Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eduardo Valarezo | + 1904 word(s) | 1904 | 2022-03-09 10:15:28 | | | |
| 2 | Catherine Yang | Meta information modification | 1904 | 2022-03-11 02:05:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Valarezo, E. Leaves of Chirimoya (Annona cherimola Mill.). Encyclopedia. Available online: https://encyclopedia.pub/entry/20433 (accessed on 08 February 2026).
Valarezo E. Leaves of Chirimoya (Annona cherimola Mill.). Encyclopedia. Available at: https://encyclopedia.pub/entry/20433. Accessed February 08, 2026.
Valarezo, Eduardo. "Leaves of Chirimoya (Annona cherimola Mill.)" Encyclopedia, https://encyclopedia.pub/entry/20433 (accessed February 08, 2026).
Valarezo, E. (2022, March 10). Leaves of Chirimoya (Annona cherimola Mill.). In Encyclopedia. https://encyclopedia.pub/entry/20433
Valarezo, Eduardo. "Leaves of Chirimoya (Annona cherimola Mill.)." Encyclopedia. Web. 10 March, 2022.
Copy Citation
Annona cherimola Mill. is a native species of Ecuador cultivated worldwide for the flavor and properties of its fruit. Hydrodistillation was used to isolate essential oil (EO) of fresh Annona cherimola leaves collected in Ecuadorian Sierra. The EO chemical composition was determined using a non-polar and a polar chromatographic column and enantiomeric distribution with an enantioselective column.
essential oil
Annona cherimola
chemical composition
1. Introduction
Worldwide, the Annonaceae family comprises more than 128 genera and approximately 2106 species and they are mainly distributed in tropical and subtropical regions [1]. For Ecuador, 25 genera, 106 species and 20 endemic species are reported [2]. In a review of the antimalarial properties of the Annonaceae family, 11 species from Annona and Xilopia genus were recognized for their antiparasitic potential. Annonaceae species used in traditional medicine, over the tropical regions, are well documented for having potential for the treatment of parasitic diseases such as Malaria, Chagas and Leishmaniasis as well as other illnesses [3]. Indeed, Annona muricata was one of the most cited species with a variety of medicinal properties including the treatment for the symptoms of malarial infection, fever, liver ailments and headaches [4].
Annona cherimola, A. crassiflora, A. muricata, A. squamosa and A. reticulata are the commercial species, highly valued by their exotic edible fruit. Furthermore, different parts of the tree from these species have been used in folk medicine to treat several conditions including gastrointestinal diseases, diabetes and hypertension [5]. Many secondary metabolites have been reported such as phenols and other bioactive compounds but, the main chemical marker of the genus is a diverse group of polyketides called acetogenins, compounds closely associated to their antiproliferative effect on cancer cell lines [5][6].
Annona cherimola Mill. is a native shrub, widely distributed in the Andean, Coastal, Amazon and Insular regions of Ecuador, between 0–3000 m a.s.l [2]. Currently, this species is cultivated in the subtropical and tropical regions worldwide, especially for its fruit, which is considered exotic. The A. cherimola species is commonly known as “chirimoya” or “chirimoyo” (Spanish language) and “custard-apple” (English language) [7]. The vernacular name “chirimoya” is derived from the Quechua (indigenous language) word “chirimuya”, “chiri” that means cold and “muya” seeds. The fruit of this species is considered one the most appreciated inside the genus. The plant has been used in traditional medicine for the treatment of boils and others skin diseases [8].
Anthropological evidence suggests that the species A. cherimola was cultivated since the times of the Incan Empire and its fruit was considered as an active ingredient in the their diet [9]. Some important compounds have been isolated from this species such as alkaloids as cherimoline, annocherine A, annocherine B, cherianoine and rumocosine H [10], and some amides as cherinonaine, cheritamide, (N-trans-feruloyltyramine, N-trans-caffeoyltyramine, N-cis-caeoyltyramine, dihydro feruloyltyramine, N-trans-feruloylmethoxytyramine, N-cis-feruloylmethoxytyramine, and N-p-coumaroyltyramine [11]. Recent studies have shown that custard apple leaves contain flavonoids and other phenolic compounds with biological properties [12][13] and that alcoholic extracts from the leaves have proapoptotic and antidepressant activities [14].
