
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Johanna Tonko | + 4452 word(s) | 4452 | 2021-09-11 16:01:14 | | | |
| 2 | Conner Chen | -224 word(s) | 4228 | 2022-03-09 03:11:00 | | |
Video Upload Options
Atrial fibrillation, commonly known as AFib or AF, is the most commonly treated type of arrhythmia. Arrhythmias are heartbeats that are slow, fast, or irregular.
1. Screening and Diagnosis
1.1. Novel Screening Tools and Their Integration in Clinical Practice
1.2. Management of Atrial High Rate Episodes (AHRE) and Subclinical Atrial Fibrillation in CIED
2. Structured Characterization of Atrial Fibrillation
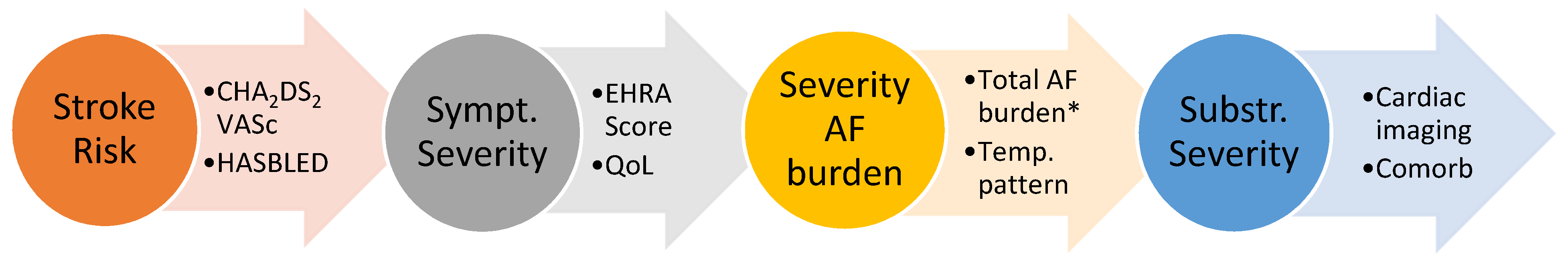
- Stroke Risk: As in previous guidelines, the recommended assessment tool for stroke risk estimation remains the well-known CHA2DS2-VASc Score. The ESC taskforce has refined some of the risk factors by adding precise blood pressure and blood glucose cut-offs as well as including hypertrophic cardiomyopathy, heart failure with preserved ejection fraction and asymptomatic moderate to severe LV dysfunction into the score. Opposed to the original score, angiographically documented significant coronary artery disease (regardless of symptom status) is now included as well. The temporal pattern and total burden of AF are not part of the stroke risk assessment in the current guidelines.
- Symptom Severity: The severity of symptoms should be evaluated in a standardized manner with the EHRA symptom score ranging from 1 to 4 or via quality-of-life questionnaires. Importantly, a symptom-rhythm correlation should be established to differentiate from symptoms due to underlying co-morbidities.
- Severity of AF burden: Assessing the AF burden includes not only the traditionally used classification of the temporal pattern into paroxysmal, persistent and permanent AF but also the total AF burden defined as the percentage of time in AF for a defined time frame. Higher AF burden have been associated with higher stroke risk [17] and mortality rates (if >6–24 h of AF per week) [18], poorer response to rhythm control therapy [19] and may represent progression of advanced atrial remodeling [20]. However, it remains unclear whether progressive AF burden is primarily a marker or a driver or both of progression of the underlying disease and adverse prognosis.
- Substrate Severity: A growing body of evidence showed that the severity and extent of left atrial structural and electrical remodeling has prognostic value for patients with AF. Technological improvements in non-invasive imaging modalities (echocardiography with TDI and strain, cardiac MRI with Late-Gadolinium Enhancement (LGE), cardiac CT) as well as invasive high density electro-anatomical contact mapping has allowed for more detailed assessment of the underlying substrate of AF. Nowadays, it is commonly acknowledged that left atrial size alone is not able to accurately define the disease state. Atrial wall fibrosis [21] and wall thickness [22], epicardial fat infiltration [23], atrial conduction velocities [24] or geometrical assessments such as sphericity [25] are a number of further parameters that have shown prognostic value and may guide treatment decisions, though most are not yet routinely assessed in daily practice.
