Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Subodh Samrat | + 1225 word(s) | 1225 | 2022-03-01 04:42:35 | | | |
| 2 | Rita Xu | Meta information modification | 1225 | 2022-03-11 03:38:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Samrat, S. Antiviral Agents against Flavivirus Protease. Encyclopedia. Available online: https://encyclopedia.pub/entry/20431 (accessed on 08 February 2026).
Samrat S. Antiviral Agents against Flavivirus Protease. Encyclopedia. Available at: https://encyclopedia.pub/entry/20431. Accessed February 08, 2026.
Samrat, Subodh. "Antiviral Agents against Flavivirus Protease" Encyclopedia, https://encyclopedia.pub/entry/20431 (accessed February 08, 2026).
Samrat, S. (2022, March 10). Antiviral Agents against Flavivirus Protease. In Encyclopedia. https://encyclopedia.pub/entry/20431
Samrat, Subodh. "Antiviral Agents against Flavivirus Protease." Encyclopedia. Web. 10 March, 2022.
Copy Citation
Flaviviruses cause a significant amount of mortality and morbidity, especially in regions where they are endemic. A recent example is the outbreak of Zika virus throughout the world. Development of antiviral drugs against different viral targets is as important as the development of vaccines. During viral replication, a single polyprotein precursor (PP) is produced and further cleaved into individual proteins by a viral NS2B-NS3 protease complex together with host proteases.
flavivirus
NS2B-NS3
Zika virus
dengue virus
West Nile virus
1. Introduction
The Flavivirus genus of the Flaviviridae family includes more than 70 related arthropod-borne viruses [1][2][3][4]. The most common and representative members are the dengue virus (DENV) with four serotypes (DENV-1, -2, -3, and -4), Zika (ZIKV), West Nile (WNV), Yellow Fever (YFV), Japanese-encephalitis (JEV), and tick-borne encephalitis (TBEV) viruses [5][6][7]. These are the causative agents for viral hemorrhagic fever and encephalitis in human beings.
Susceptible hosts are normally infected by arthropod vectors carrying flaviviruses [1][8][9][10]. Many flaviviruses are restricted to a particular region, though occasional spillovers of viruses can occur, which pose a significant challenge for international health care. For examples, WNV jumped from the Middle East into the Americas [11][12]; and ZIKV spread from Africa to Southeast Asia, the islands of Polynesia, and later to Brazil in 2015–2016, causing an epidemic [3][13][14].
Specific antiviral therapeutics or vaccines are not available to combat most flavivirus infections, except for YFV, DENV, JEV, Kyasanur forest disease virus, and TBEV [10]. In the case of DENV, vaccine design and development have been significantly impeded because of an antibody-dependent enhancement effect due to the presence of four DENV serotypes, leading to a more severe disease phenotype in subsequent natural infections [10][15]. It remains a challenge for the scientific community to develop a DENV vaccine that simultaneously elicits a balanced tetravalent immunity against all four DENV serotypes. In 2019, the United States Food and Drug Administration (FDA) approved the live-attenuated, tetravalent vaccine, Dengvaxia (from Sanofi Pasteur), but only for individuals living in endemic areas between 9–16 years of age with prior DENV infection [15][16]. These challenges increase the demand to develop therapeutics and vaccines that target different flaviviruses.
2. Flavivirus Genome Organization
The Flavivirus genome consists of a positive-sense single-stranded RNA which is ∼11 kb in length, consisting of a single long open reading frame (ORF) flanked by a 5′ untranslated region (UTR) and a 3′ UTR [3][4][17][18]. The viral genome is not polyadenylated but capped as in cellular mRNA. The single ORF encodes a PP that is further processed by proteases from the host and the viral NS2B-NS3 complex. The flavivirus protease is highly conserved and essential for virus replication. The viral PP is processed to three structural proteins (Capsid, pr-Membrane, and Envelope) and seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [4][18]. Three structural proteins form the virus shell, whereas seven NS proteins participate in the membrane-bound replication complex. Among these NS proteins, only NS3 and NS5 bear enzymatic functions [19][20][21] (Figure 1).
