Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anastasia Prodromidou | + 2467 word(s) | 2467 | 2022-02-28 10:49:57 | | | |
| 2 | Rita Xu | + 182 word(s) | 2649 | 2022-03-08 02:57:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Prodromidou, A. Tubal Endometriosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/20303 (accessed on 07 February 2026).
Prodromidou A. Tubal Endometriosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/20303. Accessed February 07, 2026.
Prodromidou, Anastasia. "Tubal Endometriosis" Encyclopedia, https://encyclopedia.pub/entry/20303 (accessed February 07, 2026).
Prodromidou, A. (2022, March 07). Tubal Endometriosis. In Encyclopedia. https://encyclopedia.pub/entry/20303
Prodromidou, Anastasia. "Tubal Endometriosis." Encyclopedia. Web. 07 March, 2022.
Copy Citation
Tubal endometriosis (EM) refers to the detection of ectopic endometrial implants on tubes. It may cause a significant defect of the tubes, translating into dysmenorrhea, pelvic pain, and infertility.
tubal endometriosis
fallopian tube
endometriosis
1. Introduction
Endometriosis (EM) is a chronic benign gynecological disease, which is defined as the presence of endometrial deposits outside the uterine cavity [1]. The estimated prevalence of the disease is approximately 10% among women of reproductive age [2]. Endometriosis is most commonly identified in the pelvis and it affects the ovaries, the pelvic peritoneum cul-de-sac and uterosacral ligaments [3]. Additionally, less common extrapelvic endometriosis sites in the gastrointestinal and urinary tract, chest and brain have also been recorded, while there are also reports of multiple endometriosis sites especially in patients with deep infiltrating (DIE) endometriosis in as high as 44% of them [3][4]. Pain and infertility are the most common primary symptoms encountered in 30–50% of women with EM [5]. Retrograde menstruation, firstly described by Sampson et al., has been considered as the most prevalent theory for the pathogenesis of EM [2]. Women with obstructive outflow diseases are considered more susceptible to retrograde menstrual flow, which could facilitate the transportation of endometriotic menstrual cells to the peritoneal cavity through the fallopian tubes [2]. The genetic and epigenetic theory, according to which already existing endometrial cells are modified and result in the development of the clinical manifestation of the disease, could explain why not all women with retrograde menstruation will develop EM [6]. Coelomic metaplasia is another theory that supports the transformation of peritoneal, pleural and ovarian mesothelial cells to endometriosis, while theories about the lymphatic and vascular spread of endometrial cells are still under investigation [7]. Treatment options may range from conservative medication hormonal-based treatment to more invasive surgical procedures. Despite the benign nature of the disease, the risk of malignant transformation reaches a proportion of approximately 1% [8]. History of EM is related to a significantly elevated risk of developing ovarian cancer [8]. The most common histological subtypes arising from EM are endometrioid adenocarcinoma, clear cell carcinoma and low-grade serous carcinoma [8][9]. Histopathologically, the malignant transformation is recognized as cytologic atypia and architectural proliferation [10]. Cytologic atypia is defined as the transition from benign EM to carcinoma and is classified as moderate (simple hyperplasia or cellular atypia) or severe (complex hyperplasia or cellular atypia that is more evident) [11]. Concerning cellular proliferation, complex hyperplasia is translated into glandular proliferation and reduced stroma, which can evolve towards ovarian cancer [11]. As mentioned above, endometrioid carcinoma or clear cell carcinoma are the most prevalent types, and their gross appearance consists of the typical histology of each type of malignancy including cribiform, glandular or solid architecture and papillary, solid or tubulocystic architecture for each cancer type, respectively [11]. Mitoses are also detected in both types. Endometriosis associated ovarian cancer (EAOC) is defined as the coexistence of malignant cells and EM either in the same ovary or EM in the one and cancer in the other ovary [10][11].
Tubal EM is defined as the detection of ectopic endometrial implants on the tubes. It may cause a significant defect of the fallopian tubes and functional and structural disorders, which may translate into dysmenorrhea, pelvic pain and infertility. Notwithstanding the multiple reports on the potential contribution of tubal EM on the pathogenesis of endometriosis related symptomatology, the exact aspects of the disease still remain elusive.
2. Excluded Studies
A total of 3 studies were excluded from tabulation and analysis after reading their full text. More specifically, the study by Chakrabarti et al. was excluded as reported a case of EM that was developed in the fallopian stump four years after salpingectomy [12]. The studies by Sinha et al. and Audebert et al. did not present separate outcomes of patients with tubal EM apart from the prevalence of the disease among their study populations and were thus excluded [13][14].