3. Treatment: The ABC Pathway
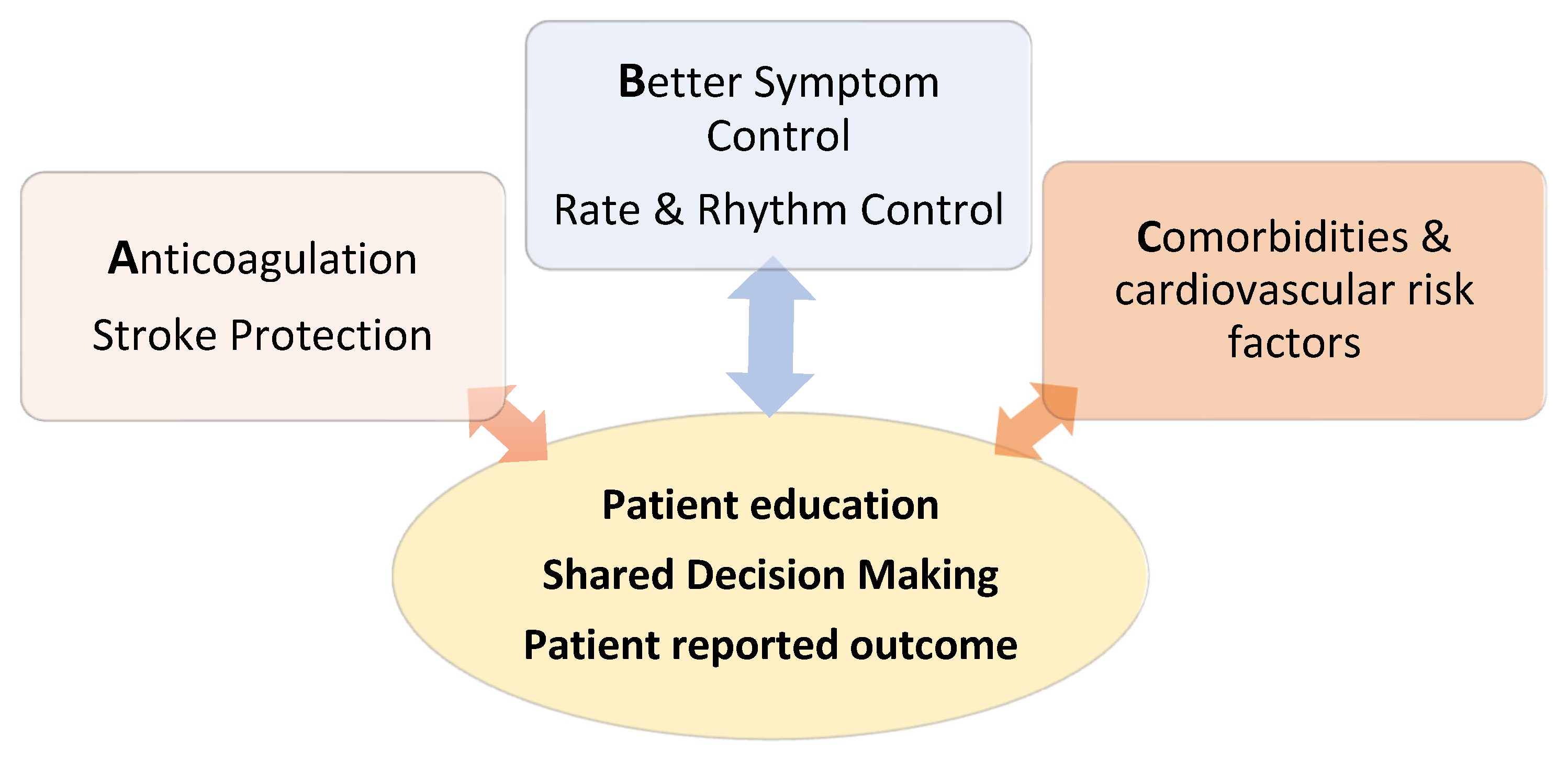
3.1. Anticoagulation
3.2. Better Symptom Control
| Rate Control | Medical Rhythm Control + | |||
|---|---|---|---|---|
| No significant SHD | CAD, VAD, HFpEF | HFrEF | ||
| 1. Line | Betablocker NDCC * (IB) |
Flecainide Propafenone Dronedarone (IA) |
Dronedarone Amiodarone (IA) |
Amiodarone (IA) |
| 2. Line | Digoxin (IB) Combinations (IIa) |
Sotalol (IIbA) |
Sotalol (IIbA) | - |
| 3. Line | Pace&Ablate (IIaB) Amiodarone (IIbB) |
|||
3.2.1. Rate Control
3.2.2. Rhythm Control
3.2.2.1. New Evidence for the Prognostic Benefit of Rhythm Control
3.2.2.2. Optimal Timing of Rhythm Control
3.2.2.3. Choice of Rhythm Control Modalities
3.2.2.4. Focus on AF and Heart Failure
3.2.2.5. Defining, Measuring and Predicting Rhythm Control Success
3.3. Cardiovascular Risk Factors and Comorbidities
The third pillar of the ABC pathway relates to systematic assessment, identification and aggressive treatment of all cardiovascular risk factors and comorbidities associated with AF. Isolated management of specific conditions alone is often insufficient, as commonly, they are not the sole contribute to AF. In addition to encouraging intense lifestyle modifications to achieve normal body weight, reduce alcohol intake and increase physical activity, a particular focus is on optimal control of hypertension, sleep apnea treatment as well as addressing other well-known cardiovascular risk factors, including diabetes mellitus, hyperlipidemia and smoking. All of these have been identified to have a negative impact on the clinical course of AF and contribute to negative atrial remodeling. The guidelines reinforce the importance of patient education and involvement in order to achieve optimal outcomes.
References
- Lowres, N.; Neubeck, L.; Salkeld, G.; Krass, I.; McLachlan, A.J.; Redfern, J.; Bennett, A.A.; Briffa, T.; Bauman, A.; Martinez, C.; et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. Thromb. Haemost. 2014, 111, 1167–1176.
