Alzheimer’s Disease (AD) is one of the main neurodegenerative diseases worldwide. Unfortunately, AD shares many similarities with other dementias at early stages, which impedes an accurate premortem diagnosis. Therefore, it is urgent to find biomarkers to allow for early diagnosis of the disease. There is increasing scientific evidence highlighting the similarities between the eye and other structures of the CNS, suggesting that knowledge acquired in eye research could be useful for research and diagnosis of AD. For example, the retina and optic nerve are considered part of the central nervous system, and their damage can result in retrograde and anterograde axon degeneration, as well as abnormal protein aggregation. In the anterior eye segment, the aqueous humor and tear film may be comparable to the cerebrospinal fluid. Both fluids are enriched with molecules that can be potential neurodegenerative biomarkers. Indeed, the pathophysiology of AD, characterized by cerebral deposits of amyloid-beta (Aβ) and tau protein, is also present in the eyes of AD patients, besides numerous structural and functional changes observed in the structure of the eyes. Therefore, all this evidence suggests that ocular changes have the potential to be used as either predictive values for AD assessment or as diagnostic tools.
1. Introduction
Alzheimer’s Disease (AD) is a degenerative disorder of the nervous system with a slow and progressive onset. AD is mainly considered an old-age condition, being the most common neurodegenerative disorder among the elderly in developed countries
[1][2][3]. However, based on age at onset, AD can be defined as either early-onset AD (<65 years) or late-onset AD (>65 years)
[4]. Overall, early-onset AD is mostly caused by autosomal dominant mutations, with the β-amyloid (Aβ) precursor protein (APP), presenilin 1 (PS1), and presenilin 2 (PS2) genes among the most studied
[4]. Remarkably, these mutations collectively represent less than 1% of total cases. In contrast, late-onset AD represents most of AD cases, although its etiology remains unclear because of the multifactorial nature of the disease, where both environmental and genetic risk (e.g., the ε4 allele of APOE (APOEε4)) factors are involved
[4][5].
The two pathophysiological hallmarks of AD are neuronal and glial abnormal protein deposits of both extracellular Aβ and intracellular filamentous aggregates of tau
[6]. It is well known that such protein accumulations trigger cellular pathways underlying neuronal death but also mediate the activation of microglia and astrocytes, which leads to further damage of surrounding tissues via inflammatory processes
[2][6][7]. As a result, there is a progressive atrophy of brain structures, including different lobes (frontal, temporal, and parietal), the entorhinal cortex, amygdala and hippocampus, among others
[6][8]. Eventually, these molecular and histopathological changes impact negatively on cortical cognitive functions, such as memory, motor, and language functions, which can promote and/or exacerbate depression or anxiety states
[2][3][6]. Although there have been significant advances in the understanding of AD over the last two decades, there are still no reliable treatments to slow down the progression of the disease and/or its onset. Moreover, the lack of an early diagnostic test to accurately determine the onset of AD makes the available treatments almost ineffective, since the neuronal damage is irreparable by the time the disease is diagnosed
[9].
Nowadays, the diagnosis of AD is based on clinical, cognitive, and functional criteria using brief cognitive tests (e.g., Mini-Mental Status Examination (MMSE) and clinical dementia rating (CDR)), neuroimaging techniques (e.g., brain scanning by computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)), and biomarker analysis in cerebrospinal fluid (CSF)
[10]. Likewise, the National Institute of Aging and the Alzheimer’s Association established complete clinical and cognitive guidelines for the diagnosis of mild cognitive impairment (MCI) or dementia associated with AD, allowing for the classification of individuals with probable AD dementia, possible AD dementia, and probable or possible AD dementia
[11]. Early detection/diagnosis is mandatory in order to obtain an effective treatment of AD patients with drugs to delay cognitive loss, as well as with non-pharmacological treatments, such as non-invasive brain stimulation (NIBS), to prolong their quality of life. NIBS approaches are currently getting attention as they show promising results by stimulating different brain regions simultaneously, which improves memory and specific cognitive functions
[12][13][14][15][16]. Interestingly, these NIBS approaches could offer a reliable therapeutic option for those AD patients not responding to drug treatments
[14].
Therefore, it is important to identify neurodegenerative biomarkers that detect cognitive decline or progression from MCI to dementia
[17][18]. In this regard, ophthalmological assessments have detected several ocular changes in patients with central nervous system (CNS) disorders
[19]. In many of these disorders, ocular manifestations often precede brain symptoms, suggesting that eye exams could offer an early diagnosis of the underlying disease
[19]. Hence, since the eye constitutes an extension of the brain, looking for early ocular manifestations in AD becomes an essential element to explore further.
Several reasons support the viability of the eye as a useful model for the study of AD. First, in comparison with other CNS structures, the eye is relatively accessible for manipulation and in vivo observation. Currently, ophthalmological imaging techniques, such as optical coherence tomography (OCT) or scanning laser ophthalmoscopy (SLO), allow for the visualization and study of the eye by a non-invasive approach
[20][21][22]. Secondly, different aspects of visual processing can be affected by AD. Patients with damage in the dorsal region have impaired functions, such as angular discrimination and motion perception, whereas patients with damage in the ventral region have impaired face, color, and shape discrimination
[23][24]. Furthermore, it has been suggested that a thinner retinal nerve fiber layer is associated with cognitive decline in subjects with MCI and AD
[25]. There are also several studies showing that cognitive decline is associated with brain atrophy and corneal nerve fiber loss
[25][26][27][28]. Finally, visual perceptual disturbances are quite common in AD. Loss of visual field, decreased contrast sensitivity, low visual acuity, impaired color vision or motion perception, visuospatial deficits, object agnosia, prosopagnosia, and impaired recognition of emotional facial expressions are some visual deficits that may be involved in AD
[29].
