Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mahesh Rachamalla | + 2856 word(s) | 2856 | 2021-09-22 04:37:37 | | | |
| 2 | Camila Xu | -28 word(s) | 2828 | 2022-03-11 01:32:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rachamalla, M. Arsenic Induced Neurotoxicity. Encyclopedia. Available online: https://encyclopedia.pub/entry/20460 (accessed on 08 February 2026).
Rachamalla M. Arsenic Induced Neurotoxicity. Encyclopedia. Available at: https://encyclopedia.pub/entry/20460. Accessed February 08, 2026.
Rachamalla, Mahesh. "Arsenic Induced Neurotoxicity" Encyclopedia, https://encyclopedia.pub/entry/20460 (accessed February 08, 2026).
Rachamalla, M. (2022, March 10). Arsenic Induced Neurotoxicity. In Encyclopedia. https://encyclopedia.pub/entry/20460
Rachamalla, Mahesh. "Arsenic Induced Neurotoxicity." Encyclopedia. Web. 10 March, 2022.
Copy Citation
Arsenic is a ubiquitous environmental contaminant widely distributed in the surrounding environmental compartments. Exposure to inorganic arsenic is known to cause major neurological effects such as cytotoxicity, chromosomal aberration, damage to cellular DNA and genotoxicity. On the other hand, long-term exposure to arsenic may cause neurobehavioral effects in the juvenile stage, which may have detrimental effects in the later stages of life. Thus, it is important to understand the toxicology and underlying molecular mechanism of arsenic which will help to mitigate its detrimental effects.
arsenic
environmental toxicity
myelination
neurotoxicity
1. Introduction
Arsenic is recognized as a primary environmental pollutant that has substantial health impacts on human and other species. It is also ranked first in the priority list of Agency for Toxic Substances and Disease Registry (ASTDR), USA till 2020 (https://www.atsdr.cdc.gov/spl/index.html, accessed on 17 July 2021) [1]. Arsenic is a ubiquitous environmental contaminant widely distributed in the surrounding environment. Further spread of Arsenic is promoted through anthropological actions including smelting, burning of fossil fuel, and use in pesticide production responsible for its increased levels in earth, water, air, agricultural and aquatic food [2][3]. Increased arsenic levels in the environment have thus become a serious human health concern is widely distributed globally [4]. Developing countries such as Bangladesh, India, Mexico, and Taiwan are highly impacted by arsenic contamination in groundwater [4][5][6]. Epidemiological studies suggest that arsenic and its related compounds are responsible for causing various types of cancers, coronary and neurological ailments [7]. Arsenic toxicity is influenced by its chemical speciation, as inorganic arsenic exhibits a higher level of toxicity compared to organo-arsenicals. Inorganic arsenic is a potent carcinogen and causes malignancies in lungs, kidneys, skin, urinary bladder, and liver [8]. Chronic arsenic exposure via drinking water is one of the major factors for the greater risk of noncancerous ailments such as pigmentation, hyperkeratosis, cardiovascular disorders, hypertension, neurological, liver and kidney disorders, and diabetes [9]. Increased arsenic in the environment also impacts the health of aquatic species [10]. Arsenic present in sediments is biologically available through diet to benthic fish [10]. Dissolved arsenic levels in aquatic ecosystems in many developing countries have been reported to be higher than the permissible limit (10 µg/L) set by World Health Organization (WHO). This might be responsible for the disturbed physiological functions such as ion regulation, gene expression, enzyme and immune functions, growth and repair of tissue matrix, reproduction, and development [10]. Several studies in rodents, fish and invertebrates suggest that increased arsenic accumulation may alter the normal physiological function of organisms by directly or indirectly promoting the initiation of disease [10][11][12][13][14]. Arsenic is a strong reducing agent and interacts with other molecules such as sulphur, chloride and oxygen. It’s interactions with carbon containing molecules results in the formation of organic arsenic [15]. In addition, binding of arsenic with certain metals and charged ions such as Ca or Mg promotes the adsorption of As(V) in the solid particulate phases [16]. In addition, the effects of arsenic and its critical interactions might acknowledge new platforms of recent understanding on the diverse activities. Thus, the complete understanding of pathological effects and molecular mechanism of arsenic are crucial to mitigate its harmful effects on various species health.
2. Metabolic Pathway of Arsenic
Scientific studies indicate that inorganic arsenic methylation was incomplete, and urinary metabolite excretion varied from person to person, though arsenic exposure was same in all the populations [17]. Inorganic arsenic is associated with toxicity and is reduced from As(V) state to As(III) by arsenate reductase enzyme. The generated trivalent species are highly active and toxic [18]. Arsenic is primarily metabolized and detoxified through oxidative methylation in the liver in the presence of methyl donor S-adenosylmethionine (SAM). The co-factor used is glutathione (GSH) with arsenic methyltrasferase resulting in the production of monomethylarsonic acid and dimethyl arsenic acid which are finally excreted through urine (Figure 1) [19].