- Jacobs, M.S.; Kaasenbrood, F.; Postma, M.J.; Van Hulst, M.; Tieleman, R.G. Cost-effectiveness of screening for atrial fibrillation in primary care with a handheld, single-lead electrocardiogram device in the Netherlands. Eurospace 2016, 20, 12–18.
- Aronsson, M.; Svennberg, E.; Rosenqvist, M.; Engdahl, J.; Al-Khalili, F.; Friberg, L.; Frykman-Kull, V.; Levin, L.-. Åke Cost-effectiveness of mass screening for untreated atrial fibrillation using intermittent ECG recording. Eurospace 2015, 17, 1023–1029.
- Svennberg, E.; Engdahl, J.; Al-Khalili, F.; Friberg, L.; Frykman, V.; Rosenqvist, M. Mass screening for untreated atrial fibril-lation: The STROKESTOP study. Circulation 2015, 131, 2176–2184.
- Svennberg, E. The STROKESTOP-STUDY Benefits of Systematic Screening for Atrial fibrillation. In Proceedings of the EHRA Congress 2021, Online, 23–25 April 2021; Available online: https://digital-congress.escardio.org/EHRA-Congress/sessions/511-late-breaking-clinical-trials (accessed on 18 May 2021).
- A Study to Determine If Identification of Undiagnosed Atrial Fibrillation in People at Least 70 Years of Age Reduces the Risk of Stroke (GUARD-AF). Identifier: NCT04126486. Available online: ClinicalTrials.gov (accessed on 18 May 2021).
- Mant, J.; Burt, J.; Griffin, S.; Hobbs, R.; McManus, R.; Williams, K.; Dymond, A.; Lund, J.; Edwards, D.; Massou, L.; et al. Abstract D.11. The SAFER study—Screening for Atrial Fibrillation with ECG to Reduce stroke. In Proceedings of the Society for Academic Primary Care Conference, University of Leeds, Leeds, UK, 15–17 July 2020; p. 49.
- Diederichsen, S.Z.; Haugan, K.J.; Køber, L.; Højberg, S.; Brandes, A.; Kronborg, C.; Graff, C.; Holst, A.G.; Nielsen, J.B.; Krieger, D.; et al. Atrial fibrillation detected by continuous electrocardiographic monitoring using implantable loop recorder to prevent stroke in individuals at risk (the LOOP study): Rationale and design of a large randomized controlled trial. Am. Heart J. 2017, 187, 122–132.