2. Cognitive Alterations and Visual Repercussions Related to AD
As previously introduced, neuropsychological examination aims to define the state of the different components of a patient’s cognitive status. Cognition is the set of brain-based activities that is allowed to be aware of ourselves, others, and the environment
[30]. Therefore, the assessment of a potential AD patient should include issues such as memory, orientation, language, behavior, functional ability, executive function, emotional and affective state, and behavioral changes. Several neuropsychological tests, including the MMSE, the CDR, the AD Assessment Scale (ADAS-cog), and the Montreal Cognitive Assessment (MoCA), are used to define the patterns of affected and preserved cognitive abilities
[31][32][33]. Importantly, amnesia is the most common form of cognitive impairment attributed to AD, as seen in patients with MCI
[34]. However, additional approaches, such as genetics (
APOEε4 carrier), neuroimaging (medial temporal lobe atrophy on MRI, temporoparietal hypometabolism and/or amyloid uptake on PET), and molecular biomarkers (Aβ and tau/p-tau levels in CSF) are also required
[35].
Visual perception helps people to acquire information about the surrounding world; when visual perception deteriorates, quality of life worsens and complicates the assessment of other cognitive deficits
[29]. AD is not an exception since most AD patients experience defects in visual recognition as a consequence of damage in the associative visual areas
[29]. In general, these early visual recognition deficits include impaired ocular fixation and difficulty in visual analysis and synthesis. In particular, such patients often show difficulty in describing the content of a complex photograph, recognizing figures presented from unusual perspectives, or identifying incomplete letters. In advanced stages of AD, patients show visual apperceptive agnosia, involving great difficulty in identifying objects and loss of the ability to reconstruct shapes
[29]. In fact, AD patients frequently report difficulty recognizing familiar faces (prosopagnosia)
[36]. These patients are also unable to locate objects in space and exhibit a lack of vision–hand coordination (optic ataxia)
[37].
3. Ocular Alterations Related to AD
The eye also displays many features of the AD pathophysiology seen in the brain (
Figure 1). In this regard, there are numerous structural and functional changes in the eye linked to AD progression
[25][26][27][28]. As mentioned above, irreversible progression, due to the lack of both an effective early diagnosis and a treatment that reverses the AD, exalts the necessity of finding early detection, diagnosis, and curative strategies. In this scenario, eyes could constitute a promising target for new strategies to detect AD progression and onset.
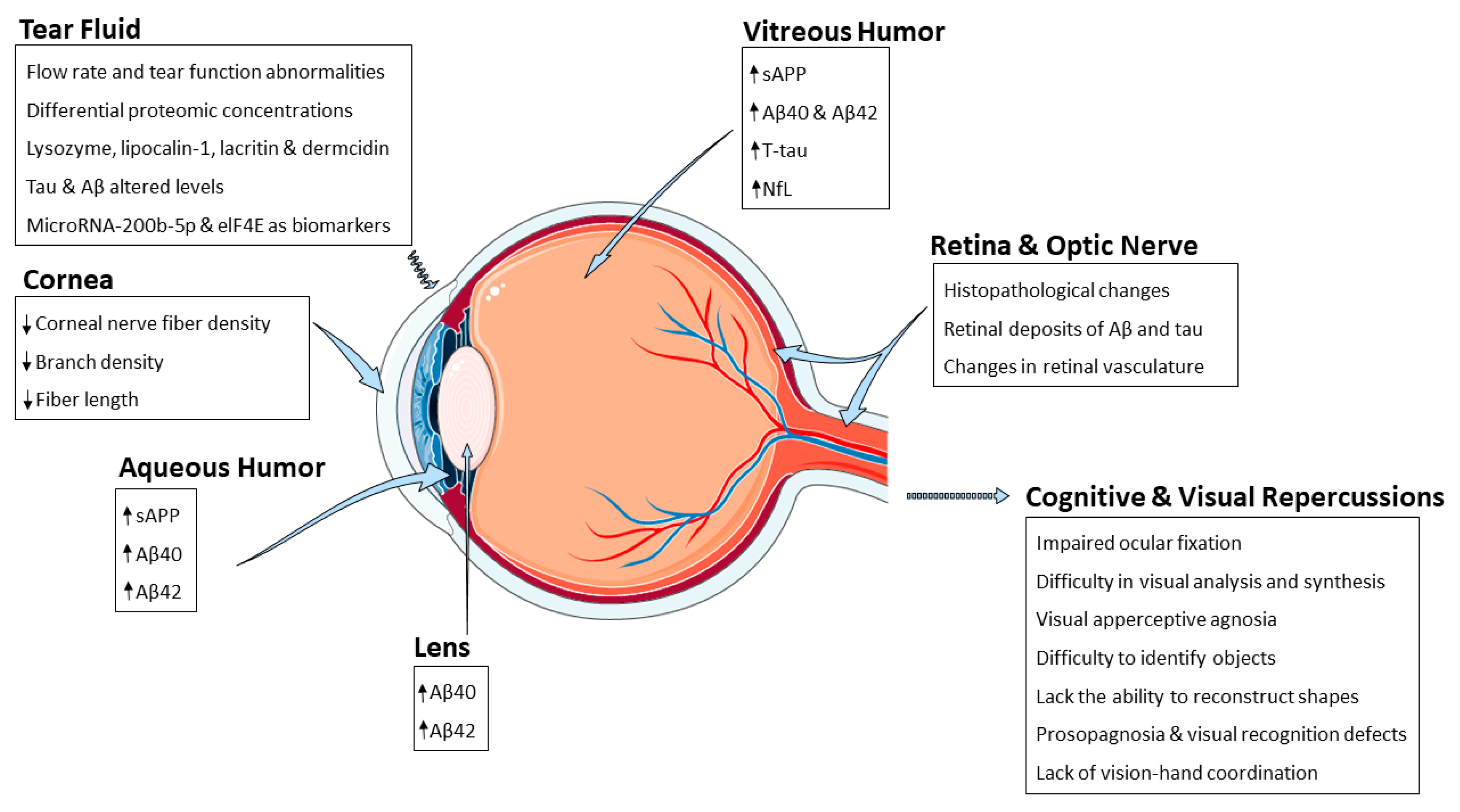 Figure 1.
