
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maroun Bou Zerdan | + 2188 word(s) | 2188 | 2022-03-08 10:15:09 | | | |
| 2 | Rita Xu | Meta information modification | 2188 | 2022-03-09 04:34:00 | | |
Video Upload Options
Breast cancer (BC) is the most common malignancy affecting women. It is a highly heterogeneous disease broadly defined by the differential expression of cell surface receptors. In the United States, triple negative breast cancer (TNBC) represents 15 to 20% of all BC. When compared with other subtypes of BC, TNBC tends to present in younger women, and has a higher mortality rate of 40% in advanced stages within the first 5 years after diagnosis. TNBC has historically had limited treatment options when compared to other types of BC.
1. Introduction
2. Triple-Negative Breast Cancer Molecular Subtyping
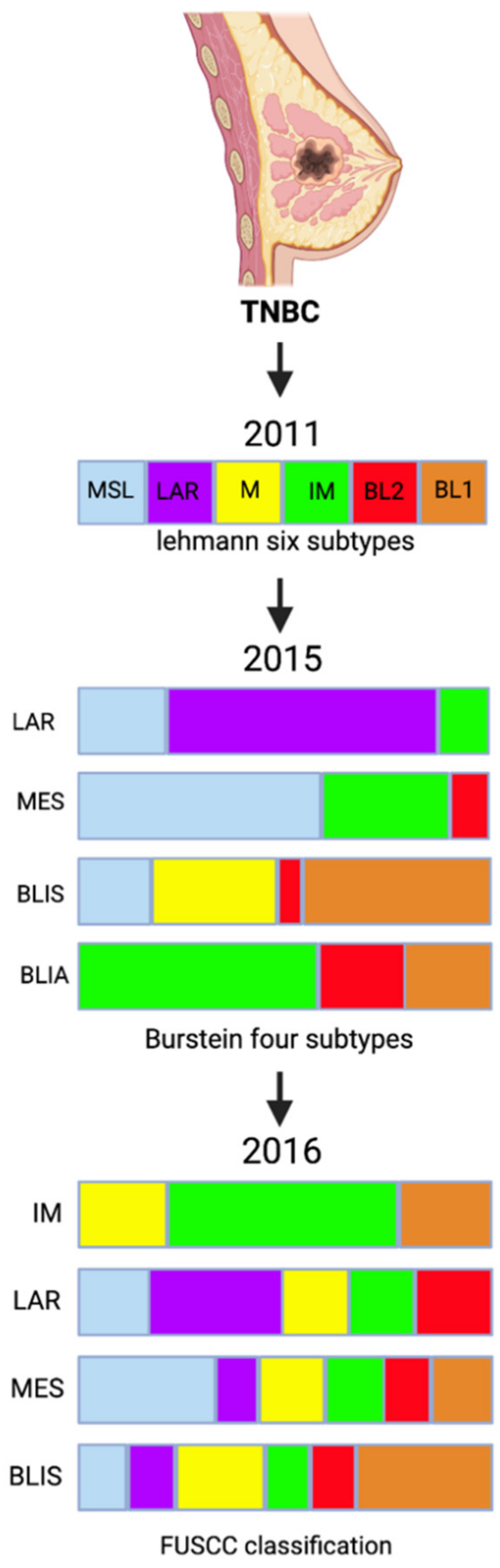
| FUSCC Classification | Pathways | |
|---|---|---|
| IM (immunomodulatory) | ↑ |
|
| LAR (luminal androgen receptor) | ↑ |
|
| MES (mesenchymal-like) | ↑ |
|
| BLIS (basal-like and immune suppressed) | ↑ |
|
| ↓ |
|
|
3. Chemotherapy for Triple Negative Breast Cancer
| Trials (References) |
Regimen 1 (R1) | Nb. of Patients | Regimen 2 (R2) | Nb of Patients | pCR Rate (R1 vs. R2) | p-Value |
|---|---|---|---|---|---|---|
| GeparOcto GBG84 [30] | P + NPLD + Cb; q1w for 18 weeks |
203 | E then P then C; q2w/3 cycles over 18 weeks |
200 | 51.7% vs. 48.5% | 0.518 |
| GALGB40603 Alliance [24] | (P q1w for 12 weeks then ddAC q2w/4 cycles) + (Cb q3w/4 cycles ± Bev. q2w/9cycles) | 221 | P q1w for 12 weeks then ddAC q2w/4 cycle | 212 | 54% vs. 41% | 0.0029 |
| GeparSixto GBG66 [26] | (P q1w for 18 weeks + NPLD q1w for 18 weeks + Bev. q3w/6 cycles) + Cb q1w for 18 weeks | 158 | P q1w for 18 weeks + NPLD q1w for 18 weeks + Bev. q3w/6 cycles | 157 | 53.2% vs. 36.9% | 0.005 |
| Zhang et al. [31] | P + Cb q3w/4–6 cycles | 47 | P + E q3w/4–6 cycles | 44 | 38.6% vs. 14.0% | 0.014 |
| Ando et al. [32] | (P q2w/2 cycles then CEF q2w/4 cycles) + Cb q3w/4 cycles | 37 | P q2w/2 cycles then CEF q2w/4 cycles | 38 | 61.2% vs. 6.3% | 0.003 |
P = Paclitaxel; NPLD = Nonpegylated Liposomal Doxorubicin; Cb = Carboplatin; E = Epirubicin; C = Cyclophosphamide; ddAC = Doxorubicin plus Cyclophosphamide; Bev. = Bevacizumab; CEF = Cyclophosphamide plus Epirubicin plus 5-fluorouracil.