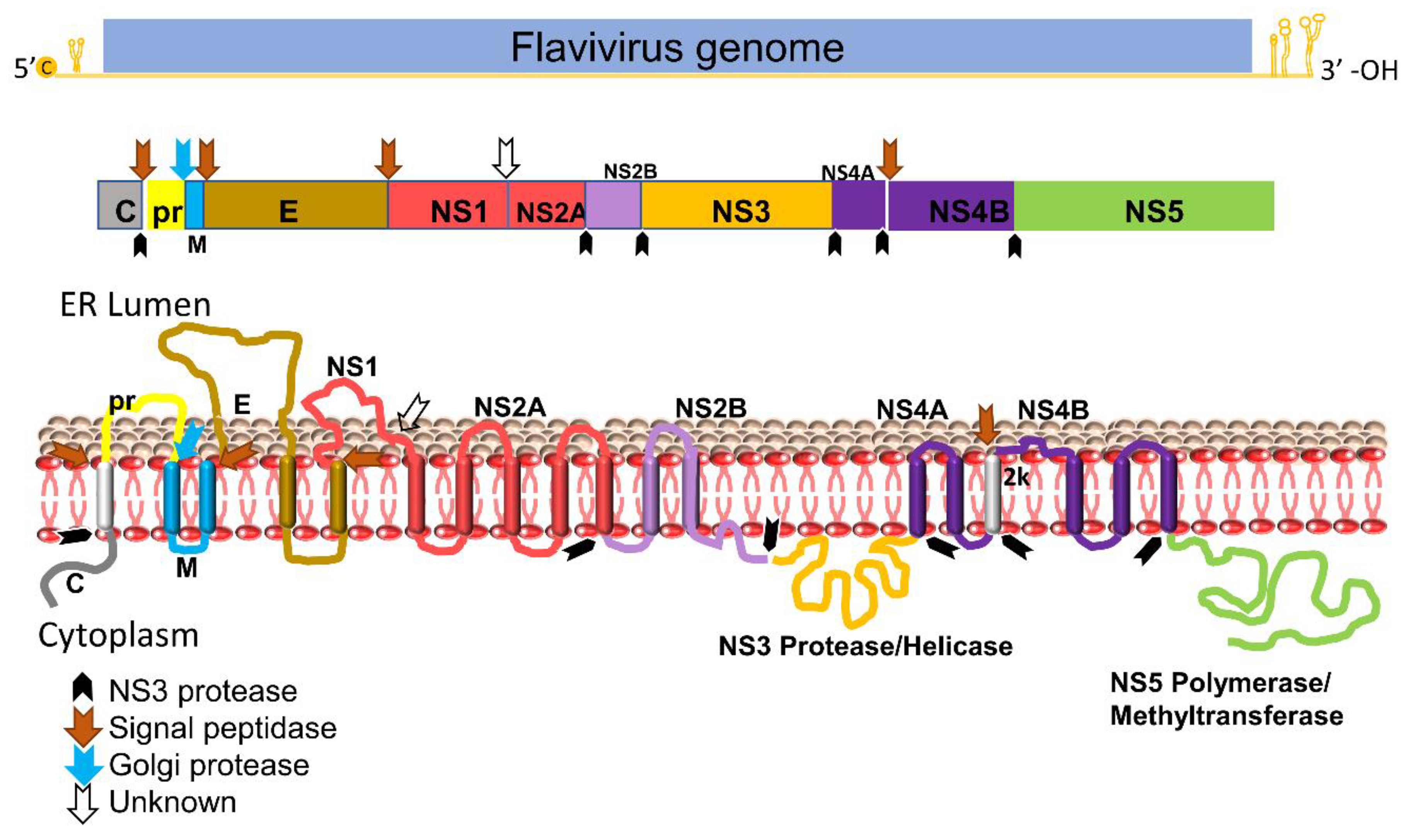
Figure 1. Schematic diagram of flavivirus PP organization, processing, and predicted membrane topology of a mature protein. Top: the representative flavivirus genome. c, RNA cap. Middle: schematic representation of the structural and nonstructural proteins within PP. Black arrows denote cleavage by the viral NS2B-NS3 protease complex, whereas the blue arrow indicates cleavage by the Golgi protease and brown arrows denote cleavage by signal peptidase. White blank arrow indicates unknown protease. Bottom: putative membrane topology of PP predicted by biochemical and cellular analyses, and protease cleavage sites (indicated by arrows).