Figure 1. Schematic representation summarizing the AD-related alterations reported so far and the different eye structures affected by such changes. Abbreviations: amyloid-β peptide (Aβ), eukaryotic initiation factor 4 e (eIF4E), neurofilament light chain (NfL), soluble amyloid precursor protein (sAPP).
3.1. Retina and Optic Nerve
The retina is a light-sensitive tissue lining the inner surface of the eye, and it is composed of 10 different layers (from interior to exterior): the inner limiting membrane, retinal nerve fiber layer (RNFL), retinal ganglion cell layer (RGCL), inner plexiform layer (IPL), inner nuclear layer, outer plexiform layer, outer nuclear layer, external limiting membrane, photoreceptor cell layer, and retinal pigment epithelium (
Figure 2)
[38]. In the central retina, there is a region called the macula that comprises multiple ganglion cell layers and supplies ~50% of the visual input to the cortex
[39]. The fovea, a thin retinal zone composed exclusively of cones, is located at the center of the macula and allows for high-acuity vision and color perception
[39].
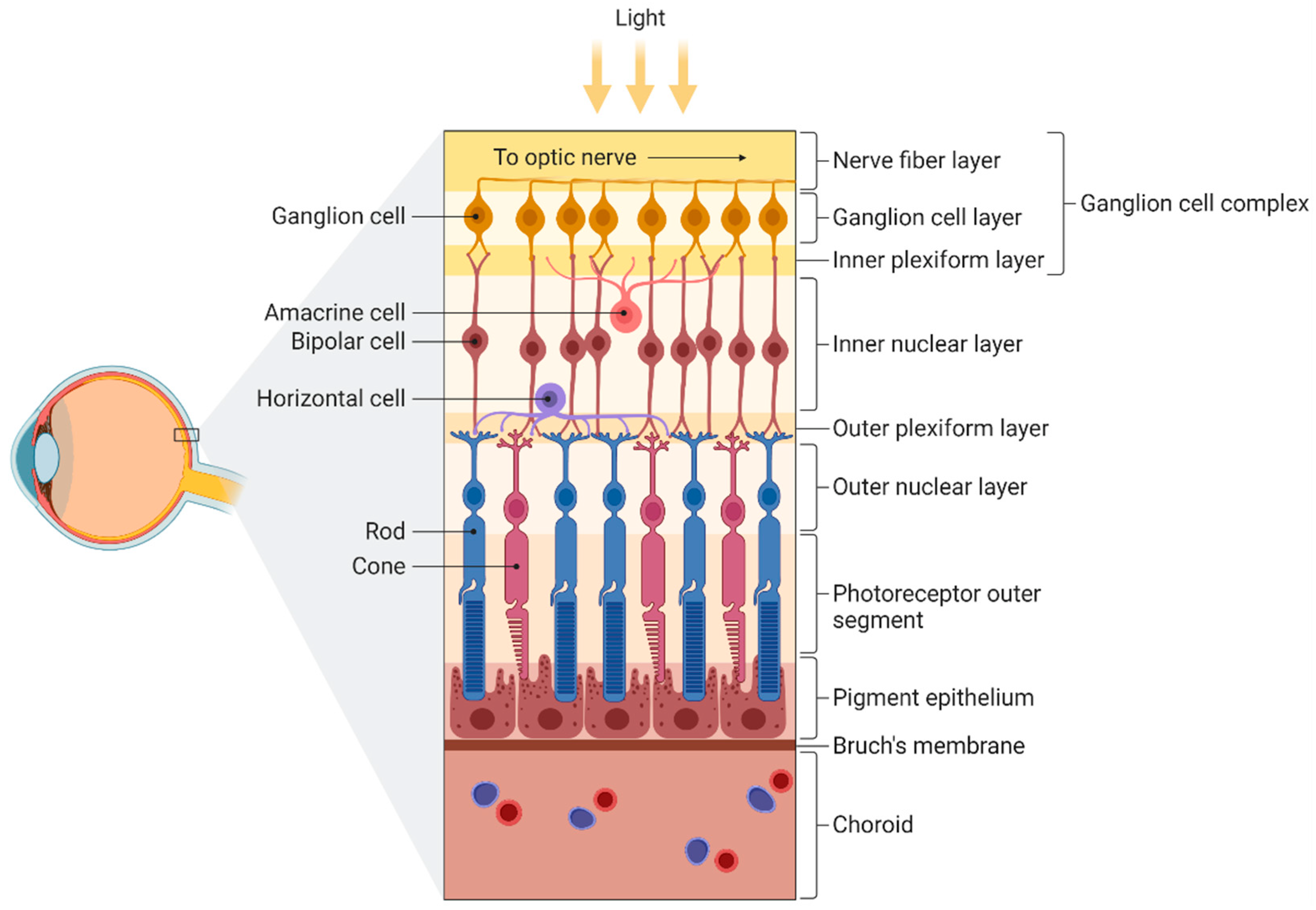 Figure 2.
Figure 2. Schematic illustration of the different layers of the retina. From inner to outer: nerve fiber layer (containing axons from ganglion cells), ganglion cell layer (containing ganglion cells), inner plexiform layer (containing dendrites from ganglion, bipolar, and amacrine cells), inner nuclear layer (containing bipolar and amacrine cells), outer plexiform layer (containing extensions from horizontal cells and both rods and cones), outer nuclear layer (containing somas of both rods and cones), photoreceptor outer segment, pigment epithelium, Bruch’s membrane, and choroid. Figure created by BioRender.com software (Toronto, ON, Canada).