4. Detecting PDL-1 Expression in TNBC
References
- Bilani, N.; Zabor, E.C.; Elson, L.; Elimimian, E.B.; Nahleh, Z. Breast cancer in the United States: A cross-sectional overview. J. Cancer Epidemiol. 2020, 2020, 6387378.
- Goldhirsch, A.; Winer, E.P.; Coates, A.; Gelber, R.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J.; Albain, K.S.; André, F.; Bergh, J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223.
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752.
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K. Differences in breast carcinoma characteristics in newly diagnosed African–American and Caucasian patients: A single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and end results database. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 110, 876–884.
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434.
- Lin, N.U.; Claus, E.; Sohl, J.; Razzak, A.R.; Arnaout, A.; Winer, E.P. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer 2008, 113, 2638–2645.
- Chaudhary, L.N.; Wilkinson, K.H.; Kong, A. Triple-negative breast cancer: Who should receive neoadjuvant chemotherapy? Surg. Oncol. Clin. 2018, 27, 141–153.
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767.
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150.
- Burstein, M.D.; Tsimelzon, A.; Poage, G.M.; Covington, K.R.; Contreras, A.; Fuqua, S.A.; Savage, M.I.; Osborne, C.K.; Hilsenbeck, S.G.; Chang, J.C. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin. Cancer Res. 2015, 21, 1688–1698.
- Liu, Y.-R.; Jiang, Y.-Z.; Xu, X.-E.; Yu, K.-D.; Jin, X.; Hu, X.; Zuo, W.-J.; Hao, S.; Wu, J.; Liu, G.-Y. Comprehensive transcriptome analysis identifies novel molecular subtypes and subtype-specific RNAs of triple-negative breast cancer. Breast Cancer Res. 2016, 18, 33.
- Jaafar, R.; Mnich, K.; Dolan, S.; Hillis, J.; Almanza, A.; Logue, S.E.; Samali, A.; Gorman, A.M. RIP2 enhances cell survival by activation of NF-ĸB in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2018, 497, 115–121.
- Abduljabbar, R.; Al-Kaabi, M.M.; Negm, O.H.; Jerjees, D.; Muftah, A.A.; Mukherjee, A.; Lai, C.F.; Buluwela, L.; Ali, S.; Tighe, P.J. Prognostic and biological significance of peroxisome proliferator-activated receptor-gamma in luminal breast cancer. Breast Cancer Res. Treat. 2015, 150, 511–522.
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-c. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61.
- Wahba, H.A.; El-Hadaad, H.A. Current approaches in treatment of triple-negative breast cancer. Cancer Biol. Med. 2015, 12, 106.
- Ismail-Khan, R.; Bui, M.M. A review of triple-negative breast cancer. Cancer Control. 2010, 17, 173–176.
- Dieci, M.V.; Del Mastro, L.; Cinquini, M.; Montemurro, F.; Biganzoli, L.; Cortesi, L.; Zambelli, A.; Criscitiello, C.; Levaggi, A.; Conte, B. Inclusion of platinum agents in neoadjuvant chemotherapy regimens for triple-negative breast cancer patients: Development of grade (Grades of Recommendation, Assessment, Development and Evaluation) recommendation by the Italian Association of Medical Oncology (AIOM). Cancers 2019, 11, 1137.
- Birgisdottir, V.; Stefansson, O.A.; Bodvarsdottir, S.K.; Hilmarsdottir, H.; Jonasson, J.G.; Eyfjord, J.E. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006, 8, R38.
- Biswas, T.; Efird, J.T.; Prasad, S.; Jindal, C.; Walker, P.R. The survival benefit of neoadjuvant chemotherapy and pCR among patients with advanced stage triple negative breast cancer. Oncotarget 2017, 8, 112712.
- Isakoff, S.J.; Mayer, E.L.; He, L.; Traina, T.A.; Carey, L.A.; Krag, K.J.; Rugo, H.S.; Liu, M.C.; Stearns, V.; Come, S.E. TBCRC009: A multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J. Clin. Oncol. 2015, 33, 1902.