3. Flavivirus Protease
NS3 carries multiple enzymatic functions, including protease, helicase, and triphosphatase activities [22][23]. The NS3 N-terminal domain (amino acids (aa) 1–180) is a trypsin-like serine protease with a classic catalytic triad such as Ser135-His51-Asp75 for DENV-2, JEV, WNV and ZIKV, and Ser138-His53-Asp77 for YFV [3][24][25][26][27][28]. A small hydrophilic proportion of NS2B is required as a co-factor for activating the NS3 protease enzymatic function [29][30][31]. The NS3 C-terminus domain (aa 180–618) is a superfamily 2 ATPase/helicase that binds to the 3′ end of transient dsRNA and unwinds it in a 3′→5′ direction [32]. In addition, the C-terminal domain of NS3 also has an RNA 5′-triphosphatase activity to cap viral RNA [21][33].
NS2B is an integral membrane protein of 14 kDa in size, with three domains: a central hydrophilic domain flanked by two transmembrane segments. The central hydrophilic region of 47 amino acids (spanning aa 49–96) interacts with the NS3 protease as a co-factor (Figure 1) [31][34]. It has been reported that flavivirus NS3 protein is catalytically inactive as a protease in the absence of NS2B in either linked [35][36][37] or unlinked formats [38][39][40][41][42]. Moreover, the NS3 protease domain expressed in E. coli is not very soluble without its co-factor NS2B. Proteases among flaviviruses have significant similarity on both structural and sequence levels [43].
The flavivirus NS2B-NS3 protease is one of the most attractive and validated targets for developing a pan flavivirus treatment. Viral proteases in general are highly promising drug targets. Examples include ten HIV-1 protease inhibitors (PIs) [44][45][46] and two HCV PIs [47][48][49]. Thus, it is plausible that a protease inhibitor for flaviviruses will be efficacious in the clinic. This is also evident by the fact that the NS2B-NS3 proteases from different flaviviruses, such as DENV, ZIKV, and WNV, show a high degree of similarity in their sequences and structures. However, lessons from HIV and HCV drug development suggest that PIs have certain drawbacks. Viral variants resistant to PIs were rapidly developed, because both HIV and HCV cause chronic infections [50]. In contrast, most flaviviruses cause acute infections, and drug resistance may be less of a concern.
The NS3 active site has been the main focus of developing flavivirus protease inhibitors. However, only limited success has been achieved, possibly due to the flat and featureless active site and requirement of a charged substrate to bind with P1 and P2 sites which denote the first and second positions of amino acids outward from the protease cleavage site to the N-terminus of the protease substrate [51]. Many of the active site inhibitors are only effective in biochemical assays and show low cellular antiviral activity and poor bioavailability in vivo [50].
4. Structural Insight of NS2B-NS3 Protease
Structural study of NS2B–NS3 led to better screening assays to identify PIs [52][53]. The NS2B and NS3 protein complex adopts two different conformations [14]. An “open” inactive conformation is present for the NS2B C-terminal portion when substrate or an active-site inhibitor is absent (Figure 2A) [14][54]. However, binding of an inhibitor or substrate triggered a “closed” conformation for the C-terminal portion of NS2B [55] (Figure 2B). The sequences of the DENV-2 and DENV-3 proteases in Figure 2A and Figure 2B, respectively, are 68% identical. Binding of NS2B to NS3 is required for NS3 function; mutations that disrupt NS2B-NS3 interactions greatly diminish the proteolytic activity of the complex [14][56][57].