The RNFL consists of axons of the ganglion cells guided to the CNS through the optic nerve (Figure 2). Moreover, there is a type of fiber called the papillomacular bundle, which carries the information that determines visual acuity and radiates from the macula area to the optic disc. The retinal ganglion cells (RGCs) form the RGCL, where they are arranged as a layer with single-cell thickness, except near the macula and the temporal side of the optic disc. Finally, the IPL is a synaptic area where axons from bipolar cells, amacrine cells, and ganglion cells converge. Therefore, the main function of this layer is to integrate inputs from motion detection, brightness changes, and the recognition of contrast and hue.
As a part of the CNS, the retina may also display the classical hallmarks of AD, as previously mentioned. The optic nerve forms a connection between the retina and the brain that may allow the crossing of amyloid precursor protein (APP) from RGCs to the cortex and vice versa. In addition, these changes underlie the atrophy and/or death of different retinal cells, as well as structural and functional modifications in the retinal morphology and vasculature
[40][41][42][43]. Considering the retina as a “window” to the brain, its visualization offers a direct and non-invasive approach to detect AD hallmarks without major disturbances in patients.
3.2. Tear Fluid
The analysis of different body fluids is a strategy to elucidate the pathophysiological mechanisms underlying a wide variety of diseases. Remarkably, the rising incidence of AD has highlighted the necessity to develop new screening and early-diagnosis techniques using less invasive and cheaper methods. In this regard, tear fluid analysis has become a promising non-invasive alternative in the search for biomarkers associated with AD.
Tear fluid consists of an aqueous–lipidic layer containing proteins, mucins, lipids, water, and electrolytes (
Figure 3). It covers and nourishes the ocular surface, playing a key role in protection against external pathogens. Even though the volume of tear samples is small, advances in proteomics and lipidomics have allowed for a better understanding of tear components and their involvement in different diseases
[44]. In this regard, biotechnological tools are being developed to optimize the extraction of samples from collection devices (such as Schirmer strips or capillaries) and to improve the detection of markers, such as β-amyloid, in tears (using biosensors)
[45]. Hopefully, these new tools will help to overcome the limitations related to the small volume of tear samples. To date, there are already numerous ocular (e.g., keratoconus, dry eye, and glaucoma) and systemic conditions (such as diabetes mellitus, thyroid dysfunction, and neurological diseases) that can be detected and evaluated by biomarkers at the lacrimal level
[46][47].
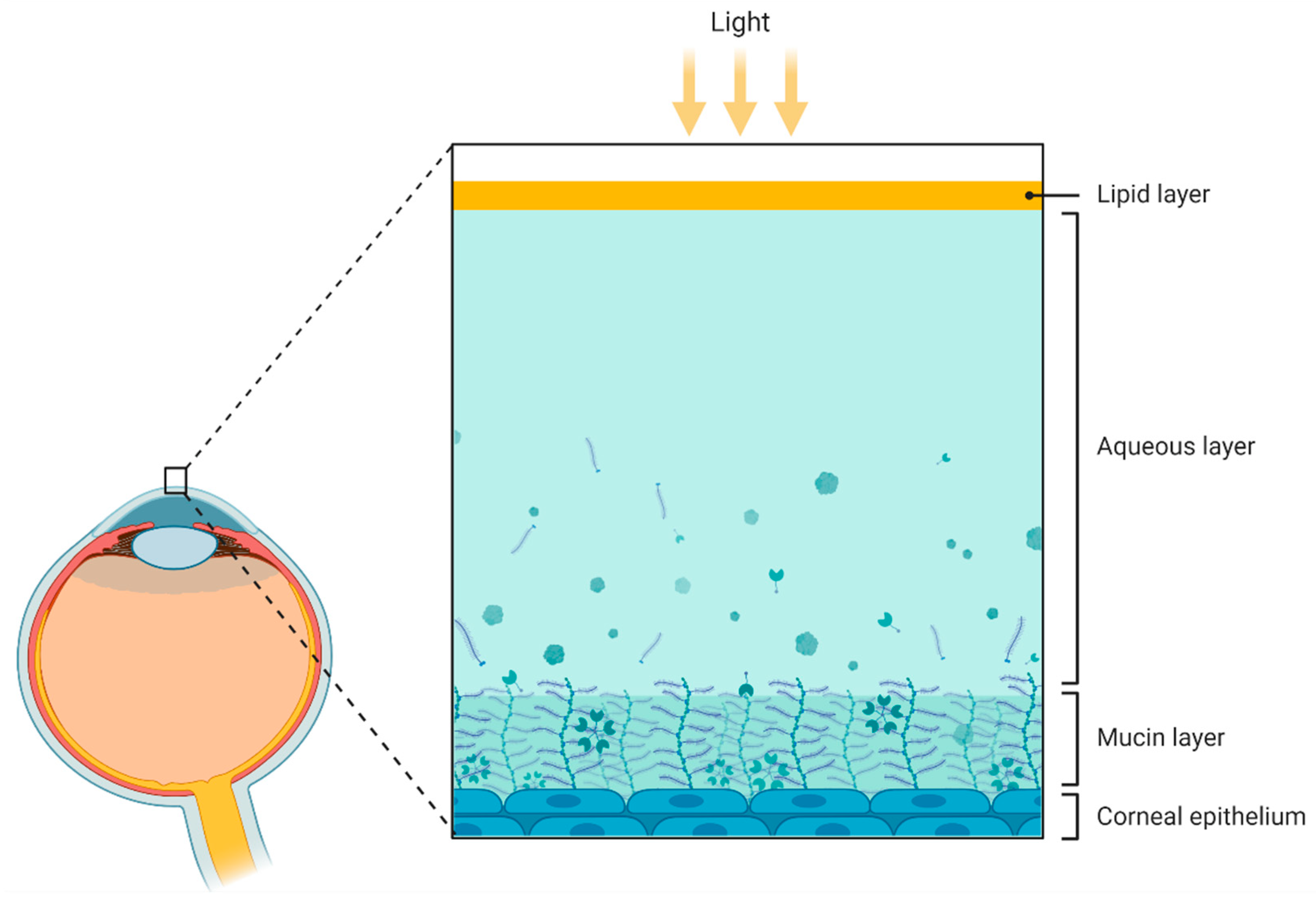 Figure 3.