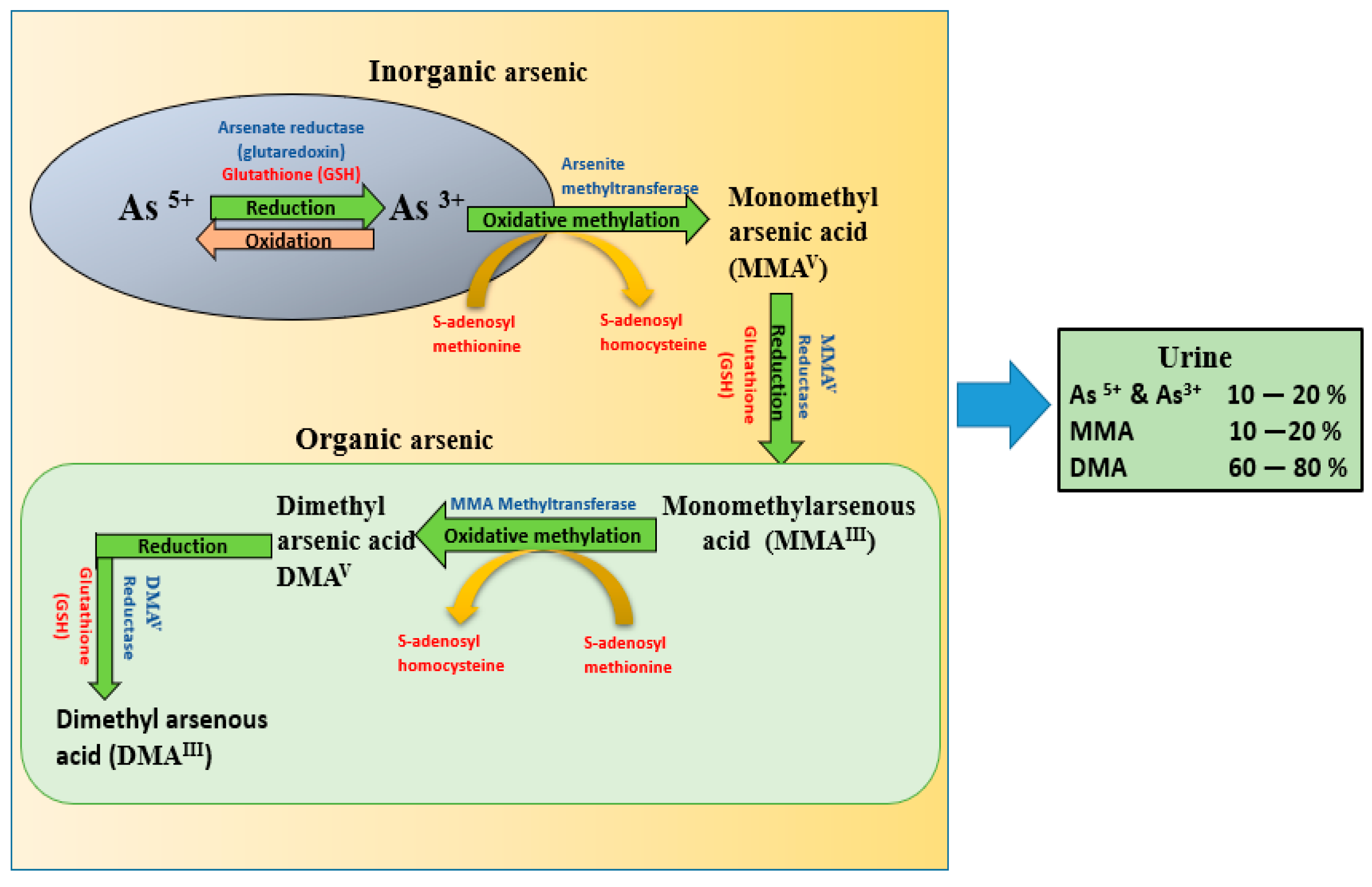 Figure 1. Metabolic pathway of inorganic arsenic demonstrating the reduction of arsenate to arsenite with the enzyme arsenate reductase mediated by glutathione (GSH), which further undergoes oxidative methylation through the enzyme arsenite methyltranferase mediated by S-adenosyl methionine, with conversion to MMA and DMA. Finally, all the metabolites are excreted through urine, among which DMA is the major metabolite (60–80%). (As- arsenic, DMA-dimethyl arsenic acid, MMA-monomethyl arsenic acid, GSH-glutathione).
Figure 1. Metabolic pathway of inorganic arsenic demonstrating the reduction of arsenate to arsenite with the enzyme arsenate reductase mediated by glutathione (GSH), which further undergoes oxidative methylation through the enzyme arsenite methyltranferase mediated by S-adenosyl methionine, with conversion to MMA and DMA. Finally, all the metabolites are excreted through urine, among which DMA is the major metabolite (60–80%). (As- arsenic, DMA-dimethyl arsenic acid, MMA-monomethyl arsenic acid, GSH-glutathione).As discussed above, the toxic potential of arsenic primarily depends on the form of arsenic in the body. Arsenic initially absorbed through various routes enters the bloodstream and is taken up by red blood cells (RBC), white blood cells (WBC), and other cells. Arsenate, on the other hand, is reduced to arsenite and is subsequently methylated to monomethyl arsonate (MMA), and dimethyl arsenate (DMA) [20]. Reduction of arsenate to arsenite is essential before methylation and requires glutathione (GSH) and methyl group transferase S-adenosyl methionine [21][22]. It is important to note that the absorbed form of arsenic depends on the nature of arsenic because all the absorbed arsenic is not in the pentavalent form. The main urinary metabolite found in urinary arsenic is DMA (60–80%). Even though methylation is the most important pathway for arsenic detoxification, its effectiveness in humans seems to decline when exposed to higher doses (Figure 1).
3. Neuronal Effects of Arsenic
Neuronal alterations due to metals/metalloids are well documented. Arsenic exposure is known to cause various neurological disorders through diverse molecular mechanisms such as cytotoxicity, increased reactive oxygen species (ROS), chromosomal aberrations and cellular DNA damage. These genotoxic effects are the major cause of degenerative changes in neurological systems [23][24]. Various epidemiological studies have revealed a correlation between increased arsenic in drinking water and neurological behavioral disorders such as decreased locomotor activity, impaired cognitive functions, and prenatal complications. Arsenic easily crosses the blood-brain barrier and can accumulate in various parts of the brain, such as the striatum and hippocampus, which further potentiates arsenic toxicity and tissue injury [25][26].