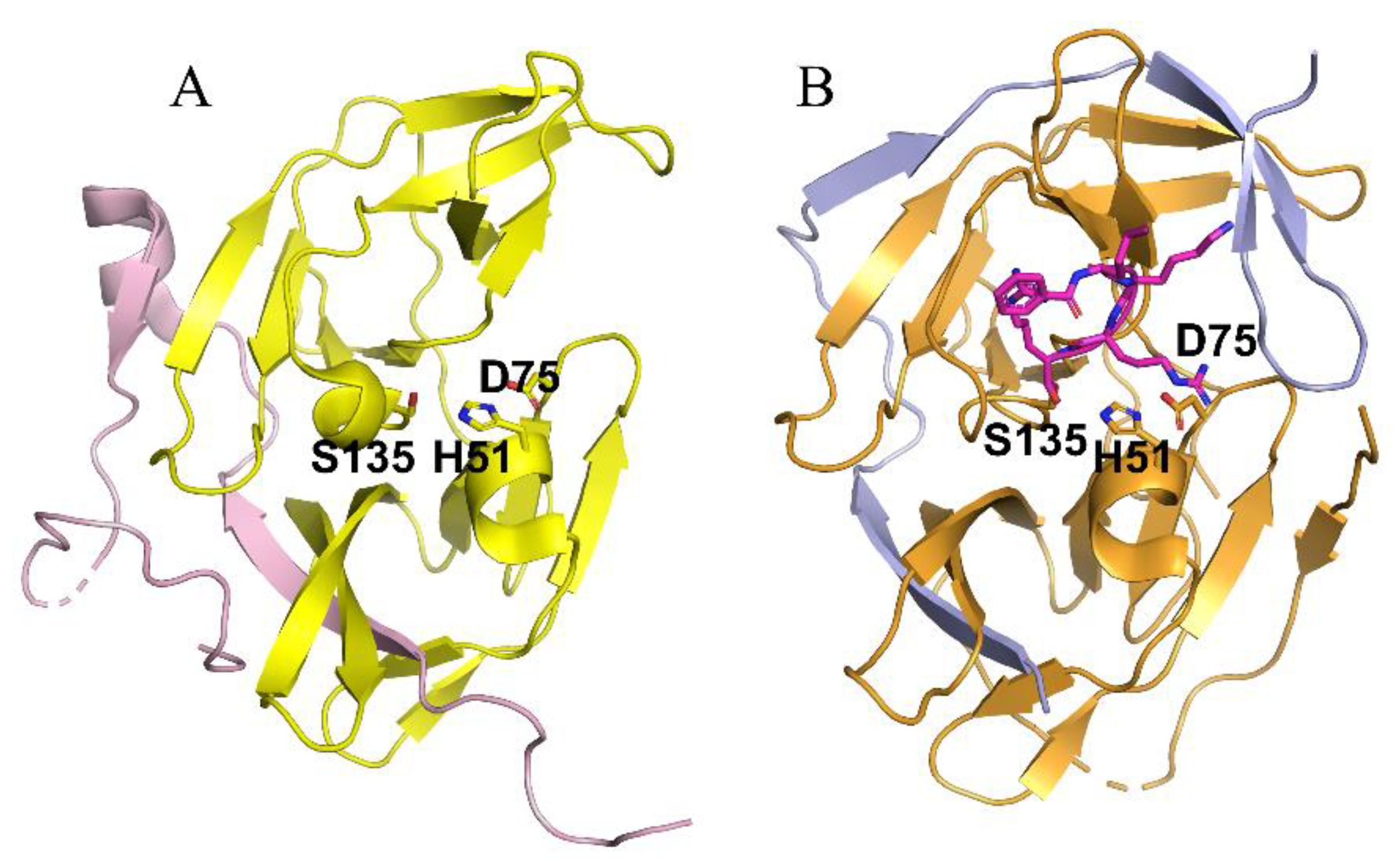
Figure 2. Crystal structures of the NS2B-NS3 proteases of DENV-2 (A) and DENV-3 (B) in the absence (A) and presence (B) of a substrate analog. (A) The open inactive conformation of the DENV-3 NS2B-NS3 protease in the unbound state (PDB ID of 2FOM). The NS2B cofactor is colored in purple and NS3 protease in yellow. (B) The closed active conformation of the DENV-3 NS2B-NS3 protease in complex with a substrate peptide analog (magenta sticks) (PDB ID of 3U1I). The NS2B cofactor is colored in blue and NS3 protease in orange. Superimposed structure of inactive and active conformation. The catalytic triad His51-Asp75-Ser135 is displayed in stick representation.
References
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812.
- Best, S.M. Flaviviruses. Curr. Biol. 2016, 26, R1258–R1260.
- Li, Z.; Zhang, J.; Li, H. Flavivirus NS2B/NS3 Protease: Structure, Function, and Inhibition. In Viral Proteases and Their Inhibitors; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 163–188.
- Brecher, M.; Zhang, J.; Li, H. The flavivirus protease as a target for drug discovery. Virol. Sin. 2013, 28, 326–336.
- Kayesh, M.E.H.; Tsukiyama-Kohara, K. Mammalian animal models for dengue virus infection: A recent overview. Arch. Virol. 2021, 167, 31–44.
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507.
- Gubler, D.J. Dengue/dengue haemorrhagic fever: History and current status. Novartis Found. Symp. 2006, 277, 3–16; discussion 16–22, 71–13, 251–253.