Figure 3. Schematic representation of the compartments presented in the tear fluid. From outer to inner: lipid layer, aqueous layer, mucin layer (containing glycoproteins called mucins), and corneal epithelium. Figure created by BioRender.com software (Toronto, ON, Canada).
Modifications in total tear proteomic concentration, as well as abnormalities in flow rate and tear function, have been reported in AD, suggesting a dysfunction in the autonomic nervous system
[48] (
Table 1). Indeed, significantly decreased levels of lysozyme, lipocalin-1, and lacritin, as well as increased levels of dermcidin, were found to be expressed in the tear fluid of AD patients
[49] (
Table 1). Remarkably, the combination of all of these factors was demonstrated as a potential AD biomarker, as they showed a sensitivity of 81% and a specificity of 77% for prediction of the disease
[49]. Likewise, CSF biomarkers were measured in tear samples to assess a new non-invasive tool to predict and detect AD. Specifically, three Aβ peptides (Aβ38, Aβ40, and Aβ42), total tau (t-tau), and phosphorylated tau (p-tau) were analyzed in patients diagnosed with clinical dementia, MCI, and subjective cognitive decline (SCD), as well as in a control group
[50] (
Table 1). Interestingly, the concentrations of all peptides were higher in tear samples of all patient groups compared to healthy subjects; however, non-statistical differences were obtained. In addition, t-tau levels were significantly higher in dementia, MCI, and SCD compared to the control group
[50]. Importantly, p-tau was also detected in all patient groups but not in control subjects. Both t-tau and p-tau showed no differential expression among the three patient groups
[50]. Moreover, the association of tear amyloid and tau levels with AD severity and neurodegeneration was also studied, highlighting the potential of tau and Aβ proteins in tear fluid as markers of AD severity
[51][52]. In this regard, a recent study also suggested the potential of t-tau and Aβ42 markers at the lacrimal level for the diagnosis/discrimination of AD
[51][52] (
Table 1). The authors determined a gradual increase in both biomarkers in tears throughout cognitive decline
[51][52]. Additionally, Kenny and colleagues observed that the total tear concentration of microRNA-200b-5p was highly upregulated in AD samples compared to the control group
[53]. Interestingly, the elongation initiation factor 4E (eIF4E), a polypeptide involved in some cellular processes (e.g., protein synthesis, mRNA stability, and RNA nuclear export), was exclusively detected in the tear samples from AD patients
[53] (
Table 1). Further studies are needed to elucidate the biological relationship between AD and biomarkers microRNA-200b-5p and eIF4E. In summary, different studies have been pointed at the relationship between tears and CSF to detect potential biomarkers; nonetheless, more studies should address this relationship.
Table 1. Studies observing changes in tear proteomic/molecular concentrations.
| Publication |
Study |
Results |
| Kalló et al. [49] |
Examination of changes in tear protein composition from patients with AD compared to control |
- −
-
Decreased levels of lysozyme, lipocalin-1, and lacritin in AD patients.
- −
-
Increased levels of dermcidin.
- −
-
Detecting all these alterations together in preclinical AD subjects might be used as a biomarker to further explore other typical AD measurements (imaging, neuropsychological testing, and CSF analyses.
|
| Gijs et al. [50] |
Examination of AD-specific biomarkers in tear fluid from SCD, MCI, and AD patients |
- −
-
Increased levels of Aβ38, Aβ40, Aβ42, t-tau and p-tau in all conditions compared to controls.
- −
-
Only t-tau level changes were significantly increased when comparing each condition to controls.
- −
-
The presence of Aβ peptides, t-tau, and p-tau was shown in tear fluid for the first time.
|
| Gijs et al. [51] |
Testing the diagnostic potential of tears as a source of AD biomarkers |
- −
-
Levels of both t-tau and Aβ42 are positively correlated with the AD stage.
- −
-
Between disease conditions, t-tau in tears of AD patients was significantly higher than in those of SCI and MCI patients.
|
| Gijs et al. [52] |
Observational study to investigate AD-specific biomarkers in tear fluid |
- −
-
Elevated levels of Aβ40 and t-tau in tear fluid from patients with cognitive impairment associated with disease severity.
- −
-
Correlated levels of AD biomarkers in tear fluid and CSF from AD patients.
|
| Kenny et al. [53] |
Examination of tear fluid to discover disease-specific protein and microRNA-based biomarkers for AD |
- −
-
eIF4E was present only in AD samples.
- −
-
Higher abundance of total microRNA in tears from AD patients compared to controls.
- −
-
Interestingly, microRNA-200b-5p could be used as a biomarker for AD.
|
3.3. Cornea
The cornea is an avascular and complex tissue comprised of several layers, such as the epithelium, epithelial basement membrane, Bowman’s layer, stroma, Descemet membrane, and endothelium (
Figure 4)
[54][55]. It is well known that the cornea must maintain transparency to benefit the eye’s refractive capacity.