2. Antibacterial Activity
Microdilution broth method was used to assess the antibacterial activity of essential oil of A. cherimola leaves. Tetracycline was used as a positive control and the maximum evaluated concentration was 1000 µg/mL. The minimum inhibitory concentration (MIC) values and the microorganisms used (seven Gram-negative bacteria and one Gram-positive bacterium) are shown in Table 1. The A. cherimola essential oil reported MIC values of 500 µg/mL against Campylobacter jejuni (ATCC 33560). EO dissolved in aqueous media caused the formation of an emulsion that difficulted the visual observation of bacterial growth particularly with C. jejuni. The reduction of 2,3,5-Triphenyl tetrazolium chloride (TTZ) yield a red color product only in the wells were bacteria developed a well growth. A blank of EO with the same range of concentrations and media was included to discard interferences due to contamination which was also confirmed by reading at 405 nm (data not shown) For the other bacteria, the essential oil did not show activity at the maximum dose tested.
Table 1. Antibacterial activity of essential oil from Annona cherimola leaves.
| Bacteria | Annona cherimola | Positive Control a |
|---|---|---|
| MIC (μg/mL) | ||
| Gram-negative | ||
| Campylobacter jejuni (ATCC 33560) | 500 | 15.65 |
| Escherichia coli (ATCC 25922) | >1000 | 1.95 |
| Klebsiella pneumoniae (ATCC 9997) | 500 | 1.95 |
| Proteus vulgaris (ATCC 8427) | >1000 | 7.81 |
| Pseudomonas aeruginosa (ATCC 27853) | >1000 | 15.62 |
| Salmonella typhimurium (LT2) | >1000 | 3.90 |
| Salmonella enterica (ATCC 29212) | >1000 | 1.95 |
| Gram-positive | ||
| Staphylococcus aureus (ATCC 25923) | >1000 | 1.95 |
a Erythromycin for Campylobacter jejuni and tetracycline for other bacteria.
3. Anticholinesterase Activity
Three different concentrations of the essential oil from A. cherimola leaves were used to determine its anticholinesterase potential. The data obtained by measuring the rate of reaction of AChE against EO are shown in Figure 1. The results plotted as Log (concentration essential oil) vs. normalized response rate of reaction allowed us to calculate the IC50 value. The IC50 value obtained for chirimoya essential oil was 41.51 ± 1.02 µg/mL. The positive control (donepezil) exhibited an IC50 value of 13.80 ± 1.01 nM.
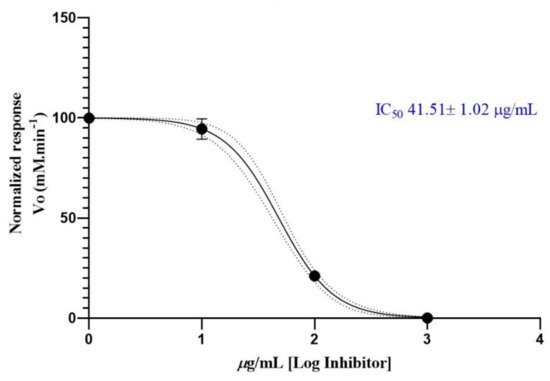
Figure 1. Half-maximum inhibitory concentration of Annona cherimola essential oil against acethylcholinesterase.
4. Enantiomeric Composition, Antioxidant Capacity and Anticholinesterase Activity of Essential Oil from Leaves of Chirimoya (Annona cherimola Mill.)
The essential oil from Annona cherimola exhibited a low yield of 2.5 ± 0.2 mL/Kg [15]. The extraction yield of essential oils is very variable between plant species and depends on different aspects related to the plant such as the part, the age and the time after plant collection and other aspects related to the isolation process such as the pretreatment of the material (drying, grinding, etc.) and the extraction time [16].