- Pfeffer, M.; Dobler, G. Emergence of zoonotic arboviruses by animal trade and migration. Parasites Vectors 2010, 3, 35.
- Enserink, M. INFECTIOUS DISEASES. An obscure mosquito-borne disease goes global. Science 2015, 350, 1012–1013.
- Holbrook, M. Historical Perspectives on Flavivirus Research. Viruses 2017, 9, 97.
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109.
- Roehrig, J. West Nile Virus in the United States—A Historical Perspective. Viruses 2013, 5, 3088–3108.
- Pierson, T.C.; Diamond, M.S. The emergence of Zika virus and its new clinical syndromes. Nature 2018, 560, 573–581.
- Brecher, M.; Li, Z.; Liu, B.; Zhang, J.; Koetzner, C.A.; Alifarag, A.; Jones, S.A.; Lin, Q.; Kramer, L.D.; Li, H. A conformational switch high-throughput screening assay and allosteric inhibition of the flavivirus NS2B-NS3 protease. PLoS Pathog. 2017, 13, e1006411.
- Solomon, T.; Mallewa, M. Dengue and other emerging flaviviruses. J. Infect. 2001, 42, 104–115.
- Norshidah, H.; Vignesh, R.; Lai, N.S. Updates on Dengue Vaccine and Antiviral: Where Are We Heading? Molecules 2021, 26, 6768.
- Gebhard, L.G.; Filomatori, C.V.; Gamarnik, A.V. Functional RNA Elements in the Dengue Virus Genome. Viruses 2011, 3, 1739–1756.
- Zhang, X.; Zhang, Y.; Jia, R.; Wang, M.; Yin, Z.; Cheng, A. Structure and function of capsid protein in flavivirus infection and its applications in the development of vaccines and therapeutics. Vet. Res. 2021, 52, 98.
- Zhao, R.; Wang, M.; Cao, J.; Shen, J.; Zhou, X.; Wang, D.; Cao, J. Flavivirus: From Structure to Therapeutics Development. Life 2021, 11, 615.
- Obi, J.O.; Gutiérrez-Barbosa, H.; Chua, J.V.; Deredge, D.J. Current Trends and Limitations in Dengue Antiviral Research. Trop. Med. Infect. Dis 2021, 6, 180.
- Bollati, M.; Alvarez, K.; Assenberg, R.; Baronti, C.; Canard, B.; Cook, S.; Coutard, B.; Decroly, E.; de Lamballerie, X.; Gould, E.A.; et al. Structure and functionality in flavivirus NS-proteins: Perspectives for drug design. Antivir. Res. 2010, 87, 125–148.
- Natarajan, S. NS3 protease from flavivirus as a target for designing antiviral inhibitors against dengue virus. Genet. Mol. Biol. 2010, 33, 214–219.
- Assenberg, R.; Mastrangelo, E.; Walter, T.S.; Verma, A.; Milani, M.; Owens, R.J.; Stuart, D.I.; Grimes, J.M.; Mancini, E.J. Crystal structure of a novel conformational state of the flavivirus NS3 protein: Implications for polyprotein processing and viral replication. J. Virol. 2009, 83, 12895–12906.
- Shi, P.Y.; Kauffman, E.B.; Ren, P.; Felton, A.; Tai, J.H.; Dupuis, A.P., 2nd; Jones, S.A.; Ngo, K.A.; Nicholas, D.C.; Maffei, J.; et al. High-throughput detection of West Nile virus RNA. J. Clin. Microbiol. 2001, 39, 1264–1271.
- Chen, X.; Yang, K.; Wu, C.; Chen, C.; Hu, C.; Buzovetsky, O.; Wang, Z.; Ji, X.; Xiong, Y.; Yang, H. Mechanisms of activation and inhibition of Zika virus NS2B-NS3 protease. Cell Res. 2016, 26, 1260–1263.
- Chambers, T.J.; Weir, R.C.; Grakoui, A.; McCourt, D.W.; Bazan, J.F.; Fletterick, R.J.; Rice, C.M. Evidence that the N-terminal domain of nonstructural protein NS3 from yellow fever virus is a serine protease responsible for site-specific cleavages in the viral polyprotein. Proc. Natl. Acad. Sci. USA 1990, 87, 8898–8902.