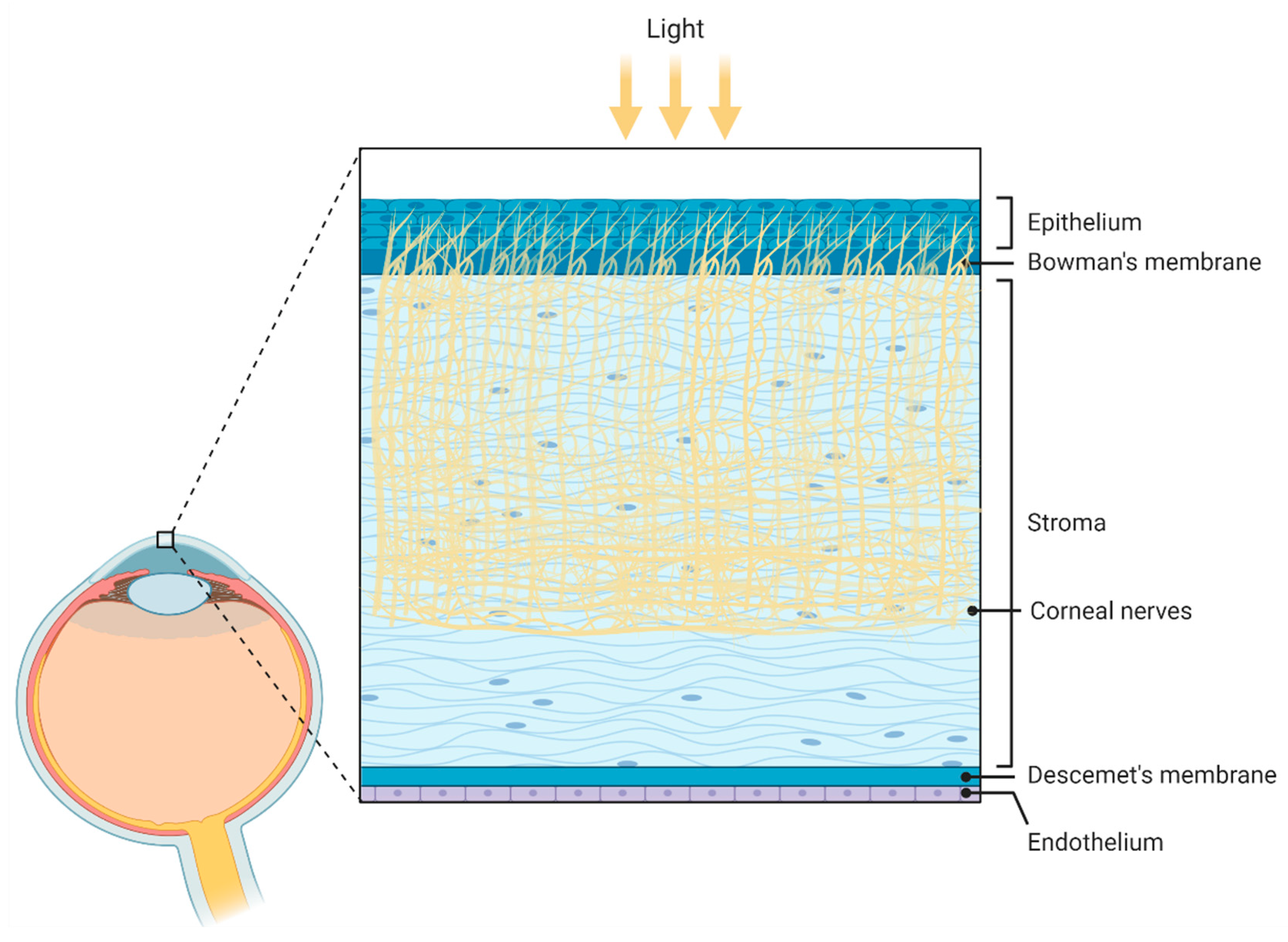 Figure 4.
Figure 4. Schematic illustration of the corneal anatomy showing its different layers and structures. From outer to inner: epithelium, Bowmans’s membrane (containing collagen fibers), stroma (containing corneal innervation), Descemet’s membrane, and endothelium. Figure created by BioRender.com software (Toronto, ON, Canada).
The cornea is considered the most sensitive structure in the human body because it contains a higher number of nerve fibers
[56]. Curiously, a density of 605.8 terminals/mm
2 was observed in the suprabasal layer of the central corneal epithelium
[57], with a corneal innervation 400 times greater than skin innervation and 40 times greater than dental pulp innervation
[58]. Activation of the corneal nerves leads to reflex activation of blinking and tearing and contributes to both the inflammatory response and the release of trophic factors.
The peripheral sensory terminals of the corneal nerves are densely integrated in the epithelium and are responsible for the maintenance and preservation of the corneal tissue and tear film
[56]. Changes in the morphology and function of the corneal nerves in response to disease, surgery, and aging have been well documented
[16][59][60]. In this regard, a wide variety of diseases, including AD, trigger negative ocular surface changes that may be linked to the harmful effects of corneal nerve dysfunction
[16][61]. Specifically, loss of corneal nerve fibers was observed in patients with Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and MCI or dementia
[26][59][60]. In AD, the morphology of corneal nerves has been studied, evidencing corneal nerve degeneration.
Ponirakis and colleagues observed a progressive and significant reduction in corneal nerve fiber density, branch density, and fiber length by corneal confocal microscopy in both MCI and dementia patients compared to age-matched healthy controls
[26]. Furthermore, this research also showed that all these corneal nerve fiber measurements were positively associated with cognitive function and functional independence in MCI and dementia patients
[26].
One of the weaknesses of studying corneal innervation in patients with AD is the limited range of the techniques used to assess the progression of corneal neuropathy. Corneal nerve morphology is evaluated by non-invasive ophthalmic confocal microscopy, and function and sensitivity are mainly tested using aesthesiometers
[62][63]. Moreover, larger studies are needed to establish the diagnostic and prognostic utility of corneal confocal microscopy in people with MCI and dementia. Currently, the use of these tools is mostly reserved for research, and their use in clinical practice is not widespread. Therefore, it is urgent to develop better tools, as well as to refine current ones, in order to use loss of corneal innervation as an early biomarker of AD
[64].
On the other hand, molecular changes in corneal tissue could occur together with the loss of corneal nerve fibers and neurological degeneration in AD. Aging-associated imbalance in the homeostatic levels of neuronal-synapse neurotransmitters (such as glutamate, GABA, and acetylcholine) is associated with the cognitive impairment of several neurological pathologies
[65]. In this line, reduced levels of acetylcholine at the brain level have been found in AD, contributing to weakening synaptic plasticity
[66]. Reduced acetylcholine levels could be compensated by central anticholinesterase drugs that prevent acetylcholine hydrolysis and increase its availability at the synapse. Regarding the cornea, it contains one of the highest concentrations of acetylcholine
[67]. In fact, this neurotransmitter is essential for corneal epithelium development and maintenance. As a result, measuring acetylcholine in the cornea of AD patients could be critical; however, no data exists to date. Currently, anticholinesterase drugs are considered the mainstay treatment for AD, although their use is limited to treating symptomatic alterations associated with memory deficiency
[66].