3. Included Studies
A total of 13 studies were finally considered eligible for inclusion [15][16][17][18][19][20][21][22][23][24][25][26][27]. Among them, four were observational, which included a total of 633 patients and mainly focused on the prevalence of tubal EM among patients with various gynecological diseases, as well as on disease-related characteristics and histopathology [15][16][17][18], while two studies focused on analyzing the genetic profile of patients with tubal EM [19][20]. The remaining seven studies were case reports [21][22][23][24][25][26][27]. The main patient and disease characteristics of the included observational studies are shown in Table 1. A summary of the findings of the case reports is also depicted in Table 2.
Table 1. Characteristics of the included observational studies and patients.
| Year; Author | 2018; Xia | 2019; Qi | 2020; Xue | 2020; Mcguinness |
|---|---|---|---|---|
| Country | China | China | China | USA |
| Type of study | PS | Cross-sectional | RS | RS |
| Study period | 06/2016–08/2017 | 06/2016–08/2017 | 01/2002–07/2019 | 07/2015–06/2018 |
| Inclusion criteria | Patients with uterine leiomyoma and adenomyosis treated with hysterectomy and salpingectomy; no hormonal medication within 3 mo; no history of tubal surgery | Premenopausal; unilateral or bilateral salpingectomy; complete data; no pregnancy; consent for participation | Salpingectomy | Surgery for EM by MIS; age < 55; no malignant cases; no previous laparotomy; no previous bil salpingectomy |
| Main outcomes | Ciliary beat frequency (CBF) | Characteristics, prevalence, clinical features, pathologic features, predictors of EM | Prevalence of tubal EM among groups | Prevalence of tubal EM among groups |
| Compared groups | AM without EM vs. EM without AM vs. control (uterine leiomyoma) | EM vs. no EM | EM vs. BN vs. MT | Salpingectomy vs. no salpingectomy |
| Indication for surgery | Leiomyoma, AM, EM | Fibroid, ovarian cyst, salpingitis/infertility, hydrosalpinx, malignancy, tubal sterilization, adenomyosis, EM | Leiomyoma, adenomyosis, endometrioid cysts, hydrosalpinx, uterine malformation, malignancy | EM, pelvic pain, cystic adnexal mass, infertility, fibroids, AUB |
| Patients (n) | 75 (20 vs. 35 vs. 20) | 1112 (161 vs. 951) | 261 (178 vs. 65 vs. 18) | 185 (97 vs. 88) |
| Patients age (years) | 44.4 ± 5.2 a vs. 43.4 ± 5.1 a vs. 47.2 ± 4.8 a (AM vs. EM vs. control) |
44.89 ± 6 a vs. 45.9 ± 5.97 a, p = 0.002 (tubal EM vs. no EM) | 44 ± 7 a (total) | 41.26 ± 7.45 a vs. 34.24 ± 7.37 a (salpingectomy vs. no salpingectomy) |
| Other EM sites | N/A | Ovarian EM L: 70/R: 53/Bil: 34 |
Ovarian EM L: 70/R: 49 |
N/A |
| Site of EM (L/R/Bil) | N/A | 84 (40.37%)/65 (52.17%)/12 (7.45%), p < 0.005 (for L/R) | 168 (55.08%)/93 (30.49%)/44 (14.43%), p < 0.001 (for L/R) | N/A |
| Prevalence of tubal EM | 24/35 (69%) for EM group | 161/1112 (14.48%) | EM group: 178 (68.2%) BN group: 65 (24.9%) MT group: 18 (6.9%) |
34/97 (35%) salpingectomy group vs. 8/88 (9%) no salpingectomy group |
| Location in tube (tubal site/histologic layer) | N/A | Proximal: 78 (48.45%) Distal: 78 (48.45%) Proximal + distal: 5 (3.1%)/ Mucosa: 88 (54.66%) Myosalpinx: 10 (6.21%) Serosa: 52 (32.3%) Mucosa + serosa: 11 (6.83%) |
N/A | N/A |
| Predisposing factors | N/A | Previous EM, multi-organ EM, uterine seromuscular EM, severity of pelvic EM, young age, AUB, previous tubal ligation | N/A | N/A |
RS: retrospective, EM: endometriosis, AM: adenomyosis, BN: benign disease, MT: malignant disease, MIS: minimally invasive surgery, AUB: abnormal uterine bleeding, PID: pelvic inflammatory disease, IUD: intrauterine device, L: left, R: right, Bil: bilateral, a Mean ± SD, N/A: not available.