- Löhr, K.; Knox, J.E.; Phong, W.Y.; Ma, N.L.; Yin, Z.; Sampath, A.; Patel, S.J.; Wang, W.L.; Chan, W.L.; Rao, K.R.R.; et al. Yellow fever virus NS3 protease: Peptide-inhibition studies. J. Gen. Virol. 2007, 88, 2223–2227.
- Chappell, K.J.; Nall, T.A.; Stoermer, M.J.; Fang, N.X.; Tyndall, J.D.; Fairlie, D.P.; Young, P.R. Site-directed mutagenesis and kinetic studies of the West Nile Virus NS3 protease identify key enzyme-substrate interactions. J. Biol. Chem. 2005, 280, 2896–2903.
- Falgout, B.; Pethel, M.; Zhang, Y.M.; Lai, C.J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J. Virol. 1991, 65, 2467–2475.
- Cahour, A.; Falgout, B.; Lai, C.J. Cleavage of the dengue virus polyprotein at the NS3/NS4A and NS4B/NS5 junctions is mediated by viral protease NS2B-NS3, whereas NS4A/NS4B may be processed by a cellular protease. J. Virol. 1992, 66, 1535–1542.
- Jan, L.R.; Yang, C.S.; Trent, D.W.; Falgout, B.; Lai, C.J. Processing of Japanese encephalitis virus non-structural proteins: NS2B-NS3 complex and heterologous proteases. J. Gen. Virol. 1995, 76 Pt 3, 573–580.
- Benarroch, D.; Selisko, B.; Locatelli, G.A.; Maga, G.; Romette, J.L.; Canard, B. The RNA helicase, nucleotide 5’-triphosphatase, and RNA 5’-triphosphatase activities of Dengue virus protein NS3 are Mg2+-dependent and require a functional Walker B motif in the helicase catalytic core. Virology 2004, 328, 208–218.
- Van Den Elsen, K.; Quek, J.P.; Luo, D. Molecular Insights into the Flavivirus Replication Complex. Viruses 2021, 13, 956.
- Clum, S.; Ebner, K.E.; Padmanabhan, R. Cotranslational Membrane Insertion of the Serine Proteinase Precursor NS2B-NS3(Pro) of Dengue Virus Type 2 Is Required for Efficient in Vitro Processing and Is Mediated through the Hydrophobic Regions of NS2B. J. Biol. Chem. 1997, 272, 30715–30723.
- Xu, T.; Sampath, A.; Chao, A.; Wen, D.; Nanao, M.; Chene, P.; Vasudevan, S.G.; Lescar, J. Structure of the Dengue Virus Helicase/Nucleoside Triphosphatase Catalytic Domain at a Resolution of 2.4 Å. J. Virol. 2005, 79, 10278–10288.
- Lescar, J.; Luo, D.; Xu, T.; Sampath, A.; Lim, S.P.; Canard, B.; Vasudevan, S.G. Towards the design of antiviral inhibitors against flaviviruses: The case for the multifunctional NS3 protein from Dengue virus as a target. Antivir. Res. 2008, 80, 94–101.
- Yamashita, T.; Unno, H.; Mori, Y.; Tani, H.; Moriishi, K.; Takamizawa, A.; Agoh, M.; Tsukihara, T.; Matsuura, Y. Crystal structure of the catalytic domain of Japanese encephalitis virus NS3 helicase/nucleoside triphosphatase at a resolution of 1.8 A. Virology 2008, 373, 426–436.
- Kim, Y.M.; Gayen, S.; Kang, C.; Joy, J.; Huang, Q.; Chen, A.S.; Wee, J.L.; Ang, M.J.; Lim, H.A.; Hung, A.W.; et al. NMR analysis of a novel enzymatically active unlinked dengue NS2B-NS3 protease complex. J. Biol. Chem. 2013, 288, 12891–12900.
- Shannon, A.E.; Chappell, K.J.; Stoermer, M.J.; Chow, S.Y.; Kok, W.M.; Fairlie, D.P.; Young, P.R. Simultaneous uncoupled expression and purification of the Dengue virus NS3 protease and NS2B co-factor domain. Protein Expr. Purif. 2016, 119, 124–129.
- Phong, W.Y.; Moreland, N.J.; Lim, S.P.; Wen, D.; Paradkar, P.N.; Vasudevan, S.G. Dengue protease activity: The structural integrity and interaction of NS2B with NS3 protease and its potential as a drug target. Biosci. Rep. 2011, 31, 399–409.