Amyloid deposition in corneal tissue has been observed in some corneal diseases, including hereditary reticular corneal dystrophy
[68]. Regarding AD, there is still a scarcity of information about the presence and concentration of amyloid and tau in the cornea. Until now, studies carried out on this topic seem to have been focused on animal models. In this line, transgenic mice showed a high cytoplasmic expression of APP and possibly Aβ in the corneal epithelium compared to wild-type controls (using mice that express the human mutation APPswe)
[69]. Likewise, APP transcripts in human corneas and in corneas of transgenic mice with AD appear to be longer and more damaging than those expressed in the brain and retina
[70].
Given the importance of Aβ deposition in AD, accounting for the role of APP, and knowing that the cornea represents an interesting structure to analyze due to its easy access, studies in humans are mandatory to evaluate predictive AD biomarkers that may be present in AD corneas—not only Aβ, but also others, such as tau protein, nerve growth factor (NGF), and acetylcholine.
3.4. Lens
The crystalline lens has been the focus of most research on the anterior eye and AD
[67], considered an ideal ocular structure for the detection of Aβ deposits. Expression of APP and Aβ in cultured crystalline lenses has been found in animal studies, suggesting that pathological mechanisms associated with AD may be linked to the development of age-related cataracts
[71]. The lens must be optically transparent to refract light on the retina, so the accumulation of protein aggregates over time leads to vision loss and worsening visual perception.
A study of Aβ40 and Aβ42 accumulation in human lenses found that both proteins show concentrations comparable to those in the brain of people with and without AD
[72]. Furthermore, both Aβ40 and Aβ42 were accumulated in the cytoplasm of supranuclear/deep cortical lens fiber cells of AD patients, suggesting that Aβ may promote regionally specific lens protein aggregation and supranuclear cataracts
[72]. Even though supranuclear cataracts are more common in patients with AD, measuring cataract severity by lens opacity seems a useless non-invasive test for determining the likelihood of developing AD
[73]. Kerbage and colleagues carried out a promising in vivo study in a small cohort of AD patients, which aimed to measure Aβ aggregates in the supranuclear region of the lens by using a laser scanning device called a fluorescent-ligand eye-scanning system (FLESS)
[74]. The FLESS was designed to detect and measure the emitted fluorescence signal of the fluorescent ligand bound to Aβ aggregates in the supranuclear region of the lens, showing that lens measurements are correlated significantly with F
18-PET amyloid brain analysis
[74]. Although the presence of Aβ in the lens is widely supported by proteomics
[72][75][76], more research in preclinical AD patients is needed to confirm whether Aβ accumulation could be used as a prognostic tool
[77][78].
3.5. Aqueous and Vitreous Humor
Aqueous humor (AH) is an important ultrafiltered blood biological fluid produced in the epithelium of the ciliary body. It is composed of proteins, electrolytes, solutes, and growth factors. AH provides nutrition to the cornea and crystalline lens and removes their excretory metabolic products
[79]. It is essential for maintenance of normal intraocular pressure and provides suitable eye shape and optical properties for the eye. Regarding vitreous humor (VH), it is a gelatinous mass that fills the space between the retina and the lens. It directly contacts and acts as an interface between the retina, the lens, and the ciliary body, contributing to the diffusion of a wide variety of compounds
[80]. Moreover, proteomic analysis showed that the surrounding tissues influence the composition of the different vitreous regions
[80]. Importantly, there is compelling evidence of AD-related ocular changes in both AH and VH levels of several biomarkers
[72][78][81][82]. For example, Prakasam and colleagues observed that soluble APP (sAPP) concentration, which is mainly secreted by the retina, was particularly high in VH in comparison with AH; likewise, Aβ40 and Aβ42 concentrations were 50% lower in AH than in VH
[82]. Goldstein and colleagues also described that Aβ40 and Aβ42 levels were detectable and measurable in AH, being comparable to those in CSF
[72]. In addition, Aβ40 and Aβ42 concentrations in VH were associated with lower cognitive function based on MMSE scores
[83]. Regarding t-tau, it was detected in VH and correlated with lower cognitive function, in agreement with findings from studies carried out in CSF
[83]. Finally, Aβ40, Aβ42, and t-tau in VH were positively correlated with levels of neurofilament light chain (NfL), a neurofilament subunit also identified in vitreous samples
[84]. NfL proteins are found in the cytoplasm of neurons and help to maintain structural stability, neuronal integrity, and impulse velocity. NfL is persistently released at low levels in normal circumstances, but higher levels of NfL were seen in both CSF and blood of AD patients
[85]. Although NfL is not currently used as a screening tool in clinical practice, it may be in the future.
4. Limitations
It is necessary to consider the significant limitations and controversial results that find in the majority of the current bibliography, preventing a feasible comparison between studies. For example, some ocular events related to AD are also shared by other ocular neurodegenerative pathologies, such as age-related macular degeneration (AMD) or glaucoma
[86]. In fact, some risk factors and pathophysiological mechanisms, as well as aging and histopathological changes, are common features in all of these conditions. For this reason, it would be mandatory to select precisely all the subjects undergoing these types of studies in order to minimize the impact of those diseases on the pathological ocular manifestations seen in the results. However, many studies do not consider some of these aspects, including significant differences in the age of participants between each condition; therefore, results are biased and not reliable for future clinical applications. Another important unsolved question is to fully understand and define the etiology of AD. Although MCI is considered the previous stage before AD development, it is a complex and heterogeneous group that comprises subjects who may not progress to AD but develop non-AD dementia or maintain MCI as part of the normal aging process. Moreover, many studies define the MCI group as cognitively normal (e.g., MMSE scores similar to control scores), whereas others include MCI subjects already showing impaired cognition (significantly lower MMSE score than controls). This corrupts the results, as these MCI cases with altered cognition already display early neuropathological changes of AD. Similarly, a standardized protocol must be established regarding the neuropsychological tools used to assess the grade of dementia in such studies. Each one study different scores and tests to define the level of dementia within groups (e.g., AD and MCI patients), which makes it difficult to compare results between studies. The well-defined guidelines from the National Institute of Aging and the Alzheimer’s Association could be established as the standard protocol to classify individuals across different studies.
