Patient-controlled analgesia (PCA) is an effective method for controlling acute pain, including postoperative pain in adults and in children from five years of age, pain resulting from labor, trauma, or other medical situations, or chronic and malignant pain. The treatment consists of a mini-computer-controlled infusion pump permitting the administration of on-demand, continuous, or combined doses of analgesic (mainly opioid) variations in response to therapy, which allows pain to be significantly controlled. Intravenous (IV)-PCA minimizes individual pharmacodynamics and pharmacokinetic differences and is widely accepted as a reference method for mild or severe postoperative pain. IV-PCA is the most studied route of PCA; other delivery methods have been extensively reported in the literature. In addition, IV-PCA usually voids the gap between pain sensation and analgesic administration, permitting better recovery and fewer side effects. The most commonly observed complications are nausea and vomiting, pruritus, respiratory depression, sedation, confusion and urinary retention. However, human factors such as pharmacy preparation and device programming can also be involved in the occurrence of these complications, while device failure is much less of an issue.
1. Introduction
Patient-controlled analgesia (PCA) has been used since the early 1970s to relieve multiple categories of pain, including acute, such as postoperative or labor pain, or chronic, such as palliative care or cancer pain
[1][2]. The goal of PCA is to efficiently deliver pain relief at a patient’s preferred dose and schedule by allowing them to administer a predetermined bolus dose of medication on-demand at the press of a button
[3]. Boluses can be administered alone or coupled with a continuous background infusion of opioids using a dedicated pump.
According to the revised international association for the study of pain definition
[4] the new definition of pain is the following: An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage. Acute pain is a type of pain that lasts less than 3 months and is generally related to soft tissue injury or damage, such as cuts. It gradually resolves as the injured tissues heal. One of the most common types of acute pain is postoperative pain, which arises in the aftermath of surgery.
Although significant improvement has been made in the anticipation and management of postoperative pain in recent decades, a non-negligible percentage of patients might still have moderate to severe postoperative pain
[5].
Patient-controlled analgesia (PCA) preceded by initial intravenous titration is an effective strategy for postoperative analgesia, as it may rapidly provide an adequate analgesic dose upon arrival at the postoperative care unit (PACU).
While intravenous and epidural administration remain the most commonly used modes of PCA, several alternative modes are also available in the clinical setting. These alternative routes of administration include oral, transdermal, inhaled and intranasal, each with its own potential benefits or risks
[3].
2. Patient-Controlled Analgesia for Acute Postoperative Pain
2.1. Interindividual Variability
Multiple studies involving morphine for postoperative analgesia display a wide range of inter- or intra-individual variability in morphine requirements but also in plasma morphine concentrations
[6][7][8][9]. By adapting to patients’ needs, PCA is highly compliant with interindividual needs.
2.2. PCA Concept
The effectiveness of PCA is mostly related to the old concept of “minimal effective analgesic concentration” (MEAC), defined as the smallest plasma concentration of morphine at which the pain is relieved