There is much evidence for the neurological impacts of arsenic in animal models. However, very few epidemiological reports are available on the influence of arsenic on adult mental health and cognitive performance [27][28]. Epidemiological and toxicological studies indicate that arsenic is a developmental neurotoxicant and is responsible for inducing intellectual and cognitive disabilities in humans [29]. Studies conducted in Bangladesh, India, Mexico, and Taiwan have indicated that chronic exposure to even very low levels of arsenic (<10 µg/L) reduced IQ and memory performance in exposed children [28]. The experimental research carried on animals has expanded the understanding of the outcomes of new and potential neurotoxic components. Arsenic can induce neurotoxic effects by altering the levels of neurotransmitters such as serotonin, dopamine and norepinephrine in the brain [30][31]. The detrimental effects of arsenic are largely influenced in the developmental stages. Exposure to arsenic could perturbate neurological complications, severely affecting memory and learning, anxiety and mood instability [32][33]. Several mechanisms correlate arsenic toxicity with reduced synaptic signaling, plasticity and neurogenesis [34].
The literature includes possible mechanisms involved in the impaired cognitive performance in adults with arsenic exposure. Peripheral nerve neuropathy, altered sensory function and reduced conduction velocity were observed in humans who were subjected to elevated levels of inorganic arsenic [35][36]. Even a single dose of 50 ppb inorganic arsenic in water in adult mice led to peripheral neuropathy, which resulted in the reduction of motor conduction velocity and abnormalities in action potentials in sensory nerves in offspring [37]. It has also been observed that arsenic exposure caused loss of neurofilaments and decreased expression of fibroblast proteins in rat sciatic nerves [38]. Arsenic-induced oxidative stress and demyelination, and morphological impacts on peripheral neurons suggest that these impacts further impair the transmission of signal transduction from the peripheral nervous system to the CNS leading to detrimental impacts on mental health [36].
The mechanisms linking arsenic exposure to neurodegeneration are complex, and the understanding of these mechanisms is continuously evolving [39]. Previous studies have suggested that arsenic might cause neurodegeneration through various mechanisms, the most studied including oxidative stress, inflammation, and mitochondrial dysfunction [40][41]. In a case control study, increased urinary arsenic excretion in patients was observed in correlation with enhanced risk of progression of Alzheimer’s disease (AD). AD is a progressive neurological disorder characterized by the formation of neurofibrillary tangles and β-amyloid (Aβ) plaques [42]. Arsenic-induced dementia and vascular injury were also reported in in-vivo studies [43]. Chronic exposure to arsenic in rats caused behavioral deficits which were associated with high levels of amyloid-β, increased advanced glycation-end products and β-secretase (BACE-1) activity in the brain [39]. Arsenic exacerbated amyloid-β and phosphorylated tau in transgenic AD rodent models, which were mediated through bioenergetic disfunction and modified redox metabolism [39]. Interestingly, arsenic reportedly induced behavioral deficits and neurodegeneration via increased production of the Aβ(1–42), amyloid precursor protein (APP) and BACE-1 [44]. These proamyloidogenic effects of arsenic were synergized when coexposed with other heavy metals, and these effects were mainly mediated by oxidative damage and neuroinflammation of brain tissues [45]. Arsenic increases proinflammatory cytokines levels in astrocytes, which are a subtype of glial cell in the central nervous system and mediate brain homeostasis and neuronal metabolism. Any imbalances/insults in glial cells lead to increased levels of amyloid precursor protein [46]. Arsenic toxicity may also synergize with DA (dopamine) to cause neurotoxicity, and cause α-synuclein aggregation, which is a hallmark of Parkinson’s disease [47].
Perinatal exposure of adult mice to 50 µg/L arsenic via drinking water was found to cause depression and depression-like behavior in the offspring [48]. The research also reported elevated serum corticosterone levels and subsequent reduction of the whole hippocampal corticotrophin-releasing factor (CRFR1), increased dorsal hippocampal serotonin 5HT1A receptor binding and receptor-effector coupling. These observations imply that perinatal exposure to arsenic may interrupt the regulatory connections between the hypothalamic-pituitary-adrenal (HPA) axis and the serotonergic system in the dorsal hippocampus. These changes significantly induced depressive behavior in offspring [31][48]. Several rodent studies indicated that chronic exposure to low and moderate levels of arsenic could significantly alter the levels of NE, DA, and 5-HT in the brain; such effects have often been reported to occur in a sex-specific manner [26][27][28][30][31][35][36][39][45][47][49][50][51][52][53][54][55][56][57]. Arsenic toxicity is more common in males (53.7%) than females (46.3%). It also causes fatal effects on the male reproductive organs and development [58]. However, low levels of arsenic (~1.321 mg) affect pregnant females and their offspring. The newborns from the arsenic exposed females showed low socio economical communications and malnutrition with an effect on growth and development [59]. Collectively, this evidence suggests that pregnant women are at a higher risk to arsenic.
To date, the neurobehavioral implications of chronic arsenic exposure have not been investigated fully in any species. Dipp et al. [51], chronically exposed different life stages of zebrafish (larval, juvenile and adult) to waterborne arsenic (50–500 µg/L) and subsequently examined motor function, social and cognitive behaviors, and anxiety-like behaviors. They reported altered motor function in embryos and adults at 500 µg/L arsenic exposure, and an increase in anxiety behavior in juveniles and adults at the same exposure. Associative learning behaviors were also impacted at 500 µg/L exposure, but only in adults [51]. The major potential mechanism of arsenic neurotoxicity could be oxidative stress. When adult zebrafish were chronically exposed to arsenic trioxide (50 µg/L for 90 D), upregulation of catalase (Cat), glutathione peroxidase (Gpx1), copper/zinc superoxide dismutase (SOD1), and manganese superoxide dismutase (SOD2) were recorded in the brain. In addition, mitochondrial cytochrome c oxidase1 (Cox1) and B-cell lymphoma 2 (Bcl2) were also upregulated, indicating the initiation of apoptosis in brain cells [60]. Even low levels of arsenic at 10 µg/L could cause long-term memory loss in zebrafish when tested with a one-trial inhibitory avoidance test. This is a behavioral task aimed at evaluating learning and memory mechanisms currently available to zebrafish, and is associated with increased protein oxidation in the brain [61].
