Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Agnieszka Barbara Najda | + 4565 word(s) | 4565 | 2022-03-07 04:31:44 | | | |
| 2 | Amina Yu | -40 word(s) | 4525 | 2022-03-09 02:29:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Najda, A. Nanomedicine-Based Delivery for Breast Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/20331 (accessed on 07 February 2026).
Najda A. Nanomedicine-Based Delivery for Breast Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/20331. Accessed February 07, 2026.
Najda, Agnieszka. "Nanomedicine-Based Delivery for Breast Cancer" Encyclopedia, https://encyclopedia.pub/entry/20331 (accessed February 07, 2026).
Najda, A. (2022, March 08). Nanomedicine-Based Delivery for Breast Cancer. In Encyclopedia. https://encyclopedia.pub/entry/20331
Najda, Agnieszka. "Nanomedicine-Based Delivery for Breast Cancer." Encyclopedia. Web. 08 March, 2022.
Copy Citation
Breast cancer is one of the most common types of cancer among women globally. It is caused by mutations in the estrogen/progesterone receptors and conventional treatment methods are commonly utilized. About 70–80 percent of individuals with the early-stage non-metastatic disease may be cured.
nanomedicine
drug therapy
breast cancer
targeted delivery
drug resistance
1. Nanomedicine: Evolving Demands for Breast Cancer Treatment
Nanomedicine in the medical or pharmaceutical field appears to be a new trend and has been proposed to minimize dose quantity and frequency while retaining a similar pharmacological profile and fewer side effects [1], as well as nanosystems that enable them to push through biological barriers like the blood-brain barrier (BBB) [1]. Among these, nano-lipid carriers for cancer therapy will lead to improved features such as higher drug loading capability, strong compatibility, scaling up viability, and regulated drug release [2]. Nanoparticles are commonly used to synthesize and prepare anti-infective, anticancer, and anti-inflammatory medicines [3]. The nanoparticles vary in size from 1–100nm. The nanoparticles combine in a multi-layered fashion, and the coating aids in resolving issues such as solubility, durability, and specificity. Using a nanoparticle-based technique, problems correlated with macromolecules such as cell toxicity, lacking specificity, cellular absorption, and the high dose may be avoided. In contrast, concerns linked to multidrug resistance (MDR) and P-glycoprotein (P-gp) efflux may also be changed [4].
The nanoparticle’s wavelength, which is usually less than 100 nm, will provide inherent stealth. Due to the drug’s lipophilic nature, it is simple to entrap and formulate into nanoparticles [5]. The conjugation of the parent first-line chemotherapeutic agent and the flavonoid with a lipid improves the drug’s lipophilicity, which aids in enhancing the entrapment efficiency when forming nanodroplets. The nano-emulsion system’s buoyant charge aids in communicating with the negative charge on the surface of cancer cells. This aids in the successful transmission of nanoparticles to the cancer cell surfaces [6]. There have been no records of the nanoparticles having any negative or harmful results since being delivered in 2D person and in vivo [7]. These are supported by an evidential study demonstrating that toxicity-related conditions are rare and have little impact on the brain, heart, lungs, or kidney. According to one study, nanoparticle aggregation in body parts such as the liver and spleen has been seen [8]. Nanomedicine is being used in many of the formulation approaches mentioned above; there are two commonly acknowledged approaches: (a) top-down method and (b) bottom-up technique. In the top-down method, the required non-material is made by using external, macroscopic raw materials. The processing of these macroscopic materials is well-controlled from the outside. Etching, ball-milling, homogenization, and the application of strong plastic deformations are all examples of this kind of method [9]. In the bottom-up method, the raw material is pre-miniaturized (at the molecular/atomic level) and then either allowed to self-assemble into nanomaterials or additional catalysts are included to aid assembly. Other molecules of interest may be introduced into the nanomaterial to create composite nanoproducts during the construction process. However, the end output must be “stabilized” by some external means in each of these ways. Otherwise, these fundamentally unstable nanoproducts tend to agglomerate while stored [9] and it is having a positive impact on the pharmaceutical healthcare industry.
In nano-delivery, the antibody can coat the nanocarrier surface for targeted distribution to HER2 cells. There have been many recent studies on HER2-targeted nanomedicine; although the HER2 antibody is the most often used targeting moiety for HER2 malignancy, other HER2 targeting ligands have been studied as well. For example, Ding et al. used a trastuzumab-mimetic peptide with promising results, indicating that this might reduce the antibody’s immunogenicity, production costs, and technical effort [10]. The nanoparticles developed by Day et al. [11] and Cai et al. [12] are both decorated with trastuzumab coating for targeted photoablation and radiation therapy. The HER2 protein, which fuels this kind of breast cancer, might be targeted to slow it down potentially. Monoclonal antibodies are synthesized proteins that attack and inhibit the proliferation of HER2 cells. These medications may be prescribed alone or in conjunction with chemotherapy by doctors. Breast cancer can also be treated with an antibody–drug combination, which delivers pinpoint accuracy chemotherapy to the cancer cells [13]. There are various nanocarriers like liposomes, carbon nanotubes, micelles, dendrimers, metallic nanoparticles (NPs), nanocrystals, polymeric NPs, and lipid-based nanocarriers, such as solid lipid nanoparticle (SLN) and NLCnanostructured lipid carrier (NLC), which are examples of some of the nanocarrier systems in Figure 1 [14]. The benefit of nanocarriers is their compact scale, which enables them to push through biological barriers like the blood-brain barrier (BBB) [15]. As well, the nanocombinatorial approaches have shown promising results to treat breast cancer [16].