Although the examination of different structures of the eye is an interesting field for the development of a non-invasive diagnostic tool, the available imaging tools should be improved for both the analysis of the anterior segment and the study of histologic changes in order to increase the accuracy of the results. Moreover, many measurements of the ocular vasculature may be affected by unrelated AD variables, such as heart rate, arterial pressure, and cardiac cycle. Unfortunately, data measurements from different imaging devices (e.g., two different types of OCT devices) cannot be fully compared, as their settings are not the same. All of this provokes the appearance of reproducibility issues, which limits the comparison of results from different labs and conclusions concerning clinical applications of the concepts.
Finally, protocols for the analysis of Aβ and tau retinal accumulation need major revision and standardization. The controversial results seen in human samples are partially due to a lack of a standard protocol for staining and tissue-mounting procedures. Thus, differences in antibodies and their dilutions, studied histological regions and their sectioning planes, and the postmortem interval influence the variability of results. Importantly, the current animal models available for AD do not recapitulate the full pathology seen in human patients. Furthermore, there is a variability in the strains of AD transgenic mice, even between populations from the same original strain, as well as different phenotypes depending on the sex of the animal (e.g., male 3xTg-AD mice develop a slower and milder phenotype than female counterparts). That is why evidence from animal models should be analyzed cautiously before drawing conclusions abouts its potential use in clinics.
5. Concluding Remarks and Future Directions
The main goal of this research was to highlight the role of ocular alterations and protein levels of ocular fluids as potential biomarkers or therapeutic targets in AD. It is compiled the most recent studies about eye and ocular alterations during AD progression. Optical examination in patients with neurodegenerative disorders is an emerging field that needs more investigation. Most of the current bibliography in this field is focused on the study of the posterior segment, which includes the retina and optic nerve. In contrast, the anterior structures have received less attention. This is surprising, given several findings suggest that the tear film, cornea, aqueous humor, and lens are also affected in AD and play a prominent role in disease progression. However, many mechanisms and events are not fully understood; therefore, more studies are needed to elucidate the underlying pathways.
Overall, despite controversy, ocular alterations associated with AD are undeniable at both structural and fluid levels. Currently, the lack of protocol standardization and uniform studies are preventing the clinical application of several interesting findings. However, AD-associated ocular changes have an enormous potential to become a non-invasive tool for early diagnosis of AD.

 Figure 1. Schematic representation summarizing the AD-related alterations reported so far and the different eye structures affected by such changes. Abbreviations: amyloid-β peptide (Aβ), eukaryotic initiation factor 4 e (eIF4E), neurofilament light chain (NfL), soluble amyloid precursor protein (sAPP).
Figure 1. Schematic representation summarizing the AD-related alterations reported so far and the different eye structures affected by such changes. Abbreviations: amyloid-β peptide (Aβ), eukaryotic initiation factor 4 e (eIF4E), neurofilament light chain (NfL), soluble amyloid precursor protein (sAPP). Figure 2. Schematic illustration of the different layers of the retina. From inner to outer: nerve fiber layer (containing axons from ganglion cells), ganglion cell layer (containing ganglion cells), inner plexiform layer (containing dendrites from ganglion, bipolar, and amacrine cells), inner nuclear layer (containing bipolar and amacrine cells), outer plexiform layer (containing extensions from horizontal cells and both rods and cones), outer nuclear layer (containing somas of both rods and cones), photoreceptor outer segment, pigment epithelium, Bruch’s membrane, and choroid. Figure created by BioRender.com software (Toronto, ON, Canada).
Figure 2. Schematic illustration of the different layers of the retina. From inner to outer: nerve fiber layer (containing axons from ganglion cells), ganglion cell layer (containing ganglion cells), inner plexiform layer (containing dendrites from ganglion, bipolar, and amacrine cells), inner nuclear layer (containing bipolar and amacrine cells), outer plexiform layer (containing extensions from horizontal cells and both rods and cones), outer nuclear layer (containing somas of both rods and cones), photoreceptor outer segment, pigment epithelium, Bruch’s membrane, and choroid. Figure created by BioRender.com software (Toronto, ON, Canada). Figure 3. Schematic representation of the compartments presented in the tear fluid. From outer to inner: lipid layer, aqueous layer, mucin layer (containing glycoproteins called mucins), and corneal epithelium. Figure created by BioRender.com software (Toronto, ON, Canada).
Figure 3. Schematic representation of the compartments presented in the tear fluid. From outer to inner: lipid layer, aqueous layer, mucin layer (containing glycoproteins called mucins), and corneal epithelium. Figure created by BioRender.com software (Toronto, ON, Canada). Figure 4. Schematic illustration of the corneal anatomy showing its different layers and structures. From outer to inner: epithelium, Bowmans’s membrane (containing collagen fibers), stroma (containing corneal innervation), Descemet’s membrane, and endothelium. Figure created by BioRender.com software (Toronto, ON, Canada).
Figure 4. Schematic illustration of the corneal anatomy showing its different layers and structures. From outer to inner: epithelium, Bowmans’s membrane (containing collagen fibers), stroma (containing corneal innervation), Descemet’s membrane, and endothelium. Figure created by BioRender.com software (Toronto, ON, Canada).