3.1. Neurotransmitter Mediated Impacts of Arsenic
There are several neurotransmitters responsible for the communication between cells within the brain. Arsenic has neurotoxic effects on these neurotransmitters and inducible effects on dopamine (DA) and serotonin (5-HT) levels due to regulation of norepinephrine (NE) levels [62]. Arsenic also alters the levels of GABA, glutamate and other biogenic amine levels, as well as biogenic amines (5-HT, NE and DA) and nitric oxide [63]. Nagaraja et al. [64] reported that inorganic arsenic consumption decreased acetylcholinesterase activity, which helps in metabolism of acetylcholine in rodents [64]. Poor outcomes in learning and memory could be mechanistically linked with altered levels of neurotransmitter release [65]. Other research groups have identified that the neurotoxic effects of arsenic are mediated via reduced glutamate levels and mGluR5 expression in the hippocampus [66]. In addition, exposure to arsenic in rats reduced the activity of acetylcholinesterase (ACHE) in the central compartments of the brain [67]. Similarly, other studies evaluated the reduced expression of homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) in mice treated with arsenic [68].
Another potential mechanism of arsenic-induced neurobehavioral alterations could be mediated by the transcriptional regulation of ectonucleotidases. The uncoupling of oxidative phosphorylation is linked with the formation of arsenate and ADP complexes [69]. Mitochondrial formation of ATP from ADP and PO4 provide cells with energy. The formation of ADP + arsenic can occur faster than ATP formation, thereby decoupling ATP production. Significant decrease in the mRNA expression of NTPDase members (entpd2_mg, entpd2_mq) and Ecto-5′-nucleotidase, eventually results in a reduction of ATP/ADP and AMP hydrolysis [70]. Arsenic-mediated alterations in the activities and mRNA levels of ectonucleotidases might be responsible for decreased adenosine levels, which could alter movement and anxiety reactions in zebrafish [70]. Arsenic could also act on the cholinergic system by interacting with thiol (-SH) groups involved in the uptake of choline and disulfide group of acetylcholinesterase [67][71][72]. Moreover, rodents exposed to arsenic showed a decrease in glutamic acid decarboxylase (GAD) expression in some areas of the brain, while glutamate (Glu) levels were increased. Increased glutamate can be excitotoxic and cause neuronal death. As-mediated disruption of cholinergic, GABAergic, and glutamatergic systems can lead to alteration in memory consolidation and retrieval [73][74]. These mechanisms could also lead to neuronal loss in the neurotransmission pathways, and thereby cause cognitive deficits.
3.2. Neurodevelopmental Defects and the Effects of Aging
It has been shown that chronic exposure to arsenic may cause detrimental neurobehavioral effects in the juvenile stage. Thus, consumption of arsenic unknowingly in childhood may have detrimental effects in later stages of life [75]. Neuropathy and peripheral neuropathy are common complications seen with arsenic toxicity. Neuropathy is a condition in which sensory function is impacted upon chronic exposure to toxicants or metabolic disorders [76]. A study in Mexico observed that urinary arsenic concentration was conversely correlated with oral IQ (verbal intellectual ability) and memory. Long term memory, attention, and capability to understand speech may be influenced by chronic exposure to arsenic in individuals with chronic malnutrition. Further, IQ of children can be decreased with increased arsenic exposure [77][78]. Additionally, by evaluating the impacts of arsenic at various life stages, studies found that early life stage exposure could lead to health impacts in adults. Clinical observations are not very well understood and poor diagnosis may lead to later complications, although as per current literature, impacts on adult life stages are much more studied than early life or prenatal exposure impacts [79].
3.3. Neurobehavioral Effects of Aging in Animal Models
Spatial learning ability of arsenic-exposed rats revealed impairment of spatial memory as concluded by inferior performance on hidden platform acquisition tests [80]. Another study of prenatal arsenic exposure before 4 months of age showed that the arsenic-exposed rats had increased errors in sensory information, and arsenic-exposed offspring had deficits in spatial working memory and reactivity to novelty [81][82]. A dose-dependent decline in body weight and brain weight were the result of arsenic exposure in young animals [83]. Another study reported that the levels of dopamine and serotonin in the brain increased with a decrease in norepinephrine following exposure to arsenic [68].
3.4. Neurobehavioral Effects of Aging in Humans
In infants, calamitous consequences have been associated with acute as well as chronic exposure. The outcomes of toxicity in children have been explained in a meta-analysis report. The meta-analysis performed in China showed that the mean IQ score of infants exposed to arsenic was six points less than that of unexposed infants [84]. The research also reported that the impact of arsenic toxicity depended on acute and chronic exposures. Moreover, upon aging the effects were largely mediated via chronic exposure. This suggests that low levels of arsenic in the early developmental stages, if neglected, might lead to severe complications with increasing of age [29]. Another clinical study on females aged 6 months demonstrated strong effects of arsenic on the neurodevelopment [85]. Further, emerging studies have shown that children up to 5 years of age are more prone to intellectual deficits due to arsenic exposure [30][86][87]. Neurobehavioral effects were also observed on chronic arsenic exposure in adolescents [75]. Adults who were exposed in early stages to arsenic performed poorer in neurobehavioral subsets indicating that infantile exposure to arsenic may affect behavioral development in the later stages of life. Studies with the geriatric population showed that a low level of arsenic exposure is linked with weaker cognition, decline in visuospatial skills, decline in language skills and information processing speed, impairment in the ability to execute tasks, and diminished short term memory [88]. Children exposed to arsenic through drinking water were found to have poor performance in information processing speed, although their verbal skills were not affected much [89][90]. Studies suggest that arsenic exposure through drinking water was associated with decreased IQ scores in youngsters aged between 6–10 years [87].