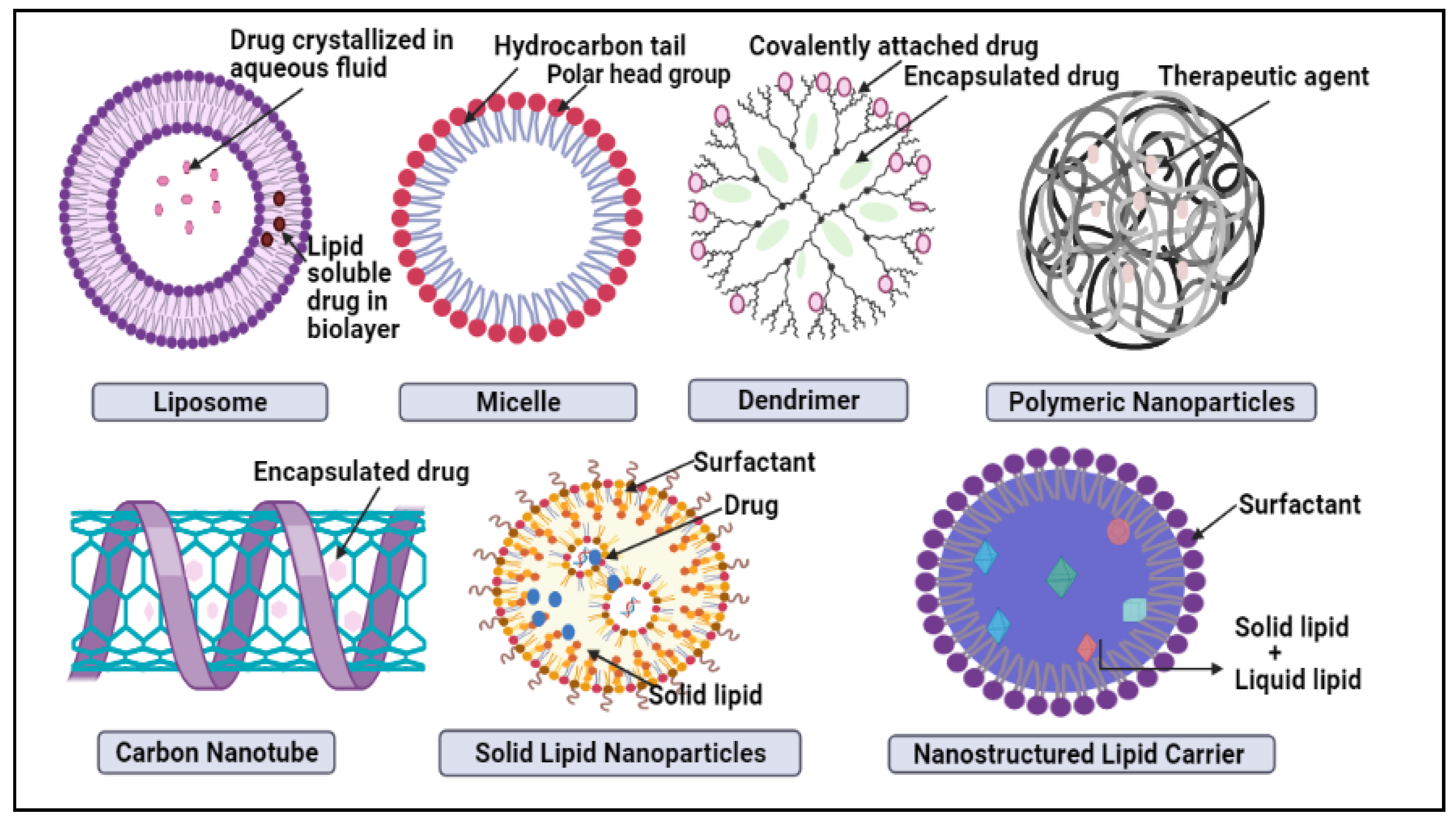
Figure 1. Schematic representation of novel nano-drug delivery approaches in breast cancer treatment.
1.1. Liposomes
Liposomes are spherical entities that are self-contained and made up of one or more concentric curved bilayer membranes as well as cholesterol. Liposomes are made up of components that are comparable to those found in the cell membrane [17]. Since one side of a phospholipid molecule is hydrophobic and the other end is hydrophilic, when coupled with water, they instantly form a spherical particle [18]. Liposomes have excellent possibilities for medication and cell delivery, owing to the amphiphilic nature of the lipids. Liposomes are necessary for regulated medication and gene delivery to the target region, as well as cell proliferation inside the pores or surrounding tissue. In vivo, the matrix phase should biodegrade at a controlled pace and elicit a low immunological and inflammatory response [19].
Insufficient drug concentrations due to poor absorption, excessive metabolism and elimination, low water solubility, and large plasma level variations due to variable bioavailability after oral administration were usually accompanied by unsatisfactory in vivo results [20]. The development of appropriate drug carrier systems is a possible solution for tackling these issues. When combined with trastuzumab, liposomal anthracyclines are effective in both progressed and early breast cancer [21]. In patients with early and HER2-over-expressing breast cancer, the use of a combination of liposomal anthracyclines and trastuzumab is of particular interest. This is the group that is most likely to benefit from anthracycline treatment [22].
Chowdhury, N. et al. [23] developed an aptamer (A6 and GFP)-labeled liposomal nanoparticle delivery system that retains and distributes doxorubicin to HER-2+ breast cancer cells. As well, it shows a significant increase in the uptake of the aptamer-labeled liposomes—by more than 60%—into both MCF-7 and SKBR-3 cells compared to non-aptamer-labeled nanoparticles and an improved uptake in HER-2 positive cells than HER-2 negative cells. Formulation shows ≈ 1.79-fold increase in the uptake of DOX in the HER-2+ cells compared to the HER-2- cells. This preliminary study indicates that aptamer-labeled nanoparticles, among several batches, showed the highest uptake as well as the targeted delivery of doxorubicin into HER-2+ breast cancer cells. Thus, an aptamer targeted approach results in a substantial reduction in the dose of DOX and improves the therapeutic benefits by promoting the target specificity.
Doxil®, Janssen Products, Titusville, NJ, USA is a pegylated liposomal DOX HCl formulation that reduces systemic toxicity while maintaining DOX antitumor properties, significantly reducing tumor growth rates, and enhancing survival rates [24]. Doxil helps alleviate the cumulative dosage limitation and allows for lower risk and longer DOX therapy, thereby significantly increasing the drug’s flexibility. In clinical trials for advanced breast cancer treatment, Doxil has been coupled with a wide range of other chemotherapeutic agents (for example, cyclophosphamide and 5-fluorouracil, cisplatin and infusional fluorouracil, cyclophosphamide followed by paclitaxel, cyclophosphamide accompanied by paclitaxel [25].
Lipodox® is a DOX HClliposome injection that is generic. Monotherapy is used for the treatment of metastatic breast cancer with a high risk of cardiac complications and can reduce the danger of infusion responses. For this process, the drug is given intravenously at a dosage of 50 mg/m2 at a rate of 1 mg/min at first and is administered once every four weeks for as long as the patient reacts well and tolerates the therapy [26]. When combined with Lapatinib, dHER2+AS15 ASCI was assessed by dose-limiting toxicities. There were two rounds of intramuscular injections every two weeks, and, between immunization rounds, there was a four-week break. For each dose of 500g of dHER2 + AS15 ASCI, two sterile glass vials were provided: one vial will contain this same lyophilized preparation containing 500 μg of recombinant dHER2 antigen, especially in combination with the immunostimulant, and the other vial will contain the dried preparation containing 500 μg of recombinant dHER2 antigen combined with the immunostimulant. The final dHER2 + AS15 ASCI for administration is obtained by reconstitution of the lyophilized product with adjunct diluents. The dose of dHER2 + AS15 ASCI is 0.5 mL [27].
Lipoplatin, an effective liposomal formulation of cisplatin, is an intriguing medication in breast cancer, especially in HER 2-negative and triple-negative patients—although its efficacy has to be confirmed [28].
EndoTAG-1/MediGene is another Liposomal paclitaxel formulation successful in TNBC with a weekly dosage of EndoTAG-1 22 mg/m2 + Paclitaxel 70 mg/m2 and a 4-month progression-free survival (PFS) rate [29]. The DPX-0907 vaccine is safe and well-tolerated. This may increase patient survival by inducing efficient anti-tumor immunity [30]. LEM-ETU is a liposomal formulation containing Mitoxantrone, an anticancer drug given intravenously every 21 days until disease progression or toxicity occurs, which requires treatment discontinuation [31].
Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer is also an effective formulation [32]. Liang, Z. et al. [33] illustrated targeted delivery of siRNA via a polypeptide-modified liposome for the treatment of gp96 over-expressed breast cancer. There are several metallic liposomalformulation, such as Ru(III) complexes [34][35] with nucleolipids (AziRu), that it were able to create with differently decorated anticancer nanosystems, which were very effective against human BCC. In the current landscape of Ru-based candidate drugs, it was demonstrated that AziRu, when inserted into a nucleolipidic structure and ad hoc nano-delivered via the positively charged lipid DOTAP, can effectively inhibit BCC proliferation in vivo while being well-tolerated, which is a critical property for anticancer drug candidates in preclinical studies to progress to the clinical stage. Thus, they demonstrated the safety and effectiveness of HoThyRu/DOTAP cationic Ru-based nanosystems in a mouse xenograft model of BC.
1.2. Dendrimers
Dendrimers are promising drug delivery devices that can solve the shortcomings of currently approved anticancer medications [36]. They can overcome drug resistance, decrease drug toxicity, and increase drug solubility and bioavailability [36]. Anticancer medicines have been loaded into micelles and dendrimers, resulting in selective drug delivery, sustained drug release, improved cellular uptake, decreased adverse side effects, and enhanced anticancer action in vitro and in vivo. Dendrimers are typically used to accomplish successful drug targeting to tumor tissues by covalently binding unique targeting moieties such as folic acid, antibody, sugar epidermal growth factor, and biotin [36]. Chemotherapeutic agents and theranostic chemotherapy applications have been successfully administered using these nanocarriers [37].
Poly-lysine, polypropylene imine (PPI), phosphorus, and carbosilane dendrimers are other forms of dendrimers used in biomedical applications, especially in oncology [38]. Polylysine (PLL) is an amphiphilic dendrimer with a branched structure made up of Penta-functional central molecules that are made up of positively charged essential amino acids, including lysine-amino-alanine, and are a fascinating new class of molecules because of their small size and natural components, which allow them to be internalized more readily than synthetic molecules [39]. The therapeutic efficacy of dendrimers and micelles for breast cancer treatment was reviewed by Sibusiso Alven et al. [40]. The latter demonstrated how they could resolve drug resistance, decrease drug toxicity, and increase drug solubility and bioavailability. Anticancer medications have been loaded into micelles and dendrimers, resulting in selective drug delivery, sustained drug release, improved cellular absorption, decreased adverse side effects, and enhanced anticancer action in vitro and in vivo [41]. The biological impact of dendrimers and micelles loaded with various recognized anticancer agents on breast cancer in vitro and in vivo are reported in their studies.
Although the larger G5 PEG1100 Dendrimer showed firm tumor and retention, drug release was poor, which restricted its anticancer effect. The smallest G4 PEG570 dendrimer was substantially effective in DOX release induced by cathepsin, but its systemic exposure and tumor uptake were minimal. Drug release kinetics, tumor absorption, systemic exposure, and retention were all improved with the intermediate-sized dendrimer. These results showed that the therapeutic effectiveness of dendrimer formulations is influenced by the polyethylene glycol (PEG) molecular weight and dendrimer scale [42].
In another study, dendrimer-loaded trastuzumab was conjugated to neratinib. The in vitro viability of SKBR-3 cells after 48 h for neratinib, neratinib-conjugated-dendrimers, and neratinib-loaded-dendrimers-trastuzumab was 40%, 36%, and 33%, respectively. Trastuzumab’s affinity for HER2 receptors expressed in SKBR-3 cells enhanced the internalization of the formulation through receptor-mediated endocytosis [43].
1.3. Micelles
Micelles are colloidal particles around 5–100 nm that are currently under investigation as carriers for hydrophobic drugs in anticancer therapy and have excellent tumor-targeted delivery properties, making them a feasible drug delivery mechanism with high translational potential [40].
NC-6300, NK911, and NC-6004 are micelle products engineered to deliver epirubicin, DOX, and cisplatin, respectively [44]. The antitumor efficacy of NC-6300 was shown in a phase I clinical trial in 2013 with a substantial reduction in cardiac toxicity, suggesting that the formulation is safe and tolerable [45]. DOX is mechanically encapsulated in the hydrophobic center of the micelles by noncovalent bonds [46]. SP1049C comprises a non-ionic Pluronic block copolymer mixture (1:8 w/w ratio) of Pluronic L61 and F127, which was shown to be more effective than doxorubicin against several tumor cell lines in vitro [47]. Compared to free doxorubicin in preclinical in vivo studies, SP1049C showed superior antitumor activity, efficacy, and an increased area under the curve (AUC) in tumor tissue in multiple animal tumor models of doxorubicin-resistant tumors and had identical AUC and Cmax in the liver, kidney, breast, lung, and plasma [48].
Sun Y. et al. [49] developed PAA-g-PEG graft micelles for high doxorubicin loading for specific target antitumor activity against mouse breast carcinoma for TNBC and discovered that articulation of DOX in the micelles, which enhanced the bioavailability of the drugs through passive targeting of the tumor and significantly reduced organ failure owing to wild tumor cell growth and metastasis and depressed the toxicity of DOX on the heart and other organs. Using a novel fluorescent cancer cell model, it was demonstrated that enhanced sensitivity of cancer stem cells to paclitaxel using poly[(D,L-lactide-co-glycolide)-co-PEG](PLGA-co-PEG) micelles of paclitaxel with CD44 surface markers [50]. Genexol-PM [51] is a new cremophor EL-free polymeric micelle formulation of paclitaxel. This single arm, multicenter phase II research was aimed to examine the effectiveness and safety of Genexol-PM in patients with histologically proven metastatic breast cancer (MBC). Forty-one women received Genexol-PM by intravenous infusion at 300 mg/m2 over 3 h every 3 weeks without premedication until disease progression or intolerability.
Taurin et al. [52] have developed a second-generation curcumin derivative, 3,5-bis(3,4,5-trimethoxybenzylidene)-1-methylpiperidine-4-one (RL71), that shows potent in vitro cytotoxicity. RL71 is hydrophobic with poor bioavailability, which limits its clinical development so it was designed styrene-co-maleic acid (SMA) micelles encapsulating 5%, 10%, or 15% RL71 by weight/weight ratio to improve its solubility and pharmacokinetic profile.