- Li, K.H.C.; White, F.A.; Tipoe, T.; Liu, T.; Wong, K.C.; Jesuthasan, A.; Baranchuk, A.; Tse, G.; Yan, B.P. The Current State of Mobile Phone Apps for Monitoring Heart Rate, Heart Rate Variability, and Atrial Fibrillation: Narrative Review. JMIR mHealth uHealth 2019, 7, e11606.
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917.
- William, A.D.; Kanbour, M.; Callahan, T.; Bhargava, M.; Varma, N.; Rickard, J.; Saliba, W.; Wolski, K.; Hussein, A.; Lindsay, B.D.; et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: The iREAD Study. Heart Rhythm 2018, 15, 1561–1565.
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. Anartificial intelligence-enabled ECG algorithm for the identification of patients with atrial fi-brillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867.
- Healey, J.; Connolly, S.; Alings, M.; Lopes, R. Apixaban for the Reduction of Thrombo-Embolism in Patients with Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA). Available online: https://clinicaltrials.gov/ct2/show/NCT01938248 (accessed on 17 August 2021).
- Kirchhof, P.; Blank, B. Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial High Rate Episodes (NOAH). Available online: https://clinicaltrials.gov/ct2/show/NCT02618577 (accessed on 17 August 2021).
- Gorenek, B.C.; Bax, J.; Boriani, G.; Chen, S.-A.; Dagres, N.; Glotzer, T.V.; Healey, J.S.; Israel, C.W.; Kudaiberdieva, G.; Levin, L.-A.; et al. ESC Scientific Document Group. Device- detected subclinical atrial tachyarrhythmias: Definition, implications and management—An European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisio-logia (SOLEACE). Europace 2017, 19, 1556–1578.
- Van Gelder, I.C.; Healey, J.S.; Crijns, H.J.; Wang, J.; Hohnloser, H.; Gold, M.R.; Capucci, A.; Lau, C.P.; Morillo, C.A.; Hobbelt, A.H.; et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in AS-SERT. Eur. Heart J. 2017, 38, 1339–1344.
- Ganesan, A.; Chew, D.; Hartshorne, T.; Selvanayagam, J.B.; Aylward, P.; Sanders, P.; McGavigan, A.D. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: A systematic review and meta-analysis. Eur. Heart J. 2016, 37, 1591–1602.
- Piccini, J.P.; Passman, R.; Turakhia, M.; Connolly, A.T.; Nabutovsky, Y.; Varma, N. Atrial fibrillation burden, progression, and the risk of death: A case-crossover analysis in patients with cardiac implantable electronic devices. Eurospace 2018, 21, 404–413.
- Ecker, V.; Knoery, C.; Rushworth, G.; Rudd, I.; Ortner, A.; Begley, D.; Leslie, S.J. A review of factors associated with maintenance of sinus rhythm after elective electrical cardioversion for atrial fibrillation. Clin. Cardiol. 2018, 41, 862–870.
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490.
- Marrouche, N.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibril-lation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506.
- Whitaker, J.; Rajani, R.; Chubb, H.; Gabrawi, M.; Varela, M.; Wright, M.; Niederer, S.; O’Neill, M. The role of myocardial wall thickness in atrial arrhythmogenesis. Eurospace 2016, 18, 1758–1772.
- Wong, C.; Ganesan, A.; Selvanayagam, J.B. Epicardial fat and atrial fibrillation: Current evidence, potential mechanisms, clinical implications, and future directions. Eur. Heart J. 2016, 38, 1294–1302.
- Fukumoto, K.; Habibi, M.; Ipek, E.; Zahid, S.; Khurram, I.M.; Zimmerman, S.L.; Zipunnikov, V.; Spragg, D.; Ashikaga, H.; Trayanova, N.; et al. Association of Left atrial Local Conduction Velocity with Late Gadolinium En-hancement on Car-diac Magnetic Resonance in Patients with Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e002897.
- Moon, J.; Lee, M.-H.; Yu, J.; Pak, H.-N.; Ha, J.-W.; Lee, M.; Kim, Y.J.; Joung, B. Prognostic implication of left atrial sphericity in atrial fibrillation patients undergoing radiofrequency catheter ablation. Pacing Clin. Electrophysiol. 2017, 40, 713–720.
