K. Preparation and Characterization of Pioglitazone Loaded PLGA Nanospheres for the Treatment of Type 2 Diabetes. Polymer (Korea) 2010, 34, 527–533.
- Laddha, U.D.; Kshirsagar, S.J. Formulation of PPAR-Gamma Agonist as Surface Modified PLGA Nanoparticles for Non-Invasive Treatment of Diabetic Retinopathy: In Vitro and in Vivo Evidences. Heliyon 2020, 6, e04589.
- Lewis, D.R.; Petersen, L.K.; York, A.W.; Zablocki, K.R.; Joseph, L.B.; Kholodovych, V.; Prud’homme, R.K.; Uhrich, K.E.; Moghe, P.V. Sugar-Based Amphiphilic Nanoparticles Arrest Atherosclerosis in Vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 2693–2698.
- Silva-Abreu, M.; Calpena, A.C.; Espina, M.; Silva, A.M.; Gimeno, A.; Egea, M.A.; García, M.L. Optimization, Biopharmaceutical Profile and Therapeutic Efficacy of Pioglitazone-Loaded PLGA-PEG Nanospheres as a Novel Strategy for Ocular Inflammatory Disorders. Pharm Res. 2018, 35, 11.
- Silva-Abreu, M.; Calpena, A.C.; Andrés-Benito, P.; Aso, E.; Romero, I.A.; Roig-Carles, D.; Gromnicova, R.; Espina, M.; Ferrer, I.; García, M.L.; et al. PPARγ Agonist-Loaded PLGA-PEG Nanocarriers as a Potential Treatment for Alzheimer’s Disease: In Vitro and in Vivo Studies. Int. J. Nanomed. 2018, 13, 5577–5590.
- Al-Majed, A.; Bakheit, A.H.H.; Abdel Aziz, H.A.; Alharbi, H.; Al-Jenoobi, F.I. Pioglitazone. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 41, pp. 379–438. ISBN 978-0-12-804784-2.
- Lee, A.; Di Mascolo, D.; Francardi, M.; Piccardi, F.; Bandiera, T.; Decuzzi, P. Spherical Polymeric Nanoconstructs for Combined Chemotherapeutic and Anti-Inflammatory Therapies. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2139–2147.
- Galliani, M.; Tremolanti, C.; Signore, G. Nanocarriers for Protein Delivery to the Cytosol: Assessing the Endosomal Escape of Poly(Lactide-Co-Glycolide)-Poly(Ethylene Imine) Nanoparticles. Nanomaterials 2019, 9, 652.
- Galliani, M.; Signore, G. Poly(Lactide-co-glycolide) Nanoparticles Co-Loaded with Chlorophyllin and Quantum Dots as Photodynamic Therapy Agents. ChemPlusChem 2019, 84, 1653–1658.
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-Loaded PLGA Nanoparticles: Systematic Study of Particle Size and Drug Content. Int. J. Pharm. 2007, 336, 367–375.
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81.
More
Information
Subjects:
Chemistry, Medicinal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
3 times
(View History)
Update Date:
14 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