In another study, it was created a nanocarrier to deliver a chemotherapeutic drug specifically to the TNBC. To form micelles for the encapsulation of docetaxel, d—tocopheryl polyethylene glycol succinate (vitamin E TPGS, or simply TPGS) was employed. Vitamin E TPGS has both a lipophilic alkyl tail and a hydrophilic polar head, which results in micelle formation above the CMC of 0.02 wt%. Additionally, TPGS micelles have a high surface area that may change for ligand conjugation to deliver specific drugs. The fibroblast cells (NIH3T3), HER2 overexpressed breast cancer cells (SK-BR-3), ER and PR overexpressed breast cancer cells (MCF7), and TNBC cells of high, moderate, and low EGFR expression (MDA MB 468, MDA MB 231 and HCC38) were employed to access in vitro cellular uptake of the coumarin 6-loaded TPGS micelles and the cytotoxicity of docetaxel formulated in the micelles. The high IC50 value, which is the drug concentration needed to kill 50% of the cells in a designated period, such as 24 h, obtained from Taxotere® showed that the TNBC cells are indeed more resistant to the free drug than the positive breast cancer cells. However, the therapeutic effects of docetaxel have been significantlyenhanced by the formulation of Cetuximab conjugated TPGS micelles, which demonstrated 205.6- and 223.8-fold higher efficiency than Taxotere® for the MDA MB 468 and MDA MB 231 cell lines, respectively [53].
1.4. Carbon Nanotubes
Carbon nanotubes are cylinders made up of one or more co-axial graphite layers with a diameter in the nanometer range that serve as instructive examples of nanomaterials with Janus-like properties [54]. Their structure can be divided into single-walled carbon nanotubes with a single cylindrical carbon wall and multi-walled carbon nanotubes with multiple wall cylinders [54]. They can offer a promising approach to gene and drug delivery for cancer therapy due to their unique electronic, thermal, and structural characteristics. Due to their thermal conductivity and optical properties, carbon nanotubes are the right candidate for killing cancer cells via local hyperthermia [55].
Carbon nanotube materials may be used as instruments for targeted and regulated drug distribution and release, contrast agents for diagnosing and identifying breast tumors, and biosensors [56].Fullerenes, carbon nanotubes, and graphene have been shown to have desirable properties for the carriage, targeted, and regulated distribution of chemotherapeutical drugs such as Taxol (paclitaxel), docetaxel (DTX), doxorubicin (DOX), and others, according to recent nanomedicine reports [57][58][59].
Nadrajan Jawahar et al. [60] developed a folic acid-conjugated raloxifene hydrochloride carbon nanotube for targeting breast cancer cells, demonstrating that the surface of the CNTs was functionalized by folic acid (FA), allowing the medicine to be delivered selectively to the cancer cells’ target sites. In vitro, drug release studies revealed that the system’s pH influenced drug release. The effectiveness of FAs physically attached to CNTs with affectivity produces apoptosis in the cancer cell line with an IC50 value of 43.57305 g/mL, according to a cytotoxicity investigation. When compared to the pure medication and the RLX-CNT formulation, the fluorescence imaging investigation revealed that the RLX had increased cellular internalization.
By using the plasma-enhanced chemical by vapor deposition (PECVD) process, Akinoglu, E. M. et al. [61] determined that a multi-walled carbon nanotube-based scaffold has a good shape for cell development and offers a biocompatible environment for human MDA-MB-231 cell lines. The current findings revealed improved cell adherence to the scaffold and displayed excellent biomimetic features and physiological adaptability, suggesting that they may be utilized to research BrCa cell line metastasis in vitro.
1.5. Polymeric Nanoparticles
Polymeric nanoparticles (PNPs) are submicron-sized structures made up of several biodegradable (for example, albumin, chitosan, and alginate) and non-biodegradable (for example, albumin, chitosan, and alginate) polymers [62]. Therapeutic agents may be encapsulated, covalently bound, or adsorbed to nanocarriers in this manner [62]. These strategies can quickly solve drug solubility problems, which are significant since a substantial percentage of potential drug candidates discovered by high-throughput screening programs are water-insoluble [63]. On the other hand, polymeric nanoparticles are distinguished from drug nanosuspensions, which are sub-micron colloidal dispersions of pure drug particles stabilized by surfactants [64]. Polymeric nanoparticles may also be tailored to individual cells and sites in the body due to their small size and the ability to functionalize their surface with polymers and suitable ligands. As a result, polymeric nanoparticles could solve drug stability problems and reduce drug-induced side effects [65]. Several PNPs were developed and used to treat cancer, especially in the distribution of anti-cancer drugs [66].
Ikmeet Kaur Grewal et al. [67] have reported a summary of recent advances in polymeric nanoparticles for breast cancer care and patents with clinical trial studies based on the recently published Web Of Science data.
1.6. Solid Lipid Nanoparticles
Lipid-based nanoparticles, including solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), will hopefully overcome the current drawbacks presented by liposomes and polymeric nanoparticles [68]. Lipid nanoparticles show the possibility of discovering new therapeutics due to their unusual size-dependent properties [69]. The ability to integrate drugs into nanocarriers introduces a modern drug delivery prototype that can be used for secondary and tertiary drug targeting [70]. As a result, SLNs hold a lot of promise for achieving the objective of controlled and site-specific drug distribution, and they’ve gotten a lot of attention from scientists [71]. SLNs consist of a solid lipid matrix that is in solid state at both room and body temperatures and are made in the same way as an oil-in-water (o/w) emulsion, except that the oil phase is substituted by a solid lipid or a mixture of solid lipids. Solid lipids in SLNs include long-chain fatty acids, fatty acid esters, and waxes. The particle size (PS) of SLNs typically varies from 80 to 1000 nm [71]. Since these small particles may not re-crystallize throughout the production process, producing SLNs with a mean PS of less than 80 nm is challenging. The SLNs dispersion may be utilized directly as a nanosuspension or may be integrated into solid dosage forms, such as tablets and pellets, by granulating the dispersion. Alternatively, a spray-drying or lyophilization procedure might be effective in order to transform an aqueous SLN dispersion into a dry product and this will improve the SLN’s long-term stability, and it can be reassembled with water to make a nanosuspension as needed [71].
One of the most important factors in breast cancer mortality is metastasis, and the most common sites for metastasis are local lymph nodes [72]. As seen in Figure 2, the rate of tumor cell migration to lymph nodes increases, resulting in the incorporation of lymph vessels, which systemically spread the cancer cells through the lymphatic route. This route can provide new possibilities for delivery of anticancer drugs to overcome first pass metabolism of the drug and can serve as a bypass route. Anticancer drugs, encapsulated in advanced lipid-based nanocarriers such as SLNs and NLCs, are better candidates for lymphatic drug delivery. Lipid digestion occurs in the intestine by m-cells and then they are converted into micelles and transformed into lipoproteins after cholesterol and phospholipid aggregation. Finally, lipoproteins by pass first-pass metabolism and are sent to the lymphatic system. Henceforth, the systemic toxicity profile associated with anticancer drugs can be avoided and breast cancer patients would benefit from such a strategy, which would be safer than conventional chemotherapy.

Figure 2. Absorption mechanism of lipid based nanocarriers through lymphatic transport system.