- Hilgenfeld, R.; Lei, J.; Zhang, L. The Structure of the Zika Virus Protease, NS2B/NS3(pro). Adv. Exp. Med. Biol. 2018, 1062, 131–145.
- Wu, C.F.; Wang, S.H.; Sun, C.M.; Hu, S.T.; Syu, W.J. Activation of dengue protease autocleavage at the NS2B-NS3 junction by recombinant NS3 and GST-NS2B fusion proteins. J. Virol. Methods 2003, 114, 45–54.
- Felicetti, T.; Manfroni, G.; Cecchetti, V.; Cannalire, R. Broad-Spectrum Flavivirus Inhibitors: A Medicinal Chemistry Point of View. ChemMedChem 2020, 15, 2391–2419.
- Wensing, A.M.; van Maarseveen, N.M.; Nijhuis, M. Fifteen years of HIV Protease Inhibitors: Raising the barrier to resistance. Antivir. Res. 2010, 85, 59–74.
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 5172–5208.
- Abdel-Rahman, H.M.; Al-karamany, G.S.; El-Koussi, N.A.; Youssef, A.F.; Kiso, Y. HIV protease inhibitors: Peptidomimetic drugs and future perspectives. Curr. Med. Chem. 2002, 9, 1905–1922.
- Wyles, D.L. Antiviral Resistance and the Future Landscape of Hepatitis C Virus Infection Therapy. J. Infect. Dis. 2013, 207, S33–S39.
- Zhang, X. Direct anti-HCV agents. Acta Pharm. Sin. B 2016, 6, 26–31.
- Meewan, I.; Zhang, X.; Roy, S.; Ballatore, C.; O’Donoghue, A.J.; Schooley, R.T.; Abagyan, R. Discovery of New Inhibitors of Hepatitis C Virus NS3/4A Protease and Its D168A Mutant. ACS Omega 2019, 4, 16999–17008.
- Ali, A.; Aydin, C.; Gildemeister, R.; Romano, K.P.; Cao, H.; Ozen, A.; Soumana, D.; Newton, A.; Petropoulos, C.J.; Huang, W.; et al. Evaluating the role of macrocycles in the susceptibility of hepatitis C virus NS3/4A protease inhibitors to drug resistance. ACS Chem. Biol. 2013, 8, 1469–1478.
- Lin, K.H.; Nalivaika, E.A.; Prachanronarong, K.L.; Yilmaz, N.K.; Schiffer, C.A. Dengue Protease Substrate Recognition: Binding of the Prime Side. ACS Infect. Dis. 2016, 2, 734–743.
- Choksupmanee, O.; Hodge, K.; Katzenmeier, G.; Chimnaronk, S. Structural Platform for the Autolytic Activity of an Intact NS2B–NS3 Protease Complex from Dengue Virus. Biochemistry 2012, 51, 2840–2851.
- Huang, Q.; Li, Q.; Joy, J.; Chen, A.S.; Ruiz-Carrillo, D.; Hill, J.; Lescar, J.; Kang, C. Lyso-myristoyl phosphatidylcholine micelles sustain the activity of Dengue non-structural (NS) protein 3 protease domain fused with the full-length NS2B. Protein Expr. Purif. 2013, 92, 156–162.
- Erbel, P.; Schiering, N.; D’Arcy, A.; Renatus, M.; Kroemer, M.; Lim, S.P.; Yin, Z.; Keller, T.H.; Vasudevan, S.G.; Hommel, U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 2006, 13, 372–373.
- Noble, C.G.; Seh, C.C.; Chao, A.T.; Shi, P.Y. Ligand-Bound Structures of the Dengue Virus Protease Reveal the Active Conformation. J. Virol. 2012, 86, 438–446.
- Hale, B.G.; Randall, R.E.; Ortín, J.; Jackson, D. The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 2008, 89, 2359–2376.
- Niyomrattanakit, P.; Winoyanuwattikun, P.; Chanprapaph, S.; Angsuthanasombat, C.; Panyim, S.; Katzenmeier, G. Identification of residues in the dengue virus type 2 NS2B cofactor that are critical for NS3 protease activation. J. Virol. 2004, 78, 13708–13716.
More
Information
Subjects:
Infectious Diseases
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
951
Revisions:
2 times
(View History)
Update Date:
11 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