The aroma of the Annona species is well recognized and has been studied in some species, however, little has been reported about the essential oil composition of Annona cherimola. In the present study, the main chemical components identified were aliphatic monoterpenes (25.68%) and aliphatic sesquiterpenes (69.40%), which was similar to the information reported by Rabelo et al. [17]. Furthermore, Rios et al. in 2003 [18] reported monoterpenes (6.09%) and sesquiterpenes (76.56%) as the main type of compounds in the A. cherimola EO. On the other hand, the same type of volatile compounds were meaningful in fruits of Annona cherimola (monoterpene 40.3% and sesquiterpene 24.3%) [19].
The major components (>5%) identified in the A. cherimola EO were germacrene D (28.77%), bicyclogermacrene (11.12%), (E)-caryophyllene (10.52%), sabinene (9.05%) and β-pinene (7.93%). The results are different to those reported by Elhawary et al. fβ-caryophyllene with 9.50%, germacrene-D with 17.71% an β-elemene with 25.02% [20], and those reported by Rios et al. reported bicyclogermacrene (18.20%), trans-caryophyllene (11.50%), α-amorphene (7.57%), α-copaene (5.63%) and germacrene D (3.75%) [18]. In addition, Pino observed that the major compounds were α-thujene (18.7 ppm), α-pinene (23 ppm), terpinen-4-ol (19.8 ppm) and germacrene D (17.6 ppm) [19]. Despite the differences in their concentrations, the main component that is common in all the studies is germacrene D. It is well known the influence of different cultivation and climatic factors over the chemical composition of the essential oils.
Due to the relevance of aromatic compounds of the Annona species Ferreira et al. in 2009 compared the essential oil and the volatile compounds of the leaves and fruits of Annona cherimola. The chemical composition for the EO was different to the volatile compounds in fruits, the main compounds in the leaves essential oil were identified in lower quantities, germacrene-D (0.11% to 0.22%), sabinene (not identified), β-pinene (0.79% to 3.60%), (E)-caryophyllene (0.23% to 0.32%) and bicyclogermacrene (not identified) while the main compounds analyzed by headspace solid phase microextraction were methyl butanoate, butyl butanoate, 3-methylbutyl butanoate, 3-methylbutyl 3-methylbutanoate and 5-hydroxymethyl-2-furfural [21].
This is the first report of enantioselective GC-MS analysis of A. cherimola EO, this analysis showed the ocurrence of five pairs of enantiomers and one enantiomerically pure chiral monoterpenoid, β-pinene. The enantiomeric ratio of an essential oil is an important information which could be related with the biological activity, metabolism and organoleptic quality of the enantiomeric pairs [22]. The enantiomeric excess (e.e %) were (−)-α-pinene (1S,5S) (63.99%), (−)-sabinene (1S,5S) (95.91%), (−)-limonene (4R) (27.50%) and (−)-germacrene D (8S) (95.91%).
Regarding their biological activity, the essential oil of Annona cherimola showed moderate antibacterial activity against Campylobacter jejuni (ATCC 33560) and Klebsiella pneumonia (ATCC 9997), both with MIC at 500 μg/mL and no activity for the other bacteria tested (MIC was higher than 1000 μg/mL). Compared to data reported in the literature, Rios et al. in 2003 reported a significant activity against two Gram-positive bacteria Staphylococcus aureus (MIC 250 μg/mL) and Enterococcus faecalis (MIC 500 μg/mL), however, the MIC values for Gram-negative bacteria were higher than 5000 μg/mL [18]. Elhawary et al. in 2013 reported the MIC of EO A. cherimola for Bacillus subtilis (130 μg/mL), Staphylococcus aureus (285 μg/mL), Escherichia coli (110 μg/mL), Pseudomonas aeruginosa (140 μg/mL), and Candida albicans (152 μg/mL) [20]. When the antibacterial activity of pure compounds was analyzed [18] the MIC of trans-caryophyllene, β-pinene, linalool, and other compounds was higher than the value for the essential oil, therefore suggesting that the antibacterial potency could be exerted by a synergistic effect among the constituents above mentioned. The essential oil of Annona species showed a wide range of biological activity, for A. vepretorum Costa et al. in 2012 reported a moderate activity (MIC 500 μg/mL) against Staphylococcus aureus and Staphylococcus epidermis and a significant activity against Candida tropicalis (MIC 100 μg/mL) [23]. Another study, in 2013, Costa et al. observed the antibacterial activity of essential oil of A. salzmannii and A. pickelii against Staphylococcus aureus, Staphylococcus epidermis and Candida tropicalis with MIC of 500 μg/mL [24].