Other formulations, such as dimethyl sulfoxide solubilization and Cremophor EL vehicles, were linked to paclitaxel-SLN action toward MCF-7 drug-resistant and drug-sensitive cells (commercial formulation). The cytotoxicity of SLNs with paclitaxel was determined to be concentration-dependent [73]. Pindiprolu et al. [72] generated niclosamide-loaded SLNs that increased cellular uptake and chemotherapeutic efficacy in TNBC. Another study, such as their use of curcumin carriers against the breast cancer cell line MDA-MB-231, has also backed up the efficacy of SLNs. When curcumin was injected into the SLNs, the findings revealed a significant improvement in the cells drug absorption capability, and curcumin–SLN also caused a greater decrease in cell viability and a rise in apoptotic cells as compared to curcumin diluted in dimethyl sulfoxide [74]. Another study was on the usage of resveratrol-loaded SLNs to treat human breast cancer cells, which was also a source of concern and investigators discovered that resveratrol SLNs are more efficient at inhibiting cell proliferation than free resveratrol. They also had a much stronger inhibitory effect on cell invasion and migration, implying that resveratrol–SLN may be a promising BreC drug [75]. The history and background of SLNs are very short as the findings were lacking in clinical studies for breast cancer treatment [75].
1.7. Nanostructured Lipid Carriers (NLC)
NLCs are the second generation of lipid-based nanocarriers formed from a mixture of solid and liquid lipids and have an unstructured matrix due to the different moieties of the constituents of NLCs [76]. NLCs were designed in order to overcome the SLNs’ limitations. NLCs have a higher drug loading capacity because of their imperfect crystal structure and could avoid drug expulsion by avoiding lipid crystallization during the manufacturing and storage periods. Due to the presence of liquid lipids in NLCs formulation, expulsion of the loaded drug after formulation and during the storage period is minimized. NLCs can also increase drug solubility in lipid matrices and they can show more controllable release profiles in comparison to SLNs [77]. Although NLCs are solid in nature, even in body temperature, they have a lower melting point than SLNs and, due to their unstructured nature and imperfection in their crystalline behaviors, provide more space for drug dissolution and payload in the liquid part of the NLC [78].
By replacing liquid lipid for a component of pure solid lipid, the strategies to keep defects in the lipid, even after lengthy storage, alleviate the issues associated with standard SLNs [79]. When a little amount of liquid lipid/oil was added to a solid lipid matrix, the crystal lattice structures were less ordered [80].
Liquid lipids contributed in widening the loading capabilities of lipid nanocarriers by increasing the number of defects wherein amorphous drug clusters might fit. As a consequence, this dual lipid framework may not only be able to accommodate higher drug loads, but it may also be able to reduce the drug’s expulsion from the lipid during storage [81].
Mingzhen Lin et al. [82] formulated a folic acid-loaded curcumin nanostructured lipid carrier using a solvent diffusion approach. The results revealed that FA-CUR-NLCs were efficient in selective delivery to cancer cells overexpressing FA receptors (FRs). CUR is also delivered to breast cancer cells via FA-CUR-NLCs, boosting anti-tumor action. As a consequence, FA-CUR-NLCs might be a more effective nanomedicine for tumor therapy.
2. Combinatorial Nanocarrier-Based Drug Delivery for Anti-Tumor Agent Amalgamation in Breast Cancer
In recent years, nanocarrier-based drug delivery has risen, with significant socioeconomic implications in various disciplines [83]. Furthermore, due to the nanocarrier’s capacity to deliver inside limited tissue, reducing dose quantity and frequency has been recommended while maintaining a similar pharmacological profile and fewer adverse effects [84]. Despite advances in surgical procedures and therapy regimes, patients with breast cancer have a poor prognosis. Henceforth, to improve the quality of life of breast cancer patients, cancer treatment strategies have shifted toward a nanomedicine drug delivery with the combinatorial approach to mitigate the toxicological issues associated with monotherapy [85]. Combining a synthetic chemotherapeutic agent with another synthetic or herbal bioactive molecule with anticancer activity has shown promising results in treating various cancers [86]. This amalgamation of a synthetic chemotherapeutic agent with an herbal anticancer bioactive molecule ameliorates the efficacy of monotherapy either synergistically or in an additive manner. Several such combinations have been reported to attenuate the toxicity associated with high-dose monotherapy [7]. These combinations have shown therapeutic benefits at a lower dose of the synthetic chemotherapeutic agent, which can easily be calculated using the combination index [87].
Boroujeni et al. [88] developed and manufactured curcumin-loaded folate-modified-chitosan-NPs with targeting capabilities by using a self-assembling approach. Curcumin release from folic acid chitosan NPs was finally demonstrated to be influenced by the concentration of the release medium, with a drop in pH from 7.4 to 5.0 speeding up the process. Curcumin-loaded NPs have been demonstrated to have an excellent potential for application in breast cancer treatment.
Day, C. M. et al. [89] additionally conjugated N-desmethyl tamoxifen and a Zinc (II) phthalocyanine moiety with the non-toxic amphiphilic spacer TEG. The novel conjugate was discovered to have a high affinity for ERs, thereby allowing it to perform both BC photodynamic and hormone treatment. The resultant conjugation has good biocompatibility and it showed an excellent cytotoxicity profile witha50% cancer cell killing impact that had powerful cancer apoptotic capabilities on the MCF-7 cell line. The pictorial representation of the action of a nanomedicine combinatorial approach and its action on breast cancer cells is given in Figure 3.

Figure 3. Action of nanomedicine combinatorial approach on breast cancer cells and effectiveness.
References
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Fernández-Carballido, A.; Torres-Suárez, A.I. Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother. Pharmacol. 2019, 84, 689–706.
- Boucharaba, A.; Guillet, B.; Menaa, F.; Hneino, M.; van Wijnen, A.J.; Clézardin, P.; Peyruchaud, O. Bioactive lipids lysophosphatidic acid and sphingosine 1-phosphate mediate breast cancer cell biological functions through distinct mechanisms. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2009, 18, 173–184.
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251.
- Zhang, H.; Hu, H.; Zhang, H.; Dai, W.; Wang, X.; Wang, X.; Zhang, Q. Effects of PEGylated paclitaxel nanocrystals on breast cancer and its lung metastasis. Nanoscale 2015, 7, 10790–10800.
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.; Najda, A.; Alanazi, I.S.; Germoush, M.O. and Mohamed, H.R. The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders. Molecules 2021, 26, 7109.
- Dykman, L.A.; Khlebtsov, N.G. Uptake of engineered gold nanoparticles into mammalian cells. Chem. Rev. 2014, 114, 1258–1288.
- Yang, M.; Gu, Y.; Tang, X.; Wang, T.; Liu, J. Advancement of lipid-based nanocarriers and combination application with physical penetration technique. Curr. Drug Deliv. 2019, 16, 312–324.
- Plaza-Oliver, M.; Santander-Ortega, M.J.; Lozano, M.V. Current approaches in lipid-based nanocarriers for oral drug delivery. Drug Deliv. Transl. Res. 2021, 11, 471–497.
- Tagde, P.; Tagde, S.; Bhattacharya, T.; Tagde, P.; Chopra, H.; Akter, R.; Kaushik, D.; Rahman, M. Blockchain and artificial intelligence technology in e-Health. Environ. Sci. Pollut. Res. 2021, 28, 52810–52831.