- Hart, R.G.; Pearce, L.A.; Aguilar, M.I. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have non-valvu-lar atrial fibrillation. Ann. Intern. Med. 2007, 146, 857–867.
- Borre, E.; Goode, A.; Raitz, G.; Shah, B.; Lowenstern, A.; Chatterjee, R.; Sharan, L.; Lapointe, N.M.A.; Yapa, R.; Davis, J.K.; et al. Predicting Thromboembolic and Bleeding Event Risk in Patients with Non-Valvular Atrial Fibrillation: A Systematic Review. Thromb. Haemost. 2018, 118, 2171–2187.
- Ruff, C.T.; Giugliano, R.P.; Braunwald, E.; Hoffman, E.B.; Deenadayalu, N.; Ezekowitz, M.D.; Camm, A.J.; Weitz, J.I.; Lewis, B.S.; Parkhomenko, A.; et al. Comparison of the efficacy and safety of new oral anticoagulatns with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet 2014, 383, 955–962.
- Boersma, L.V.; Bosschaert, M. Left atrial appendage occlusion for AF patients unable to Use oral Anticoagulation Therapy (COMPARE-LAAO). ClinicalTrials.gov Identifier: NCT04676880. Available online: https://clinicaltrials.gov/ct2/show/NCT04676880 (accessed on 23 May 2021).
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. N. Engl. J. Med. 2021, 384, 2081–2091.
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524.
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I.; et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343.
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; Van Eickels, M.; et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434.
- Lopes, R.D.; Hong, H.; Harskamp, R.E.; Bhatt, D.L.; Mehran, R.; Cannon, C.P.; Granger, C.B.; Verheugt, F.W.A.; Li, J.; Ten Berg, J.M.; et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: A network meta-analysis of randomized controlled trials. JAMA Cardiol. 2019, 4, 747–755.
- Fiedler, K.A.; Maeng, M.; Mehilli, J.; Schulz-Schüpke, S.; Byrne, R.A.; Sibbing, D.; Hoppmann, P.; Schneider, S.; Fusaro, M.; Ott, I.; et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implan-tation: The ISAR-TRIPLE trial. J. Am. Coll. Cardiol. 2015, 65, 1619–1629.
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.-P.; Adriaenssens, T.; Vrolix, M.; Heestermans, A.A.; Vis, M.M.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet 2013, 381, 1107–1115.
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524.
- Hunter, R.J.; Berriman, T.J.; Diab, I.; Kamdar, R.; Richmond, L.; Baker, V.; Goromonzi, F.; Sawhney, V.; Duncan, E.; Page, S.P.; et al. A Randomized Controlled Trial of Catheter Ablation Versus Medical Treatment of Atrial Fibrillation in Heart Failure (The CAMTAF Trial). Circ. Arrhythm. Electrophysiol. 2014, 7, 31–38.
- Khan, M.N.; Jaïs, P.; Cummings, J.; Di Biase, L.; Sanders, P.; Martin, D.O.; Kautzner, J.; Hao, S.; Themistoclakis, S.; Fanelli, R.; et al. Pulmonary-Vein Isolation for Atrial Fibrillation in Patients with Heart Failure. N. Engl. J. Med. 2008, 359, 1778–1785.
- Prabhu, S.; Taylor, A.J.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.A.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961.
- Jones, D.G.; Haldar, S.K.; Hussain, W.; Sharma, R.; Francis, D.P.; Rahman-Haley, S.L.; McDonagh, T.A.; Underwood, S.R.; Markides, V.; Wong, T. A randomized trial to assess catheter ablation versus rate control in the man-agement of persistent atrial fibrillation in heart failure. J. Am. Coll. Cardiol. 2013, 61, 1894–1903.
- Muhammad, Z.K.; Safi, K.; Adeel, A.; Muhammad, S.Z.; Muhammad, U.K.; Muhammad, S.K.; Edo, K.; Mohamad, A. Meta-Analysis of Catheter Ablation versus Medical Therapy in Patients with AF without Heart Failure. J. Atr. Fibrillation 2020, 12, 2266.
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316.
- Turakhia, M.P.; Santangeli, P.; Winkelmayer, W.C.; Xu, X.; Ullal, A.J.; Than, C.T.; Schmitt, S.; Holmes, T.H.; Frayne, S.M.; Phibbs, C.S.; et al. Increased mortality associated with Digoxin in contemporary pa-tients with atrial fibrillation: Findings from the TREAT-AF Study. J. Am. Coll. Cardiol. 2014, 64, 660–668.