The MIC and minimal microbicidal concentration (MMC) showed that the positive enantiomers of pinene exerted a microbicidal effect against all the fungi and bacteria tested with MIC values ranging from 117 to 4150 µg/mL. However, with concentrations up to 20 mg/mL of the negative enantiomers, no antimicrobial activity was observed [25]. The MIC values against three Gram-positive (B. cereus, E. faecalis and S. aureus) and four Gram-negative (E. coli, K. pneumoniae, M. catarrhalis and P. aeruginosa) bacteria were in the ranges of 3 to 27 mg/mL for (+)-limonene and 2 to 27 mg/mL for (−)-limonene. The greatest difference was obtained against Staphylococcus aureus ATCC 12600 where the (+)-limonene showed a MIC of 14 mg/mL and the (−)-limonene a MIC of 4 mg/mL [26]. Omran et al. found that (−)-limonene had better antifungal activity than (+)-limonene against Aspergillus niger, Aspergillus sp., Candida albicans and Penicillium sp. [27]. It was not possible to find previous studies about the antifungal or antibacterial activity of the (+) and (−) enantiomers of the main compound germacrene D, however, Stranden et al. determined that the two enantiomers of this compound mediate the same kind of information to the receptor neurons of the moth Helicoverpa armigera, but (−)-germacrene D had approximately 10 times stronger effect than (+)-germacrene D [28]. The difference in biological activity of the enantiomers is maintained even when they are mixed with other compounds [26]. The enantiomers of a compound have different biological activities, then, the enantiomeric distribution of the compounds could influence the biological activity for an essential oil.
Regarding their antioxidant effect, the Annona cherimola essential oil showed an SC50 of 470 μg/mL for the DPPH assay while the SC50 was >1000 μg/mL in the ABTS assay. Costa et al. reported as strong the antioxidant activity of EO A. salzamannii and A. pickelii measured by a TLC-based DPPH assay, however, the individual components β-pinene and α-pinene did not show antioxidant activity [24]. Araújo, et al. [29] and Costa, et al. [23] reported a weak antioxidant activity for the EO of A. vepretorum. Another study, Gyesi, et al. in 2019 [30] reported an SC50 of 244.8 μg/mL from the DPPH assay for the EO of A. muricata. The differences between the antioxidant activity of EO and pure compounds could correspond to synergistic effects among the components in the essential oil.
The acetylcholinesterase inhibitory activity of A. cherimola EO has not been previously reported. Chirimoya EO showed an AChE IC50 value of 41.51 μg/mL, this inhibitory activity could be considered very strong compared to the related EO of Piper carpunya (IC50 of 36.42 μg/mL) [31]. The inhibition of AChE due to EO is of relevant interest in the treatment of Alzheimer disease since different studies report in vitro and clinical AChE inhibitory activity. Benny and Tomas summarize the neuroprotective effects of EO and its relevance on Alzheimer disease stating that EO could rebuild the antioxidant status of brain which confer neuroprotective effect as in the case of EO of Coriandrum sativum L., Syzygium aromaticum (L.), Juniperus communis, Rosmarinis officinalis (L.), and other species. The same activity has been observed for pure compounds such as thymol, linalool, α-terpinene, α-terpineol, carvacrol, (E)-β-caryophyllene, α-pinene, and eugenol [32].
References
- The Plant List. Annonaceae. Available online: http://www.theplantlist.org/ (accessed on 12 September 2021).