- Myburgh, E.J.; Langenhoven, L.; Grant, K.A.; van der Merwe, L.; Kotze, M. Clinical overestimation of HER2 positivity in early estrogen and progesterone receptor–positive breast cancer and the value of molecular subtyping using Blue Print. J. Glob. Oncol. 2017, 3, 314–322.
- Day, E.S.; Bickford, L.R.; Slater, J.H.; Riggall, N.S.; Drezek, R.A.; West, J.L. Antibody-conjugated gold-gold sulfide nanoparticles as multifunctional agents for imaging and therapy of breast cancer. Int. J. Nanomed. 2010, 5, 445.
- Cai, Z.; Chattopadhyay, N.; Yang, K.; Kwon, Y.L.; Yook, S.; Pignol, J.-P.; Reilly, R.M. 111In-labeled trastuzumab-modified gold nanoparticles are cytotoxic in vitro to HER2-positive breast cancer cells and arrest tumor growth in vivo in athymic mice after intratumoral injection. Nucl. Med. Biol. 2016, 43, 818–826.
- Bhatia, P.; Vasaikar, S.; Wali, A. A landscape of nanomedicine innovations in India. Nanotechnol. Rev. 2018, 7, 131–148.
- Patel, K.; Patel, K. Challenges and Recent Progress of Nano Sized Drug Delivery Systems for Lung Cancer Therapy: A Review. Himal. J. Health Sci. 2020, 5, 58–62.
- Fuhrhop, J.-H.; Wang, T. Bolaamphiphiles. Chem. Rev. 2004, 104, 2901–2938.
- Tandel, H.; Bhatt, P.; Jain, K.; Shahiwala, A.; Misra, A. In-vitro and in-vivo tools in emerging drug delivery scenario: Challenges and updates. In In-Vitro and In-Vivo Tools in Drug Delivery Research for Optimum Clinical Outcomes; Mishra, A., shahiwala, A., Eds.; CRC Press, Tailor & Francis Group: Boca Raton, FL, USA, 2018; pp. 19–42.
- Zhang, H.; Yu, N.; Chen, Y.; Yan, K.; Wang, X. Cationic liposome codelivering PI3K pathway regulator improves the response of BRCA1-deficient breast cancer cells to PARP1 inhibition. J. Cell. Biochem. 2019, 120, 13037–13045.
- Alavi, M.; Hamidi, M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab. Pers. Ther. 2019, 34.
- Zhao, M.; Ding, X.F.; Shen, J.Y.; Zhang, X.P.; Ding, X.W.; Xu, B. Use of liposomal doxorubicin for adjuvant chemotherapy of breast cancer in clinical practice. J. Zhejiang Univ.-SCIENCE B 2017, 18, 15–26.
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.-J. Versatile types of organic/inorganic nanohybrids: From strategic design to biomedical applications. Chem. Rev. 2018, 119, 1666–1762.
- Yang, B.; Song, B.P.; Shankar, S.; Guller, A.; Deng, W. Recent advances in liposome formulations for breast cancer therapeutics. Cell. Mol. Life Sci. 2021, 78, 5225–5243.
- Di Wu, M.S.; Xue, H.Y.; Wong, H.L. Nanomedicine applications in the treatment of breast cancer: Current state of the art. Int. J. Nanomed. 2017, 12, 5879–5892.
- Chowdhury, N.; Chaudhry, S.; Hall, N.; Olverson, G.; Zhang, Q.-J.; Mandal, T.; Dash, S.; Kundu, A. Targeted Delivery of Doxorubicin Liposomes for Her-2+ Breast Cancer Treatment. AAPS PharmSciTech 2020, 21, 1–12.
- Iqbal, J.; Abbasi, B.A.; Ahmad, R.; Mahmood, T.; Ali, B.; Khalil, A.T.; Kanwal, S.; Shah, S.A.; Alam, M.M.; Badshah, H.; et al. Nanomedicines for developing cancer nanotherapeutics: From benchtop to bedside and beyond. Appl. Microbiol. Biotechnol. 2018, 102, 9449–9470.
- Rau, K.-M.; Lin, Y.-C.; Chen, Y.-Y.; Chen, J.-S.; Lee, K.-D.; Wang, C.-H.; Chang, H.-K. Pegylated liposomal doxorubicin (Lipo-Dox®) combined with cyclophosphamide and 5-fluorouracil is effective and safe as salvage chemotherapy in taxane-treated metastatic breast cancer: An open-label, multi-center, non-comparative phase II study. BMC Cancer 2015, 15, 1–8.
- Burade, V.; Bhowmick, S.; Maiti, K.; Zalawadia, R.; Ruan, H.; Thennati, R. Lipodox®(generic doxorubicin hydrochloride liposome injection): In vivo efficacy and bioequivalence versus Caelyx®(doxorubicin hydrochloride liposome injection) in human mammary carcinoma (MX-1) xenograft and syngeneic fibrosarcoma (WEHI 164) mouse models. BMC Cancer 2017, 17, 1–12.
- Solinas, C.; Aiello, M.; Migliori, E.; Willard-Gallo, K.; Emens, L.A. Breast cancer vaccines: Heeding the lessons of the past to guide a path forward. Cancer Treat. Rev. 2020, 84, 101947.
- Boulikas, T. Clinical overview on Lipoplatin™: A successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs 2009, 18, 1197–1218.
- Awada, A.; Bondarenko, I.N.; Bonneterre, J.; Nowara, E.; Ferrero, J.M.; Bakshi, A.V.; Wilke, C.; Piccart, M.; CT4002 Study Group. A randomized controlled phase II trial of a novel composition of paclitaxel embedded into neutral and cationic lipids targeting tumor endothelial cells in advanced triple-negative breast cancer (TNBC). Ann. Oncol. 2014, 25, 824–831.
- Karkada, M.; Berinstein, N.L.; Mansour, M. Therapeutic vaccines and cancer: Focus on DPX-0907. Biol. Targets Ther. 2014, 8, 27.
- Pilla, L.; Rivoltini, L.; Patuzzo, R.; Marrari, A.; Valdagni, R.; Parmiani, G. Multipeptide vaccination in cancer patients. Expert Opin. Biol. Ther. 2009, 9, 1043–1055.
- Yang, Y.; Zhao, Z.; Xie, C.; Zhao, Y. Dual-targeting liposome modified by glutamic hexapeptide and folic acid for bone metastatic breast cancer. Chem. Phys. Lipids 2020, 228, 104882.
- Liang, Z.; Du, L.; Zhang, E.; Zhao, Y.; Wang, W.; Ma, P.; Dai, M.; Zhao, Q.; Xu, H.; Zhang, S.; et al. Targeted-delivery of siRNA via a polypeptide-modified liposome for the treatment of gp96 over-expressed breast cancer. Mater. Sci. Eng. C 2021, 121, 111847.
- Piccolo, M.; Ferraro, M.G.; Raucci, F.; Riccardi, C.; Saviano, A.; Russo Krauss, I.; Trifuoggi, M.; Caraglia, M.; Paduano, L.; Montesarchio, D.; et al. Safety and Efficacy Evaluation In Vivo of a Cationic Nucleolipid Nanosystem for the Nanodelivery of a Ruthenium (III) Complex with Superior Anticancer Bioactivity. Cancers 2021, 13, 5164.