- Whitbeck, M.G.; Charnigo, R.J.; Khairy, P.; Ziada, K.; Bailey, A.L.; Zegarra, M.M.; Shah, J.; Morales, G.; Macaulay, T.; Sorrell, V.L.; et al. Increased mortality among patients taking digoxin—Analysis from the AFFIRM study. Eur. Heart J. 2012, 34, 1481–1488.
- Gheorghiade, M.; Fonarow, G.; Van Veldhuisen, D.J.; Cleland, J.G.; Butler, J.; Epstein, A.E.; Patel, K.; Aban, I.B.; Aronow, W.S.; Anker, S.D.; et al. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: Findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur. Heart J. 2013, 34, 1489–1497.
- Kotecha, D.; Holmes, J.; Krum, H.; Altman, D.G.; Manzano, L.; Cleland, J.G.F.; Lip, G.Y.H.; Coats, A.J.S.; Andersson, B.; Kirchhof, P.; et al. Efficacy of beta blockers in patients with heart failure plus atrial fi-brillation: An indi-vidual-patient data meta-analysis. Lancet 2014, 384, 2235–2243.
- Bavendiek, U.; Berliner, D.; Davila, L.A.; Schwab, J.; Maier, L.; Philipp, S.; Rieth, A.J.; Westenfeld, R.; Pirkowski, C..; Weber, K.; et al. DIGIT-HF Investigators and Committees. Rationale and design of the DIGIT-HF trial (DIGi-toxin to Improve ouTcomes in patients with advanced chronic Heart Failure): A randomized, double-blind, pla-cebo-controlled study. Eur J. Heart Fail. 2019, 21, 676–684.
- Willems, S.; Meyer, C.; De Bono, J.; Brandes, A.; Eckardt, L.; Elvan, A.; Van Gelder, I.; Goette, A.; Gulizia, M.; Haegeli, L.; et al. Cabins, castles, and constant hearts: Rhythm control therapy in patients with atrial fibrillation. Eur. Heart J. 2019, 40, 3793–3799.
- Marijon, E.; Le Heuzey, J.-Y.; Connolly, S.; Yang, S.; Pogue, J.; Brueckmann, M.; Eikelboom, J.; Themeles, E.; Ezekowitz, M.; Wallentin, L.; et al. Response to Letter Regarding Article, “Causes of Death and Influencing Factors in Patients With Atrial Fibrillation: A Competing-Risk Analysis From the Randomized Evaluation of Long-Term Anticoagulant Therapy Study”. Circulation. 2014, 130, e85.
- Kim, D.; Yang, P.S.; You, S.C.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.H.; et al. Treatment timing and the effect of rhythm control strategy in patients with atrial fibril-lation: Nationwide cohort study. BMJ 2021, 373, n991.
- Kim, D.; Yang, P.-S.; Sung, J.-H.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Pak, H.-N.; Lee, M.-H.; et al. Less dementia after catheter ablation for atrial fibrillation: A nationwide cohort study. Eur. Heart J. 2020, 41, 4483–4493.
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427.
- Abushouk, A.I.; Ali, A.A.; Mohamed, A.A.; El-Sherif, L.; Abdelsamed, M.-A.; Mohamed, M.K.; Sayed, M.K.; Mohamed, N.A.; Osman, A.A.; Shaheen, S.M.; et al. Rhythm Versus Rate Control for Atrial Fibrillation: A Meta-analysis of Randomized Controlled Trials. Biomed. Pharmacol. J. 2018, 11, 609–620.
- Roy, D.; Talajic, M.; Nattel, S.; Wyse, D.G.; Dorian, P.; Lee, K.L.; Bourassa, M.G.; Arnold, J.M.O.; Buxton, A.E.; Camm, A.J.; et al. Rhythm Control versus Rate Control for Atrial Fibrillation and Heart Failure. N. Engl. J. Med. 2008, 358, 2667–2677.
- Corley, S.; Epstein, A.; DiMarco, J.P.; Domanski, M.J.; Geller, N.; Greene, H.L.; Josephson, R.A.; Kellen, J.C.; Klein, R.C.; Krahn, A.D.; et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004, 109, 1509–1513.