References
- Kurosawa, K.; Egashira, K.; Tani, M.; Jahiruddin, M.; Moslehuddin, A.Z.M.; Rahman, Z.M. Groundwater–soil–crop relationship with respect to arsenic contamination in farming villages of Bangladesh—A preliminary study. Environ. Pollut. 2008, 156, 563–565.
- Smith, E.; Juhasz, A.; Weber, J.; Naidu, R. Arsenic uptake and speciation in rice plants grown under greenhouse conditions with arsenic contaminated irrigation water. Sci. Total Environ. 2008, 392, 277–283.
- Ayotte, J.D.; Montgomery, D.L.; Flanagan, S.M.; Robinson, K.W. Arsenic in Groundwater in Eastern New England: Occurrence, Controls, and Human Health Implications. Environ. Sci. Technol. 2003, 37, 2075–2083.
- Karagas, M.R.; Stukel, T.; Tosteson, T.D. Assessment of cancer risk and environmental levels of arsenic in New Hampshire. Int. J. Hyg. Environ. Health 2002, 205, 85–94.
- Mukherjee, A.; Sengupta, M.K.; Hossain, M.A.; Ahamed, S.; Das, B.; Nayak, B.; Lodh, D.; Rahman, M.M.; Chakraborti, D. Arsenic contamination in groundwater: A global perspective with emphasis on the Asian scenario. J. Health Popul. Nutr. 2006, 24, 142–163.
- Tchounwou, P.B.; Centeno, J.A.; Patlolla, A.K. Arsenic toxicity, mutagenesis, and carcinogenesis—A health risk assessment and management approach. Mol. Cell. Biochem. 2004, 255, 47–55.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Drinking-Water Disinfectants and Con-taminants, including Arsenic, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC Monogr. Eval. Carcinog. Risks Hum. 2004, 84, 1–477.
- National Research Council. Arsenic in Drinking Water; The National Academies Press: Washington, DC, USA, 1999.
- Hong, H.; Wu, H.; Chen, J.; Wu, B.; Yu, H.; Yan, B.; Liang, Y. Cytotoxicity induced by iodinated haloacetamides via ROS accumulation and apoptosis in HepG-2 cells. Environ. Pollut. 2018, 242, 191–197.
- Zhang, Y.; Sun, H.; Zhang, J.; Ndayambaje, E.; Lin, H.; Chen, J.; Hong, H. Chronic exposure to dichloroacetamide induces biochemical and histopathological changes in the gills of zebrafish. Environ. Toxicol. 2019, 34, 781–787.
- Pedlar, R.; Ptashynski, M.; Wautier, K.; Evans, R.; Baron, C.; Klaverkamp, J. The accumulation, distribution, and toxicological effects of dietary arsenic exposure in lake whitefish (Coregonus clupeaformis) and lake trout (Salvelinus namaycush). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 131, 73–91.
- Datta, S.; Ghosh, D.; Saha, D.R.; Bhattacharaya, S.; Mazumder, S. Chronic exposure to low concentration of arsenic is immunotoxic to fish: Role of head kidney macrophages as biomarkers of arsenic toxicity to Clarias batrachus. Aquat. Toxicol. 2009, 92, 86–94.
- Dauphiné, D.C.; Smith, A.H.; Yuan, Y.; Balmes, J.R.; Bates, M.N.; Steinmaus, C. Case-Control Study of Arsenic in Drinking Water and Lung Cancer in California and Nevada. Int. J. Environ. Res. Public Health 2013, 10, 3310–3324.
- Maimaitiyiming, Y.; Zhu, H.-H.; Yang, C.; Naranmandura, H. Biotransformation of arsenic trioxide by AS3MT favors eradication of acute promyelocytic leukemia: Revealing the hidden facts. Drug Metab. Rev. 2020, 52, 425–437.
- Torres, J.; Santos, P.; Ferrari, C.; Kremer, C.; Kremer, E. Solution Chemistry of Arsenic Anions in the Presence of Metal Cations. J. Solut. Chem. 2017, 46, 2231–2247.
- Santra, S.C.; Samal, A.C.; Bhattacharya, P.; Banerjee, S.; Biswas, A.; Majumdar, J. Arsenic in Foodchain and Community Health Risk: A Study in Gangetic West Bengal. Procedia Environ. Sci. 2013, 18, 2–13.
- Kharroubi, W.; Ahmed, S.H.; Nury, T.; Andreoletti, P.; Sakly, R.; Hammami, M.; Lizard, G. Mitochondrial dysfunction, oxidative stress and apoptotic induction in microglial BV-2 cells treated with sodium arsenate. J. Environ. Sci. 2017, 51, 44–51.
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905–23926.
- Gbaruko, B.C.; Ana, G.; Nwachukwu, J.K. Ecotoxicology of arsenic in the hydrosphere: Implications for public health. Afr. J. Biotechnol. 2008, 7, 4737–4742.
- Engström, K.S.; Broberg, K.; Concha, G.; Nermell, B.; Warholm, M.; Vahter, M. Genetic Polymorphisms Influencing Arsenic Metabolism: Evidence from Argentina. Environ. Health Perspect. 2007, 115, 599–605.
- Pavlogeorgatos, G.; Vasilis, K. The importance of mercury determination and speciation to the health of the general population. Glob. NEST Int. J. 2003, 4, 107–125.
- Jha, A.; Noditi, M.; Nilsson, R.; Natarajan, A. Genotoxic effects of sodium arsenite on human cells. Mutat. Res. Mol. Mech. Mutagen. 1992, 284, 215–221.