- Piccolo, M.; Misso, G.; Ferraro, M.G.; Riccardi, C.; Capuozzo, A.; Zarone, M.R.; Maione, F.; Trifuoggi, M.; Stiuso, P.; D’Errico, G.; et al. Exploring cellular uptake, accumulation and mechanism of action of a cationic Ru-based nanosystem in human preclinical models of breast cancer. Sci. Rep. 2019, 9, 1–15.
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Ferreira, E.I.; El Seoud, O.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814.
- Narmani, A.; Mohammadnejad, J.; Yavari, K. Synthesis and evaluation of polyethylene glycol-and folic acid-conjugated polyamidoamine G4 dendrimer as nanocarrier. J. Drug Deliv. Sci. Technol. 2019, 50, 278–286.
- Li, J.; Liang, H.; Liu, J.; Wang, Z. Poly (amidoamine)(PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int. J. Pharm. 2018, 546, 215–225.
- Janiszewska, J.; Posadas, I.; Játiva, P.; Bugaj-Zarebska, M.; Urbanczyk-Lipkowska, Z.; Ceña, V. Second generation amphiphilic poly-lysine dendrons inhibit glioblastoma cell proliferation without toxicity for neurons or astrocytes. PLoS ONE 2016, 11, e0165704.
- Alven, S.; Aderibigbe, B.A. The Therapeutic Efficacy of Dendrimer and Micelle Formulations for Breast Cancer Treatment. Pharmaceutics 2020, 12, 1212.
- Mahmoudi, A.; Sadi, K.S.; Malaekeh-Nikouei, B. Surface engineered dendrimers as novel option for enhanced pharmaceutical and biomedical potential. In Dendrimer-Based Nanotherapeutics; Academic Press: Cambridge, MA, USA, 2021; pp. 225–252.
- Mehta, D.; Leong, N.; McLeod, V.M.; Kelly, B.D.; Pathak, R.; Owen, D.J.; Porter, C.J.; Kaminskas, L.M. Reducing dendrimer generation and PEG chain length increases drug release and promotes anticancer activity of PEGylated polylysine dendrimers conjugated with doxorubicin via a cathepsin-cleavable peptide linker. Mol. Pharm. 2018, 15, 4568–4576.
- Aleanizy, F.S.; Alqahtani, F.Y.; Seto, S.; Al Khalil, N.; Aleshaiwi, L.; Alghamdi, M.; Alquadeib, B.; Alkahtani, H.; Aldarwesh, A.; Alqahtani, Q.H. Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy. Int. J. Nanomed. 2020, 15, 5433.
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268.
- Takahashi, A.; Yamamoto, Y.; Yasunaga, M.; Koga, Y.; Kuroda, J.I.; Takigahira, M.; Harada, M.; Saito, H.; Hayashi, T.; Kato, Y. NC-6300, an epirubicin-incorporating micelle, extends the antitumor effect and reduces the cardiotoxicity of epirubicin. Cancer Sci. 2013, 104, 920–925.
- Danson, S.; Ferry, D.; Alakhov, V.; Margison, J.; Kerr, D.; Jowle, D.; Brampton, M.; Halbert, G.; Ranson, M. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br. J. Cancer 2004, 90, 2085–2091.
- Venne, A.; Li, S.; Mandeville, R.; Kabanov, A.; Alakhov, V. Hypersensitizing effect of pluronic L61 on cytotoxic activity, transport, and subcellular distribution of doxorubicin in multiple drug-resistant cells. Cancer Res. 1996, 56, 3626–3629.
- Alakhov, V.; Klinski, E.; Li, S.; Pietrzynski, G.; Venne, A.; Batrakova, E.; Bronitch, T.; Kabanov, A. Block copolymer-based formulation of doxorubicin. From cell screen to clinical trials. Colloids Surf. BBiointerfaces 1999, 16, 113–134.
- Sun, Y.; Zou, W.; Bian, S.; Huang, Y.; Tan, Y.; Liang, J.; Fan, Y.; Zhang, X. Bioreducible PAA-g-PEG graft micelles with high doxorubicin loading for targeted antitumor effect against mouse breast carcinoma. Biomaterials 2013, 34, 6818–6828.
- Gener, P.; Gouveia, L.P.; Sabat, G.R.; de Sousa Rafael, D.F.; Fort, N.B.; Arranja, A.; Fernández, Y.; Prieto, R.M.; Ortega, J.S.; Arango, D. Biology; Medicine, Fluorescent CSC models evidence that targeted nanomedicines improve treatment sensitivity of breast and colon cancer stem cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1883–1892.
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.-B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250.
- Taurin, S.; Nehoff, H.; Diong, J.; Larsen, L.; Rosengren, R.J.; Greish, K. Curcumin-derivative nanomicelles for the treatment of triple negative breast cancer. J. Drug Target. 2013, 21, 675–683.
- Kutty, R.V.; Feng, S.-S. Cetuximab conjugated vitamin E TPGS micelles for targeted delivery of docetaxel for treatment of triple negative breast cancers. Biomaterials 2013, 34, 10160–10171.
- Sargent, L.M.; Shvedova, A.; Hubbs, A.; Salisbury, J.; Benkovic, S.; Kashon, M.; Lowry, D.; Murray, A.; Kisin, E.; Friend, S.; et al. Induction of aneuploidy by single-walled carbon nanotubes. Environ. Mol. Mutagenesis 2009, 50, 708–717.
- Servant, A.; Jacobs, I.; Bussy, C.; Fabbro, C.; Da Ros, T.; Pach, E.; Ballesteros, B.; Prato, M.; Nicolay, K.; Kostarelos, K. Gadolinium-functionalised multi-walled carbon nanotubes as a T1 contrast agent for MRI cell labelling and tracking. Carbon 2016, 97, 126–133.
- Hashemi, M.; Yadegari, A.; Yazdanpanah, G.; Omidi, M.; Jabbehdari, S.; Haghiralsadat, F.; Yazdian, F.; Tayebi, L. Normalization of doxorubicin release from graphene oxide: New approach for optimization of effective parameters on drug loading. Biotechnol. Appl. Biochem. 2017, 64, 433–442.
- Huson, H.B.; Sanghani, M.V.; Mayra Lupe Llamas, C.; Leslie, E.; Botnick, M. Long-term community-based results of breast-conserving therapy in early-stage breast cancer. J. Community Supportive Oncol. 2016, 14, 249–254.
- Nasrollahi, F.; Varshosaz, J.; Khodadadi, A.A.; Lim, S.; Jahanian-Najafabadi, A. Targeted delivery of docetaxel by use of transferrin/poly (allylamine hydrochloride)-functionalized graphene oxide nanocarrier. ACS Appl. Mater. Interfaces 2016, 8, 13282–13293.