- Jørgesen, P.M.; León-Yáñez, S. Catalogue of the Vascular Plants of Ecuador. Available online: http://legacy.tropicos.org/ProjectAdvSearch.aspx?projectid=2 (accessed on 11 July 2020).
- Frausin, G.; Lima, R.B.S.; Hidalgo, A.F.; Maas, P.; Pohlit, A.M. Plants of the annonaceae traditionally used as antimalarials: A review. Rev. Bras. Frutic. 2014, 36, 315–337.
- Tajbakhsh, E.; Kwenti, T.E.; Kheyri, P.; Nezaratizade, S.; Lindsay, D.S.; Khamesipour, F. Antiplasmodial, antimalarial activities and toxicity of African medicinal plants: A systematic review of literature. Malar. J. 2021, 20, 349.
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Pérez-Herrera, A.; Rodríguez-Fernández, D.J. Biological Activities of Plants from Genus Annona; IntechOpen: London, UK, 2018; p. 168.
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625.
- Arunjyothi, B.; Venkatesh, K.; Chakrapani, P.; Anupalli, R.R. Phytochemical and Pharmacological potential of Annona cherimola—A Review. Int. J. Phytomed. 2012, 3, 9.
- Gayoso Bazán, G.; Chang Chávez, L. Annona cherimola Mill. “chirimoya” (Annonaceae), una fruta utilizada como alimento en el Perú prehispánico. Arnaldoa 2017, 24, 619–634.
- Gupta-Elera, G.; Garrett, A.R.; Martinez, A.; Robison, R.A.; O’Neill, K.L. The antioxidant properties of the cherimoya (Annona cherimola) fruit. Food Res. Int. 2011, 44, 2205–2209.
- Chen, C.-Y.; Chang, F.-R.; Pan, W.-B.; Wu, Y.-C. Four alkaloids from Annona cherimola. Phytochemistry 2001, 56, 753–757.
- Chen, C.-Y.; Chang, F.-R.; Wu, Y.-C. Cherinonaine, a novel dimeric amide from the stems of Annona cherimola. Tetrahedron Lett. 1998, 39, 407–410.
- Díaz-de-Cerio, E.; Aguilera-Saez, L.M.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Fernández, I.; Arráez-Román, D. Characterization of bioactive compounds of Annona cherimola L. leaves using a combined approach based on HPLC-ESI-TOF-MS and NMR. Anal. Bioanal. Chem. 2018, 410, 3607–3619.
- Albuquerque, T.G.; Santos, F.; Sanches-Silva, A.; Beatriz Oliveira, M.; Bento, A.C.; Costa, H.S. Nutritional and phytochemical composition of Annona cherimola Mill. fruits and by-products: Potential health benefits. Food Chem. 2016, 193, 187–195.
- Ammoury, C.; Younes, M.; El Khoury, M.; Hodroj, M.H.; Haykal, T.; Nasr, P.; Sily, M.; Taleb, R.I.; Sarkis, R.; Khalife, R.; et al. The pro-apoptotic effect of a Terpene-rich Annona cherimola leaf extract on leukemic cell lines. BMC Complement. Altern. Med. 2019, 19, 365.
- Molares, S.; González, S.B.; Ladio, A.; Agueda Castro, M. Etnobotánica, anatomía y caracterización físico-química del aceite esencial de Baccharis obovata Hook. et Arn. (Asteraceae: Astereae). Acta Bot. Bras. 2009, 23, 578–589.
- Valarezo, E.; Ojeda-Riascos, S.; Cartuche, L.; Andrade-González, N.; González-Sánchez, I.; Meneses, M.A. Extraction and Study of the Essential Oil of Copal (Dacryodes peruviana), an Amazonian Fruit with the Highest Yield Worldwide. Plants 2020, 9, 1658.
- Rabelo, S.V.; Quintans, J.d.S.S.; Costa, E.V.; Guedes da Silva Almeida, J.R.; Quintans Júnior, L.J. Chapter 24—Annona Species (Annonaceae) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 221–229.