- Wang, Y.; Zhao, F.; Liao, Y.; Jin, Y.; Sun, G. Effects of arsenite in astrocytes on neuronal signaling transduction. Toxicology 2012, 303, 43–53.
- Sinha, D.; Prasad, P. Health effects inflicted by chronic low-level arsenic contamination in groundwater: A global public health challenge. J. Appl. Toxicol. 2019, 40, 87–131.
- Itoh, T.; Zhang, Y.F.; Murai, S.; Saito, H.; Nagahama, H.; Miyate, H.; Saito, Y.; Abe, E. The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol. Lett. 1990, 54, 345–353.
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health Effects of Chronic Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 245–252.
- Rahman, M.D.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Khabiruddin, M.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human disease, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940.
- Tolins, M.; Ruchirawat, M.; Landrigan, P. The Developmental Neurotoxicity of Arsenic: Cognitive and Behavioral Consequences of Early Life Exposure. Ann. Glob. Health 2014, 80, 303–314.
- Calderon, J.; Navarro, M.; Capdevillea, M.; Diaza, M.; Golden, A.; Leyvaa, I.; Aburtod, V.; Barriga, F.D. Exposure to Arsenic and Lead and Neuropsychological Development in Mexican Children. Environ. Res. 2001, 85, 69–76.
- Li, Z.; Li, X.; Qian, Y.; Guo, C.; Wang, Z.; Wei, Y. The sustaining effects of e-waste-related metal exposure on hypothalmus-pituitary-adrenal axis reactivity and oxidative stress. Sci. Total Environ. 2020, 739, 139964.
- Alao, M.E.; Perin, J.; Brooks, W.A.; Hossain, L.; Goswami, D.; Zaman, K.; Yunus, M.; Khan, A.F.; Jahan, Y.; Ahmed, D.; et al. Urinary arsenic is associated with wasting and underweight status in young children in rural Bangladesh. Environ. Res. 2020, 195, 110025.
- Florea, A.-M.; Splettstoesser, F.; Büsselberg, D. Arsenic trioxide (As2O3) induced calcium signals and cytotoxicity in two human cell lines: SY-5Y neuroblastoma and 293 embryonic kidney (HEK). Toxicol. Appl. Pharmacol. 2007, 220, 292–301.
- Malavasi, F.; Deaglio, S.; Funaro, A.; Ferrero, E.; Horenstein, A.L.; Ortolan, E.; Vaisitti, T.; Aydin, S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008, 88, 841–886.
- Yang, Y.-W.; Liou, S.-H.; Hsueh, Y.-M.; Lyu, W.-S.; Liu, C.-S.; Liu, H.-J.; Chung, M.-C.; Hung, P.-H.; Chung, C.-J. Risk of Alzheimer’s disease with metal concentrations in whole blood and urine: A case–control study using propensity score matching. Toxicol. Appl. Pharmacol. 2018, 356, 8–14.
- Sharma, B.; Sharma, P. Arsenic toxicity induced endothelial dysfunction and dementia: Pharmacological interdiction by histone deacetylase and inducible nitric oxide synthase inhibitors. Toxicol. Appl. Pharmacol. 2013, 273, 180–188.
- Martinez, E.J.; Kolb, B.L.; Bell, A.; Savage, D.D.; Allan, A.M. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. NeuroToxicology 2008, 29, 647–655.
- Vahidnia, A.; Romijn, F.; Tiller, M.; Van Der Voet, G.B.; De Wolff, F.A. Arsenic-induced toxicity: Effect on protein composition in sciatic nerve. Hum. Exp. Toxicol. 2006, 25, 667–674.
- Barguilla, I.; Peremartí, J.; Bach, J.; Marcos, R.; Hernández, A. Role of As3mt and Mth1 in the genotoxic and carcinogenic effects induced by long-term exposures to arsenic in MEF cells. Toxicol. Appl. Pharmacol. 2020, 409, 115303.
- Medda, N.; Patra, R.; Ghosh, T.K.; Maiti, S. Neurotoxic mechanism of arsenic: Synergistic effect of effect of mitochondrial instability, oxidative stress and hormonal - neurotransmitter impairment. Biol. Trace Elem. Res. 2020, 198, 8–15.
- Niño, S.A.; Martel-Gallegos, G.; Castro-Zavala, A.; Ortega-Berlanga, B.; Delgado, J.M.; Hernández-Mendoza, H.; Romero-Guzmán, E.; Ríos-Lugo, J.; Rosales-Mendoza, S.; Jiménez-Capdeville, M.E.; et al. Chronic Arsenic Exposure Increases Aβ(1–42) Production and Receptor for Advanced Glycation End Products Expression in Rat Brain. Chem. Res. Toxicol. 2017, 31, 13–21.
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356.
- Gouras, G.K.; Olsson, T.T.; Hansson, O. β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics 2015, 12, 3–11.
- Virk, D.; Kumar, A.; Jaggi, A.S.; Singh, N. Ameliorative role of rolipram, PDE-4 inhibitor, against sodium arsenite–induced vascular dementia in rats. Environ. Sci. Pollut. Res. 2021.
- Ashok, A.; Rai, N.K.; Tripathi, S.; Bandyopadhyay, S. Exposure to As-, Cd-, and Pb-Mixture Induces Aβ, Amyloidogenic APP Processing and Cognitive Impairments via Oxidative Stress-Dependent Neuroinflammation in Young Rats. Toxicol. Sci. 2014, 143, 64–80.
- Li, K.; Li, J.; Zheng, J.; Qin, S. Reactive Astrocytes in Neurodegenerative Diseases. Aging Dis. 2019, 10, 664–675.
- Shavali, S.; Sens, D.A. Synergistic neurotoxic effects of arsenic and dopamine in human dopaminergic neuroblastoma SH-SY5Y cells. Toxicol. Sci. 2008, 102, 254–261.