[10]. To achieve this concentration, a preliminary titration is necessary, while further adjustments with automatic bolus administered by the patient permit navigation in what is generally described as an analgesic corridor. Trespassing the upper limit of this corridor results in opioid side effects, while exiting the lower limit of this corridor results in the inefficiency of pain relief
[11] (
Figure 1).
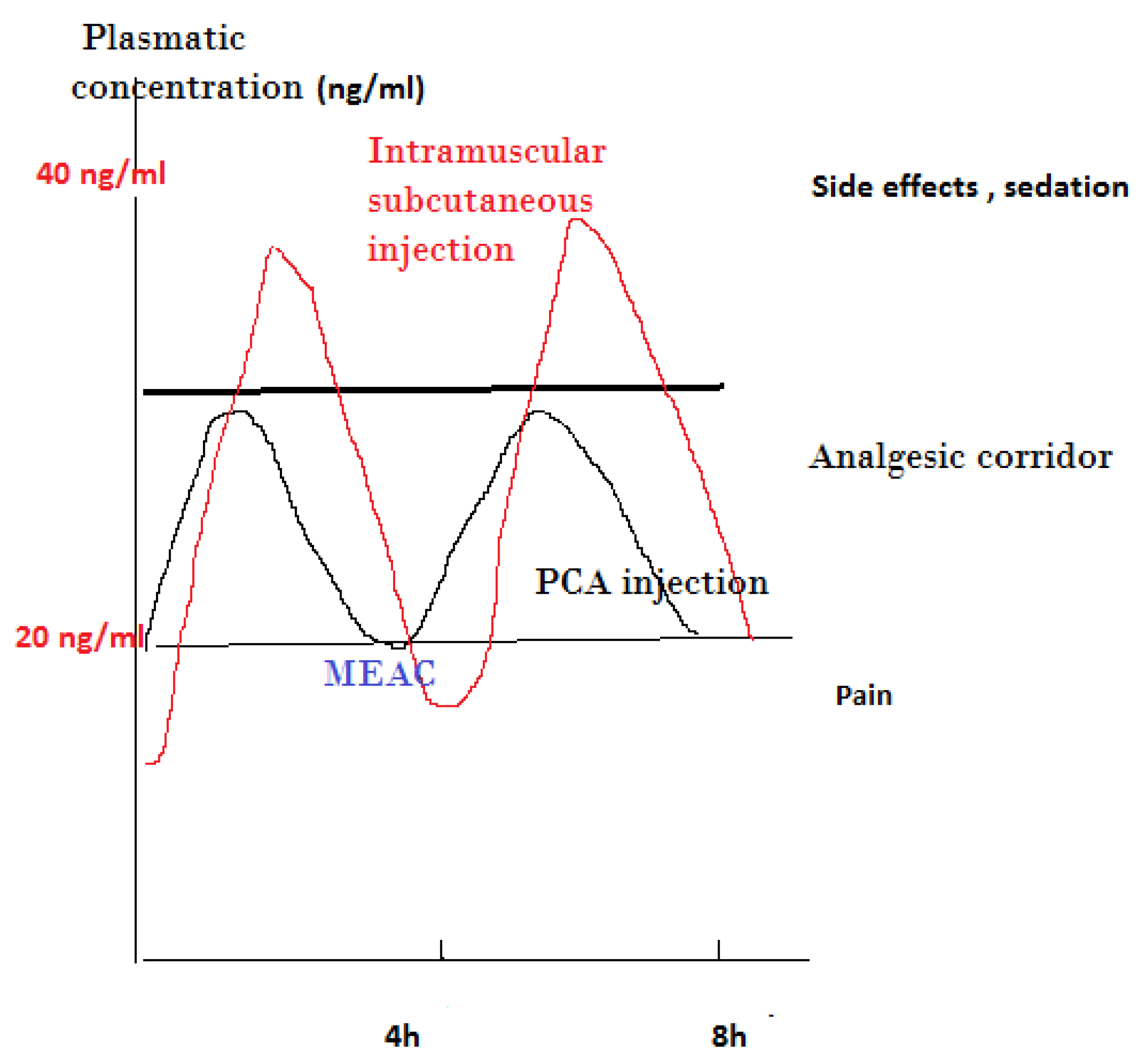
Figure 1. Presentation of analgesic corridor concept: subcutaneous or intramuscular injection vs. PCA IV injection of lower dose of opioid. MEAC, minimum effective analgesic concentration.
To reach this target, a controlled and progressive increase in the plasma level of opioids is necessary, which is achieved by titration. Titration means that the drug is administered as a bolus of small doses
[11]. Titration of morphine in the operating room during wound closure or PACU is generally the first step of IV opioid management. Morphine by titration in PACU requires previous pain assessment by caretakers, generally a numeric verbal scale less than 3–4 on an 11-grade scale (0 no pain, 10 worst ‘maximal’). Titration provides relatively rapid analgesia and the ability to adapt the dose to interindividual variability requirements until the establishment of a clinically acceptable analgesia pattern is obtained; however, on some occasions, another opioid can be used before adequate pain relief is achieved
[12].
Morphine, a hydrophilic agent and hydromorphone, remains the most commonly used drug for postoperative analgesic titration compared to lipophilic opioids such as fentanyl, sufentanil, alfentanil, or remifentanil
[12], as these opioids have a faster onset but also a shorter duration of action.
The maximum concentration after a bolus injection is around six minutes, explaining the delay between further titration injection or the “lock out” period necessary while PCA is used
[9][13]. During this lockout time, the PCA pump does not permit further delivery, permitting each bolus to reach the peak effect before the next bolus reducing the risk of overdose.
IV morphine titration allows the dose to be adapted to the patients’ needs and can provide reliable immediate relief of postoperative pain after a wide range of surgical interventions in both young and elderly patients
[11]. Titration needs individual adaptation, as the subsequent injection should consider also what has already been administered. Factors affecting early morphine requirements include ethnicity, emergency, major surgery, long-lasting surgery and high pain score upon arrival to PACU
[14].
It should be emphasized that morphine titration to alleviate acute postoperative pain might not always be effective or possible due to the early appearance of side effects or other complicating factors, such as tachyphylaxis; therefore, it is appropriate to define an alert dose for titration to use alternative methods to alleviate pain
[11].
2.3. Side Effects of Intravenous (IV)-PCA
Most side effects of opioids administered via PCA are related to opioids, such as nausea and vomiting, sedation, apnea, hypoxemia, hypoventilation, pruritus and postoperative delirium (POD).
The ventilatory depressant effects of morphine are the most serious side effects. Although they can be patient-related, they are mostly related to a default in preparation, prescription, or administration, as well as a device failure.
A recent comparison of the side effects of different opioids given in an equianalgesic dose revealed that no significant or clinically relevant difference should be expected in terms of nausea, vomiting and pruritus when comparing different types of opioids
[15].