- Packer, D.L.; Mark, D.B.; Robb, R.A.; Monahan, K.H.; Bahnson, T.D.; Poole, J.E.; Noseworthy, P.A.; Rosenberg, Y.D.; Jeffries, N.; Mitchell, L.B.; et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation. JAMA 2019, 321, 1261–1274.
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennet, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or drug therapy for initial treatment of AF. N. Engl. J. Med. 2021, 384, 305–315.
- Wazni, O.M.; Dandamudi, G.; Sood, N.; Hoyt, R.; Tyler, J.; Durrani, S.; Biebauer, M.; Makati, K.; Halperon, B.; Gauri, A.; et al. Cryoballoon Ablation as initial Theapy for atrial Fibrillation. N. Engl. J. Med. 2021, 384, 316–324.
- Velagic, V.; Pavlovic, N.; Chierchia, G.-B.; Hermida, J.-S.; Healey, S.; Arena, G.; Badenco, N.; Meyer, C.; Chen, J.; Iacopino, S.; et al. Abstract 13915: Cryoballoon Catheter Ablation versus Antiarrhythmic Drug as a First-Line Therapy for Patients With Paroxysmal Atrial Fibrillation: Results of the Cryo-FIRST Study. Circle 2020, 142, 13915.
- Chew, D.S.; Black-Maier, E.; Loring, Z.; Noseworthy, P.A.; Packer, D.L.; Exner, D.V.; Mark, D.B.; Piccini, J.P. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: A systematic review and meta-analysis of observational studies. Circ. Arrhythm. Electrophysiol. 2020, 13, e008128.
- Pallisgaard, J.L.; Gislason, G.; Hansen, J.; Johannessen, A.; Torp-Pedersen, C.; Rasmussen, P.V.; Hansen, M.L. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: A nationwide Danish cohort study. Eur. Heart J. 2018, 39, 442–449.
- Walters, T.E.; Nisbet, A.; Morris, G.M.; Tan, G.; Mearns, M.; Teo, E.; Lewis, N.; Ng, A.; Gould, P.; Lee, G.; et al. Progression of atrial remodeling in patients with high-burden atrial fibrillation: Implications for early ablative therapy. Heart Rhythm 2016, 13, 331.
- Pluymaekers, N.; Dudink, E.; Luermans, J.; Meeder, J.G.; Lenderink, T.; Widdershoven, J.; Bucx, J.J.J.; Rienstra, M.; Kamp, O.; Opstal, J.M.V.; et al. RACE ACWAS Investigators. Early or delayed cardioversion in recent-onset atrial fibrilla-tion. N. Engl. J. Med. 2019, 380, 1499–1508.
- Jaïs, P.; Cauchemez, B.; Macle, L.; Daoud, E.; Khairy, P.; Subbiah, R.; Hocini, M.; Extramiana, F.; Sacher, F.; Bordachar, P.; et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: The A4 study. Circulation 2008, 118, 2498–2505.
- Pappone, C.; Augello, G.; Sala, S.; Gugliotta, F.; Vicedomini, G.; Gulleta, S.; Paglino, G.; Mazzone, P.; Sora, N.; Greiss, I.; et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: The APAF Study. J. Am. Coll. Cardiol. 2006, 48, 2340–2347.
- Packer, D.L.; Kowal, R.C.; Wheelan, K.R.; Irwin, J.M.; Champagne, J.; Guerra, P.G.; Dubuc, M.; Reddy, V.; Nelson, L.; Holcomb, R.G.; et al. STOP AF Cryoablation Investigators. Cryoballoon ablation of pulmonary veins for parox-ysmal atrial fibrillation: First results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013, 61, 1713–1723.
- Wilber, D.J.; Pappone, C.; Neuzil, P.; Paola, A.D.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. ThermoCool AF Trial Investigators. Comparison of antiarrhythmic drug therapy and radiofre-quency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA 2010, 303, 333–340.
- Mont, L.; Bisbal, F.; Hernandez-Madrid, A.; Pérez-Castellano, N.; Viñolas, V.; Arenal, A.; Arribas, F.; Fernández-Lozano, I.; Bodegas, A.; Cobos, A.; et al. SARA investigators. Catheter ablation vs antiarrhythmic drug treatment of persistent atrial fibrillation: A multicentre, randomized, controlled trial (SARA study). Eur. Heart J. 2014, 35, 505–507.