- Li, D.; Lu, C.; Wang, J.; Hu, W.; Cao, Z.; Sun, D.; Xia, H.; Ma, X. Developmental mechanisms of arsenite toxicity in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2009, 91, 229–237.
- Niño, S.A.; Morales-Martínez, A.; Chi-Ahumada, E.; Carrizales, L.; Delgado, R.S.; Pérez-Severiano, F.; Díaz-Cintra, S.; Jiménez-Capdeville, M.E.; Zarazua, S. Arsenic Exposure Contributes to the Bioenergetic Damage in an Alzheimer’s Disease Model. ACS Chem. Neurosci. 2018, 10, 323–336.
- Liu, X.; Piao, F.; Li, Y. Protective Effect of Taurine on the Decreased Biogenic Amine Neurotransmitter Levels in the Brain of Mice Exposed to Arsenic. Adv. Exp. Med. Biol. 2013, 776, 277–287.
- Dipp, V.R.; Valles, S.; Ortiz-Kerbertt, H.; Suarez, J.V.; Bardullas, U. Neurobehavioral Alterations in Zebrafish Due to Long-Term Exposure to Low Doses of Inorganic Arsenic. Zebrafish 2018, 15, 575–585.
- Ellinsworth, D.C. Arsenic, reactive oxygen, and endothelial dysfunction. J. Pharmacol. Exp. Ther. 2015, 353, 458–464.
- Pou, S.; Pou, W.; Bredt, D.; Snyder, S.; Rosen, G. Generation of superoxide by purified brain nitric oxide synthase. J. Biol. Chem. 1992, 267, 24173–24176.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84.
- Kato, K.; Hayashi, H.; Hasegawa, A.; Yamanaka, K.; Okada, S. DNA damage induced in cultured human alveolar (L-132) cells by exposure to dimethylarsinic acid. Environ. Health Perspect. 1994, 102, 285–288.
- Xu, M.; Niu, Q.; Hu, Y.; Feng, G.; Wang, H.; Li, S. Proanthocyanidins Antagonize Arsenic-Induced Oxidative Damage and Promote Arsenic Methylation through Activation of the Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 1–19.
- Sun, Y.; Yin, Y.; Zhang, J.; Yu, H.; Wang, X.; Wu, J.; Xue, Y. Hydroxyl radical generation and oxidative stress in Carassius auratus liver, exposed to pyrene. Ecotoxicol. Environ. Saf. 2008, 71, 446–453.
- Kim, Y.-J.; Kim, J.-M. Arsenic Toxicity in Male Reproduction and Development. Dev. Reprod. 2015, 19, 167–180.
- Wang, B.; Liu, J.; Liu, B.; Liu, X.; Yu, X. Prenatal exposure to arsenic and neurobehavioral development of newborns in China. Environ. Int. 2018, 121, 421–427.
- Sarkar, S.; Mukherjee, S.; Chattopadhyay, A.; Bhattacharya, S. Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: Expression of antioxidant genes. Ecotoxicol. Environ. Saf. 2014, 107, 1–8.
- de Castro, M.R.; Lima, J.V.; de Freitas, D.P.S.; de Souza Valente, R.; Dummer, N.S.; de Aguiar, R.B.; dos Santos, L.C.; Marins, L.F.; Geracitano, L.A.; Monserrat, J.M. Behavioral and neurotoxic effects of arsenic exposure in zebrafish (Danio rerio, Teleostei: Cyprinidae). Comp. Biochem. Physiol. C Toxicol. 2009, 150, 337–342.
- Mejía, J.; Barriga, F.D.; Calderón, J.; Ríos, C.; Jiménez-Capdeville, M. Effects of Lead–Arsenic Combined Exposure on Central Monoaminergic Systems. Neurotoxicol. Teratol. 1997, 19, 489–497.
- Yadav, R.S.; Shukla, R.K.; Sankhwar, M.L.; Patel, D.K.; Ansari, R.W.; Pant, A.B.; Islam, F.; Khanna, V.K. Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. NeuroToxicology 2010, 31, 533–539.
- Nagaraja, T.; Desiraju, T. Effects on Operant Learning and Brain Acetylcholine Esterase Activity in Rats following Chronic Inorganic Arsenic Intake. Hum. Exp. Toxicol. 1994, 13, 353–356.
- Grozio, A.; Sociali, G.; Sturla, L.; Caffa, I.; Soncini, D.; Salis, A.; Raffaelli, N.; De Flora, A.; Nencioni, A.; Bruzzone, S. CD73 Protein as a Source of Extracellular Precursors for Sustained NAD+ Biosynthesis in FK866-treated Tumor Cells. J. Biol. Chem. 2013, 288, 25938–25949.
- Jiang, S.; Su, J.; Yao, S.; Zhang, Y.; Cao, F.; Wang, F.; Wang, H.; Li, J.; Xi, S. Fluoride and Arsenic Exposure Impairs Learning and Memory and Decreases mGluR5 Expression in the Hippocampus and Cortex in Rats. PLoS ONE 2014, 9, e96041.
- Kannan, G.M.; Tripathi, N.; Dube, S.N.; Gupta, M.; Flora, S.; Flora, S.J.S. Toxic Effects of Arsenic (III) on Some Hematopoietic and Central Nervous System Variables in Rats and Guinea Pigs. J. Toxicol. Clin. Toxicol. 2001, 39, 675–682.
- Gresser, M. ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J. Biol. Chem. 1981, 256, 5981–5983.
- Nagaraja, T.; Desiraju, T. Regional alterations in the levels of brain biogenic amines, glutamate, GABA, and GAD activity due to chronic consumption of inorganic arsenic in developing and adult rats. Bull. Environ. Contam. Toxicol. 1993, 50.