Considering POD, a recent study did not find a difference in its incidence between morphine IV-PCA and fentanyl patient-controlled epidural analgesia (PCEA). After a propensity score matching patient characteristics, it was concluded that POD occurs regardless of the route and dose of opioid administration
[16].
Naloxone is reported to decrease the overall incidence of opioid side effects. A pooled analysis study examining IV naloxone (either as a continuous infusion or IV-PCA) revealed a decrease in pruritus and nausea with no increase in pain scores. Overall, the use of IV naloxone is not associated with any significant changes in opioid consumption nor with the risk of sedation or emesis
[3][17]. In other more recent study, low and ultra-low doses such as 0.25 µg/kg are also reported to reduce pain intensity due to morphine consumption, pruritus and nausea
[18][19]; finally, Pieters
[20] found that a high dose naloxone of 0.5 mg/kg/h was not more efficient in reducing opioid side effects, despite partially reversing the analgesic effect of opioid by yielding an increase in demand on postoperative day 2.
While IV-PCA can be used in most types of surgery, it may not provide the best quality of analgesia, especially in dynamic conditions. For example, in open abdominal surgery, epidural analgesia has been considered the gold standard for perioperative analgesia and provides significantly better pain relief both at rest and in dynamic situations
[21]. However, after the implementation of enhanced recovery after surgery (ERAS) protocols more than a decade ago and a shift from open to laparoscopic surgery, the advantage of epidural anesthesia has diminished, requiring the use of other opioids and methods, such as intravenous ketamine, peripheral nerve blocks, continuous wound infiltration, intrathecal morphine, intravenous and non-invasive PCA
[22].
Although the literature praising PCA-IV for its efficiency in controlling multiple entities of postoperative pain is abundant, the quality of pain relief as determined by assessment of pain intensity scores was only slightly superior to specific non-PCA technique-controlled parenteral opioid (iv, IM, SC) regimens; in two meta-analyses
[6][7], PCA is reported to provide superior quality of analgesia with only moderate to low evidence. In addition, despite involving a slightly higher dose of opioid consumption, no increase in opioid-induced side effects is disclosed. Modern surgeries support the use of multimodal regimens in many situations.
As effective and safe alternatives to traditional PCA and with the added benefits of being non-invasive, easy to use and making early patient mobilization possible, newer PCA systems may complement multimodal pain management or replace certain regimens in hospitalized patients with acute postoperative pain
[23] (
Table 1).
Table 1. Bolus dose and lockout period with different opioids medications.
| Analgesic |
Bolus Dose |
Lockout Period (Minutes) |
| Morphine |
1 mg |
5–10 |
| Fentanyl |
10 µg |
5–10 |
| Hydromorphone |
0.25 mg |
5–10 |
| Remifentanil |
0.5 µg/kg |
2 |
| Sufentanil |
5 µg |
5–10 |
Explaining the PCA pump system to patients is generally performed before surgery at the anesthetic consultation; however, re-instruction after surgery is reported to be effective for optimizing PCA to increase the quality of analgesia. In this way, patients use this technique more efficiently, especially when multiple variables, including dynamic pain, are integrated
[24].
In terms of patient’s satisfaction, PCA is again reported to have an edge over non-PCA methods, which does not necessarily mean that the quality of analgesia is superior to the alternative technique
[25].
2.4. Comparison of Different PCA Medications
Although several opioids have been studied for use in IV-PCA, there is not enough significant clinical evidence to consider the relevant superiority of other opioids on postoperative analgesia compared to morphine IV
[6][7] (
Table 1 and
Table 2).
Table 2. Comparison of different opioids when used in a PCA mode
[26][27][28].
| |
Efficiency |
Side Effects |
| Oxycodone |
As potent as morphine |
May have fewer severe side effects |
| Hydromorphone |
|
Higher incidence of CNS side effects, excitation at higher dose |
| Fentanyl |
High potency +, may require more need for basal infusion rate |
Lesser incidence of respiratory depression in comparison to morphine, but more programming errors |
| Sufentanil |
High potency ++, high therapeutic index, more predictable profile, more need for basal infusion |
Lower incidence of PONV in comparison to fentanyl |
| Tramadol |
Ten times less potent than morphine |
More PONV in some type of surgeries (e.g., spinal fusion) |
| Remifentanil |
Very short duration, studies mainly in labor |
Higher respiratory depression, less satisfaction in comparison to epidural analgesia |
CNS, central nervous system; PONV, postoperative nausea and vomiting. +: moderate indication, ++: acceptable indication.
3. Conclusions
Despite the lack of new evidence, IV-PCA administration of opioids, especially morphine, has only a light superiority for quality of analgesia compared to non-PCA opioid-based analgesic regimens. Nevertheless, morphine IV-PCA, as a component of multimodal analgesia for acute postoperative pain, remains a well-accepted/adopted technique by patients and health care providers, resulting in a high level of satisfaction, while the incidence of side effects is equivalent to non-PCA opioid-based techniques. This modality of analgesia will still be valid and useful for the coming years, pending careful selection of patients with appropriate indications.