- Ríos, M.Y.; Castrejón, F.; Robledo, N.; León, I.; Rojas, G.; Navarro, V. Chemical Composition and Antimicrobial Activity of the Essential Oils from Annona cherimola (Annonaceae). Rev. Soc. Quim. Mex. 2003, 47, 139–142.
- Pino, J.A. Volatile Components of Cuban Annona Fruits. J. Essent. Oil Res. 2000, 12, 613–616.
- Elhawary, S.S.; El Tantawy, M.E.; Rabeh, M.A.; Fawaz, N.E. DNA fingerprinting, chemical composition, antitumor and antimicrobial activities of the essential oils and extractives of four Annona species from Egypt. J. Nat. Sci. Res. 2013, 13, 59–68.
- Ferreira, L.; Perestrelo, R.; Câmara, J.S. Comparative analysis of the volatile fraction from Annona cherimola Mill. cultivars by solid-phase microextraction and gas chromatography—Quadrupole mass spectrometry detection. Talanta 2009, 77, 1087–1096.
- Barba, C.; Santa-María, G.; Herraiz, M.; Martínez, R.M. Direct enantiomeric analysis of Mentha essential oils. Food Chem. 2013, 141, 542–547.
- Costa, E.V.; Dutra, L.M.; Nogueira, P.C.d.L.; Moraes, V.R.D.S.; Salvador, M.J.; Ribeiro, L.H.G.; Gadelha, F.R. Essential Oil from the Leaves of Annona vepretorum: Chemical Composition and Bioactivity. Nat. Prod. Commun. 2012, 7, 1934578X1200700240.
- Costa, E.V.; Dutra, L.M.; Salvador, M.J.; Ribeiro, L.H.G.; Gadelha, F.R.; de Carvalho, J.E. Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceae), and their antitumour and trypanocidal activities. Nat. Prod. Res. 2013, 27, 997–1001.
- Silva, A.C.R.d.; Lopes, P.M.; Azevedo, M.M.B.d.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of a-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316.
- Vuuren, S.F.v.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Fragr. J. 2007, 22, 540–544.
- Mahdavi Omran, S.; Moodi, M.A.; Norozian Amiri, S.M.B.; Mosavi, S.J.; Ghazi Mir Saeed, S.A.M.; Jabbari Shiade, S.M.; Kheradi, E.; Salehi, M. The Effects of Limonene and Orange Peel Extracts on Some Spoilage Fungi. Int. J. Mol. Clin. Microbiol. 2011, 1, 82–86.
- Stranden, M.; Borg-Karlson, A.-K.; Mustaparta, H. Receptor Neuron Discrimination of the Germacrene D Enantiomers in the Moth Helicoverpa armigera. Chem. Senses 2002, 27, 143–152.
- Araújo, C.d.S.; de Oliveira, A.P.; Lima, R.N.; Alves, P.B.; Diniz, T.C.; da Silva, A.J.R.G. Chemical constituents and antioxidant activity of the essential oil from leaves of Annona vepretorum Mart. (Annonaceae). Pharmacogn. Mag. 2015, 11, 615–618.
- Gyesi, J.N.; Opoku, R.; Borquaye, L.S. Chemical composition, total phenolic content, and antioxidant activities of the essential oils of the leaves and fruit pulp of Annona muricata L. (Soursop) from Ghana. Biochem. Res. Int. 2019, 2019, 4164576.
- Valarezo, E.; Rivera, J.X.; Coronel, E.; Barzallo, M.A.; Calva, J.; Cartuche, L.; Meneses, M.A. Study of Volatile Secondary Metabolites Present in Piper carpunya Leaves and in the Traditional Ecuadorian Beverage Guaviduca. Plants 2021, 10, 338.
- Benny, A.; Thomas, J. Essential oils as treatment strategy for Alzheimer’s disease: Current and future perspectives. Planta Med. 2019, 85, 239–248.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
910
Revisions:
2 times
(View History)
Update Date:
11 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