- Byrski, T.; Gronwald, J.; Huzarski, T.; Grzybowska, E.; Budryk, M.; Stawicka, M.; Mierzwa, T.; Szwiec, M.; Wiśniowski, R.; Siolek, M. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J. Clin. Oncol. 2010, 28, 375–379.
- Silver, D.P.; Richardson, A.L.; Eklund, A.C.; Wang, Z.C.; Szallasi, Z.; Li, Q.; Juul, N.; Leong, C.-O.; Calogrias, D.; Buraimoh, A. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J. Clin. Oncol. 2010, 28, 1145.
- Byrski, T.; Dent, R.; Blecharz, P.; Foszczynska-Kloda, M.; Gronwald, J.; Huzarski, T.; Cybulski, C.; Marczyk, E.; Chrzan, R.; Eisen, A. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res. 2012, 14, R110.
- Sikov, W.M.; Berry, D.A.; Perou, C.M.; Singh, B.; Cirrincione, C.T.; Tolaney, S.M.; Kuzma, C.S.; Pluard, T.J.; Somlo, G.; Port, E.R. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J. Clin. Oncol. 2015, 33, 13.
- Sikov, W.M.; Polley, M.-Y.; Twohy, E.; Perou, C.M.; Singh, B.; Berry, D.A.; Tolaney, S.M.; Somlo, G.; Port, E.R.; Ma, C.X. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT)+/-carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). Am. Soc. Clin.Oncol. 2019, 37, 591.
- Von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 747–756.
- Poggio, F.; Bruzzone, M.; Ceppi, M.; Pondé, N.; La Valle, G.; Del Mastro, L.; De Azambuja, E.; Lambertini, M. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: A systematic review and meta-analysis. Ann. Oncol. 2018, 29, 1497–1508.
- Loibl, S.; Sikov, W.; Huober, J.; Rugo, H.S.; Wolmark, N.; O’Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; Lorenzo, J.P.; et al. Event-Free Survival (EFS), Overall Survival (OS), and Safety of Adding Veliparib (V) Plus Carboplatin (Cb) or Carboplatin Alone to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer (TNBC) after ≥4 Years of Follow-Up: BrighTNess, a Randomized Phase III Trial. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-congress-2021/event-free-survival-efs-overall-survival-os-and-safety-of-adding-veliparib-v-plus-carboplatin-cb-or-carboplatin-alone-to-neoadjuvant-chem (accessed on 12 October 2021).
- Chen, H.; Marsiglia, W.M.; Cho, M.-K.; Huang, Z.; Deng, J.; Blais, S.P.; Gai, W.; Bhattacharya, S.; Neubert, T.A.; Traaseth, N.J. Elucidation of a four-site allosteric network in fibroblast growth factor receptor tyrosine kinases. eLife 2017, 6, e21137.
- Schneeweiss, A.; Moebus, V.; Tesch, H.; Hanusch, C.; Denkert, C.; Luebbe, K.; Huober, J.; Klare, P.; Kuemmel, S.; Untch, M. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto—GBG 84): A randomised phase III trial. Eur. J. Cancer 2019, 106, 181–192.
- Zhang, P.; Yin, Y.; Mo, H.; Zhang, B.; Wang, X.; Li, Q.; Yuan, P.; Wang, J.; Zheng, S.; Cai, R. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: A randomized phase 2 trial. Oncotarget 2016, 7, 60647.
- Ando, M.; Yamauchi, H.; Aogi, K.; Shimizu, S.; Iwata, H.; Masuda, N.; Yamamoto, N.; Inoue, K.; Ohono, S.; Kuroi, K. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Res. Treat. 2014, 145, 401–409.
- Erber, R.; Hartmann, A. Understanding PD-L1 testing in breast cancer: A practical approach. Breast Care 2020, 15, 481–490.
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690.
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550.
- Ancevski Hunter, K.; Socinski, M.A.; Villaruz, L.C. PD-L1 Testing in Guiding Patient Selection for PD-1/PD-L1 Inhibitor Therapy in Lung Cancer. Mol Diagn 2018, 22, 1–10.
- U.S Food & Drug Administration. List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/list-cleared-or-approved-companion-diagnostic-devices-in-vitro-and-imaging-tools (accessed on 12 October 2021).
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028.
- Roach, C.; Zhang, N.; Corigliano, E.; Jansson, M.; Toland, G.; Ponto, G.; Dolled-Filhart, M.; Emancipator, K.; Stanforth, D.; Kulangara, K. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non–small-cell lung cancer. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 392.
- Scheel, A.H.; Dietel, M.; Heukamp, L.C.; Jöhrens, K.; Kirchner, T.; Reu, S.; Rüschoff, J.; Schildhaus, H.-U.; Schirmacher, P.; Tiemann, M. Harmonized PD-L1 immunohistochemistry for pulmonary squamous-cell and adenocarcinomas. Mod. Pathol. 2016, 29, 1165–1172.