- Delurgio, D.B.; Crossen, K.J.; Gill, J.; Blauth, C.; Oza, S.R.; Magnano, A.R.; Mostovych, M.A.; Halkos, M.E.; Tschopp, D.R.; Kerendi, F.; et al. Hybrid Convergent Procedure for the Treatment of Persistent and Long-Standing Persistent Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2020, 13, 009288.
- Chen, M.S.; Marrouche, N.F.; Khaykin, Y.; Gillinov, A.M.; Wazni, O.; Martin, D.O.; Rossillo, A.; Verma, A.; Cummings, J.; Ercyyes, D.; et al. Pulmonary vein isolation for the treatment of atrial fibrillation in patients with impaired sys-tolic function. J. Am. Coll. Cardiol. 2004, 43, 1004–1009.
- Di Biase, L.; Mohanty, P.; Mohanty, S.; Santangeli, P.; Lakkireddy, D.; Reddy, M.; Jais, P.; Themistocklais, S.; Russo, A.D. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with conges-tive heart failure and an implanted device: Results from the AATAC multicenter randomized trial. Circulation 2016, 133, 1637–1644.
- Packer, D.L.; Piccini, J.P.; Monahan, K.H.; Al-Khalidi, H.R.; Silverstein, A.P.; Noseworthy, P.A.; Poole, J.E.; Bahnson, T.D.; Lee, K.L.; Mark, D.B.; et al. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure. Circulation 2021, 143, 1377–1390.
- Kuck, K.-H.; Merkley, B.; Zahn, R.; Arentz, T.; Seidl, K.; Schlüter, M.; Tilz, R.R.; Piorkowski, C.; Gellér, L.; Kleemann, T.; et al. Catheter Ablation versus best medical therapy in Patients with Persistent Atrial Fibrillation and Congestive Heart Failure: The randomized AMICA trial. Circ. Arrhythm. Electrophysiol. 2019, 12, e007731.
- Cochrane Central Register of Controlled Trials. CAMERA-MRI II trial: Catheter Ablation versus Medical Rate Control of Atrial Fibrillation with Systolic Heart Failure and Myocardial Fibrosis—An MRI Guided Multi-Centre Randomised Controlled Clinical Trial. CN-02165295. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-02165295/full (accessed on 20 May 2021).
- Black-Maier, E.; Ren, X.; Steinberg, B.A.; Green, C.L.; Barnett, A.S.; Rosa, N.S.; Al-Khatib, S.M.; Atwater, B.D.; Daubert, J.P.; Frazier-Mills, C.; et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018, 15, 651–657.
- Kelly, J.P.; DeVore, A.D.; Wu, J.J.; Hammill, B.G.; Sharma, A.; Cooper, L.B.; Felker, G.M.; Piccini, J.P.; Allen, L.A.; Heidenreich, P.A.; et al. Rhythm Control Versus Rate Control in Patients with Atrial Fibrillation and Heart Failure with Pre-served Ejection Fraction: Insights from Get with The Guidelines—Heart Failure. JAHA 2019, 8, e011560.
- Terricabras, M.; Verma, A.; Morillo, C.A. Measuring Success in Ablation of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2018, 11, e006582.
- Camm, J.; Reiffel, J. Defining endpoints in clinical trials on atrial fibrillation. Eur. Heart J. Suppl. 2008, 10, H55–H78.
- Jackson, N.; Mahmoodi, E.; Leitch, J.; Varlow, M.; Davies, A.; Collins, N.; Leigh, L.; Pldmeadow, C.; Boyle, A. Effect of Outcome Measures on the Apparent Efficacy of Ablation for Atrial fi-brillation: Why “Success” is an Inappropriate Term. Heart Lung Circ. 2021, 30, 1166–1173.
- Dretzke, J.; Chuchu, N.; Agarwal, R.; Herd, C.; Chua, W.; Fabritz, L.; Bayliss, S.; Kotecha, D.; Deeks, J.; Kirchhof, P.; et al. Predicting recurrent atrial fibrillation after catheter ablation: A systematic review of prognostic models. Eurpace 2020, 22, 748–760.