- Shi, J.; Wang, B.; Wang, L.; Lu, T.; Fu, Y.; Zhang, H.; Zhang, Z. Fullerene (C60)-based tumor-targeting nanoparticles with “off-on” state for enhanced treatment of cancer. J. Control. Release 2016, 235, 245–258.
- Jawahar, N.; De, A.; Jubee, S.; Reddy, E.S. Folic acid-conjugated raloxifene hydrochloride carbon nanotube for targeting breast cancer cells. Drug Dev. Res. 2020, 81, 305–314.
- Akinoglu, E.; Ozbilgin, K.; Sonmez, P.K.; Ozkut, M.; Giersig, M.; Inan, S.; Gumustepe, E.; Kurtman, C. Biocompatibility of vertically aligned multi-walled carbon nanotube scaffolds for human breast cancer cell line MDA-MB-231. Prog. Biomater. 2017, 6, 189–196.
- Crucho, C.I.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. 2017, 80, 771–784.
- Cano, A.; Sánchez-López, E.; Ettcheto, M.; López-Machado, A.; Espina, M.; Souto, E.B.; Galindo, R.; Camins, A.; García, M.L.; Turowski, P. Current advances in the development of novel polymeric nanoparticles for the treatment of neurodegenerative diseases. Nanomedicine 2020, 15, 1239–1261.
- Guterres, S.S.; Alves, M.P.; Pohlmann, A.R. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights 2007, 2, 117739280700200002.
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102.
- Devulapally, R.; Sekar, N.M.; Sekar, T.V.; Foygel, K.; Massoud, T.F.; Willmann, J.K.; Paulmurugan, R. Polymer nanoparticles mediated codelivery of antimiR-10b and antimiR-21 for achieving triple negative breast cancer therapy. ACS Nano 2015, 9, 2290–2302.
- Grewal, I.K.; Singh, S.; Arora, S.; Sharma, N. Polymeric nanoparticles for breast cancer therapy: A comprehensive review. Biointerface Res. Appl. Chem. 2020, 11, 11151–11171.
- Andey, T.; Sudhakar, G.; Marepally, S.; Patel, A.; Banerjee, R.; Singh, M. Lipid nanocarriers of a lipid-conjugated estrogenic derivative inhibit tumor growth and enhance cisplatin activity against triple-negative breast cancer: Pharmacokinetic and efficacy evaluation. Mol. Pharm. 2015, 12, 1105–1120.
- Ahmad, I.; Pandit, J.; Sultana, Y.; Mishra, A.K.; Hazari, P.P.; Aqil, M. Optimization by design of etoposide loaded solid lipid nanoparticles for ocular delivery: Characterization, pharmacokinetic and deposition study. Mater. Sci. Eng. C 2019, 100, 959–970.
- Aljaeid, B.M.; Hosny, K.M. Miconazole-loaded solid lipid nanoparticles: Formulation and evaluation of a novel formula with high bioavailability and antifungal activity. Int. J. Nanomed. 2016, 11, 441.
- Xu, W.; Bae, E.J.; Lee, M.-K. Enhanced anticancer activity and intracellular uptake of paclitaxel-containing solid lipid nanoparticles in multidrug-resistant breast cancer cells. Int. J. Nanomed. 2018, 13, 7549.
- Pindiprolu, S.K.S.; Chintamaneni, P.K.; Krishnamurthy, P.T.; Ratna Sree Ganapathineedi, K. Formulation-optimization of solid lipid nanocarrier system of STAT3 inhibitor to improve its activity in triple negative breast cancer cells. Drug Dev. Ind. Pharm. 2019, 45, 304–313.
- Rahman, M.; Mohammed, S. Breast cancer metastasis and the lymphatic system. Oncol. Lett. 2015, 10, 1233–1239.
- Rompicharla, S.V.K.; Bhatt, H.; Shah, A.; Komanduri, N.; Vijayasarathy, D.; Ghosh, B.; Biswas, S. Formulation optimization, characterization, and evaluation of in vitro cytotoxic potential of curcumin loaded solid lipid nanoparticles for improved anticancer activity. Chem. Phys. Lipids 2017, 208, 10–18.
- Wang, W.; Zhang, L.; Chen, T.; Guo, W.; Bao, X.; Wang, D.; Ren, B.; Wang, H.; Li, Y.; Wang, Y. Anticancer effects of resveratrol-loaded solid lipid nanoparticles on human breast cancer cells. Molecules 2017, 22, 1814.
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured lipid carriers for delivery of chemotherapeutics: A review. Pharmaceutics 2020, 12, 288.
- Müller, R.H.; Alexiev, U.; Sinambela, P.; Keck, C.M. Nanostructured lipid carriers (NLC): The second generation of solid lipid nanoparticles. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–185.
- Cortesi, R.; Valacchi, G.; Muresan, X.M.; Drechsler, M.; Contado, C.; Esposito, E.; Grandini, A.; Guerrini, A.; Forlani, G.; Sacchetti, G. Nanostructured lipid carriers (NLC) for the delivery of natural molecules with antimicrobial activity: Production, characterisation and in vitro studies. J. Microencapsul. 2017, 34, 63–72.
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161.
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613.
- Ding, X.; Xu, X.; Zhao, Y.; Zhang, L.; Yu, Y.; Huang, F.; Yin, D.; Huang, H. Tumor targeted nanostructured lipid carrier co-delivering paclitaxel and indocyanine green for laser triggered synergetic therapy of cancer. RSC Adv. 2017, 7, 35086–35095.
- Lin, M.; Teng, L.; Wang, Y.; Zhang, J.; Sun, X. Curcumin-guided nanotherapy: A lipid-based nanomedicine for targeted drug delivery in breast cancer therapy. Drug Deliv. 2016, 23, 1420–1425.
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Control. Release 2016, 227, 23–37.
- Quader, S.; Kataoka, K. Nanomaterial-enabled cancer therapy. Mol. Ther. 2017, 25, 1501–1513.
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-brain delivery methods using nanotechnology. Pharmaceutics 2018, 10, 269.
- Elzoghby, A.O.; El-Lakany, S.A.; Helmy, M.W.; Abu-Serie, M.M.; Elgindy, N.A. Shell-crosslinked zein nanocapsules for oral codelivery of exemestane and resveratrol in breast cancer therapy. Nanomedicine 2017, 12, 2785–2805.
- Tagde, P.; Tagde, S.; Tagde, P.; Bhattacharya, T.; Monzur, S.M.; Rahman, M.; Otrisal, P.; Behl, T.; Abdel-Daim, M.M.; Aleya, L.; et al. Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2. Biomedicines 2021, 9, 1266.
- Esfandiarpour-Boroujeni, S.; Bagheri-Khoulenjani, S.; Mirzadeh, H.; Amanpour, S. Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohydr. Polym. 2017, 168, 14–21.
- Day, C.M.; Hickey, S.M.; Song, Y.; Plush, S.E.; Garg, S. Novel tamoxifen nanoformulations for improving breast cancer treatment: Old wine in new bottles. Molecules 2020, 25, 1182.
More
Information
Subjects:
Allergy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
774
Revisions:
2 times
(View History)
Update Date:
09 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