- Baldissarelli, L.A.; Capiotti, K.M.; Bogo, M.R.; Ghisleni, G.; Bonan, C.D. Arsenic alters behavioral parameters and brain ectonucleotidases activities in zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 566–572.
- Valles, S.; Hernández-Sánchez, J.; Dipp, V.R.; Huerta-González, D.; Olivares-Bañuelos, T.N.; Gonzalez-Fraga, J.A.; Bardullas, U. Exposure to low doses of inorganic arsenic induces transgenerational changes on behavioral and epigenetic markers in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2020, 396, 115002.
- Chattopadhyay, S.; Bhaumik, S.; Chaudhury, A.N.; Das Gupta, S. Arsenic induced changes in growth development and apoptosis in neonatal and adult brain cells in vivo and in tissue culture. Toxicol. Lett. 2002, 128, 73–84.
- Barros, D.M.; e Souza, T.M.; De David, T.; Choi, H.; Aguzzoli, A.; Madche, C.; Ardenghi, P.; Medina, J.H.; Izquierdo, I. Simultaneous modulation of retrieval by dopaminergic D1, β-noradrenergic, serotonergic-1A and cholinergic muscarinic receptors in cortical structures of the rat. Behav. Brain Res. 2001, 124, 1–7.
- Izquierdo, I.; Bevilaqua, L.R.; Rossato, J.I.; Bonini, J.S.; Medina, J.H.; Cammarota, M. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006, 29, 496–505.
- Tsai, S.-Y.; Chou, H.-Y.; The, H.-W.; Chen, C.-M.; Chen, C.-J. The Effects of Chronic Arsenic Exposure from Drinking Water on the Neurobehavioral Development in Adolescence. NeuroToxicology 2003, 24, 747–753.
- Mukherjee, S.C.; Rahman, M.M.; Chowdhury, U.K.; Sengupta, M.K.; Lodh, D.; Chanda, C.R.; Saha, K.C.; Chakraborti, D. Neuropathy in Arsenic Toxicity from Groundwater Arsenic Contamination in West Bengal, India. J. Environ. Sci. Health Part A 2003, 38, 165–183.
- Shokoohi, R.; Khazaei, M.; Karami, M.; Seidmohammadi, A.; Berijani, N.; Khotanlou, H.; Torkshavand, Z. The relationship between chronic exposure to arsenic through drinking water and hearing function in exposed population aged 10–49 years: A cross-sectional study. Ecotoxicol. Environ. Saf. 2021, 211, 111939.
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Factor-Litvak, P.; van Geen, A.; Slavkovich, V.; Lolacono, N.J.; Cheng, Z.; Hussain, I. Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 2004, 112, 1329–1333.
- Watanabe, C.; Inaoka, T.; Matsui, T.; Ishigaki, K.; Murayama, N.; Ohtsuka, R. Effects of arsenic on younger generations. J. Environ. Sci. Health Part A 2003, 38, 129–139.
- Luo, J.-H.; Qiu, Z.-Q.; Shu, W.-Q.; Zhang, Y.-Y.; Zhang, L.; Chen, J.-A. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol. Lett. 2009, 184, 121–125.
- Rodríguez, V.; Carrizales, L.; Mendoza, M.; Fajardo, O.; Giordano, M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol. Teratol. 2002, 24, 743–750.
- Martinez-Finley, E.J.; Ali, A.-M.S.; Allan, A.M. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: Consequence of lower corticosterone receptor levels? Pharmacol. Biochem. Behav. 2009, 94, 271–277.
- Kozul-Horvath, C.D.; Zandbergen, F.; Jackson, B.P.; Enelow, R.I.; Hamilton, J.W. Effects of Low-Dose Drinking Water Arsenic on Mouse Fetal and Postnatal Growth and Development. PLoS ONE 2012, 7, e38249.
- Dong, J.; Su, S.-Y. The Association between Arsenic and Children’s Intelligence: A Meta-analysis. Biol. Trace Elem. Res. 2008, 129, 88–93.
- Liang, C.; Wu, X.; Huang, K.; Yan, S.; Li, Z.; Xia, X.; Pan, W.; Sheng, J.; Tao, R.; Tao, Y.; et al. Domain- and sex-specific effects of prenatal exposure to low levels of arsenic on children’s development at 6 months of age: Findings from the Ma’anshan birth cohort study in China. Environ. Int. 2019, 135, 105112.
- von Ehrenstein, O.S.; Poddar, S.; Yuan, Y.; Mazumder, D.G.; Eskenazi, B.; Basu, A.; Hira-Smith, M.; Ghosh, N.; Lahiri, S.; Haque, R.; et al. Children’s Intellectual Function in Relation to Arsenic Exposure. Epidemiology 2007, 18, 44–51.
- Rocha-Amador, D.; Navarro, M.E.; Carrizales, L.; Morales, R.; Calderon, J. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad. Saúde Pública 2007, 23, S579–S587.
- O’Bryant, S.E.; Edwards, M.; Menon, C.V.; Gong, G.; Barber, R. Long-Term Low-Level Arsenic Exposure Is Associated with Poorer Neuropsychological Functioning: A Project FRONTIER Study. Int. J. Environ. Res. Public Health 2011, 8, 861–874.
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Factor-Litvak, P.; Kline, J.; Van Geen, A.; Slavkovich, V.; LoIacono, N.J.; Levy, D.; et al. Water Arsenic Exposure and Intellectual Function in 6-Year-Old Children in Araihazar, Bangladesh. Environ. Health Perspect. 2007, 115, 285–289.
- Wright, R.O.; Amarasiriwardena, C.; Woolf, A.D.; Jim, R.; Bellinger, D.C. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. NeuroToxicology 2006, 27, 210–216.
- Tseng, C.-H. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol. Appl. Pharmacol. 2004, 197, 67–83.
More
Information
Subjects:
Neurosciences; Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
11 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