Table 2. Main characteristics of patients from case reports.
| Year; Author | Age (Years) | Primary Symptom | Parity | Imaging Findings | Pre-Surgical Diagnosis (Indication for Surgery)/Operative Procedure-Findings | Menopausal Status | History of EM/IO EM Findings | Histological Findings | Side/Site of Tubal EM |
|---|---|---|---|---|---|---|---|---|---|
| 2013; Wenger | 18 | Acute pelvic pain, oligomenorrhea, persistent dysmenorrhea and dyspareunia | Nulli | TVUS: hypoechoic structure 13 × 10 in the rectovaginal septum, MRI: oval-shaped nodule 30 × 20 mm hypertense structure on T1, hemoglobin products in T2 | DIE/DL-multiple red, black, and white scarred EM implants in uterosacral ligaments, R tubal cyst, fallopian tube torsion, R distal portion salpingectomy and adhesiolysis | Pre | No/EM implants identified during surgery | Tubal endometrioma with multiple sclerotic and calcified areas, stroma cells and hemosiderin-laden macrophages | R distal portion |
| 2012; Lim | 30 | 5 month dysmenorrhea and dull lower abdominal pain | Nulli (virgin) | Thick-walled, complex cystic structures 21 × 21 mm and 53 × 34 mm (R and L ovary) | Pelvic EM/DL-bilateral torted tubes and cystic dilation at the distal portion salpingectomy and adhesiolysis | Pre | No/EM implant (spot) identified during surgery | Extensive hemorrhagic infarction secondary to torsion and hematosalpinx with endometrial glands detection |
Bilateral distal portion |
| 2011; Kahyaoglu | 33 | 18 years infertility and mild EM, pelvic pain and vaginal bleeding after embryo transfer | Nulli | TVUS: R tubal ectopic ring | Ectopic pregnancy/Emergent laparoscopy- bilateral salpingectomy | Pre | Yes (pelvic peritoneum) | Bilateral tubal ectopic pregnancy with endometriotic implants | Bilateral |
| 2010; Ozturk | 31 | Secondary infertility | Primi | TVUS: R hydrosalpinx 37 × 12 mm | Hydrosalpix/DL-dilated R tubal uterine mimicking hydrosalpinx, R salpingectomy | Pre | No/No IO EM implants | Intraluminal tubal EM | R mucosa |
| 2004; Datta | 34 | Primary infertility | Nulli | TVUS: Polycystic ovaries, HSG: normal | Unexplained infertility/DL -atypical endometriotic deposit on R tube mimicking ectopic pregnancy, ovarian drilling | Pre | No/EM uterosacral implants identified during surgery | Not performed | R |
| 2003; Ohara | 49 | Anemia, acute abdominal pain | Nulli | US: R elongated sausage-shaped cystic mass 6.2 × 3.3 cmm, CA 125: 57.7 U/mL | Hematosalpinx/Emergent laparotomy-R elongated distended dark purple tube with occluded fimbrial end triple twisted, TAH-RSO | Pre | No/EM implants identified during surgery | Extensive hemorrhagic infarction secondary to torsion and endometrial glands in the haematosalpinx | R |
| 2002; De la Torre | 60 | Abdominal distension and pelvic pain | N/A | US: Tumor with solid and cystic components 10 cm, CT: L para-aortic node 1 cm | Ovarian cancer/Exploratory laparotomy- TAH BSO PL PaL | Post | N/A | Transitional areas between the newly formed and endometriotic epithelium lined the cystic cavity of tubal wall-Clear cell fallopian tube carcinoma with tubal EM | L-proximal portion 1 cm from uterine ostium |
N/A: not available, DL: diagnostic laparoscopy, TVUS: transvaginal ultrasound, DIE: deep infiltrating endometriosis, R: right, L: left, EM: endometriosis, HSG: hysterosalpingography, TAH: total abdominal hysterectomy, RSO: right salpingoophorectomy, BSO PL Pal: bilateral salpingoophorectomy pelvic lympadenectomy and para-aortic lympadenectomy.
The PRISMA search flow diagram schematically presents the stages of study selection and inclusion of the studies (Figure 1).
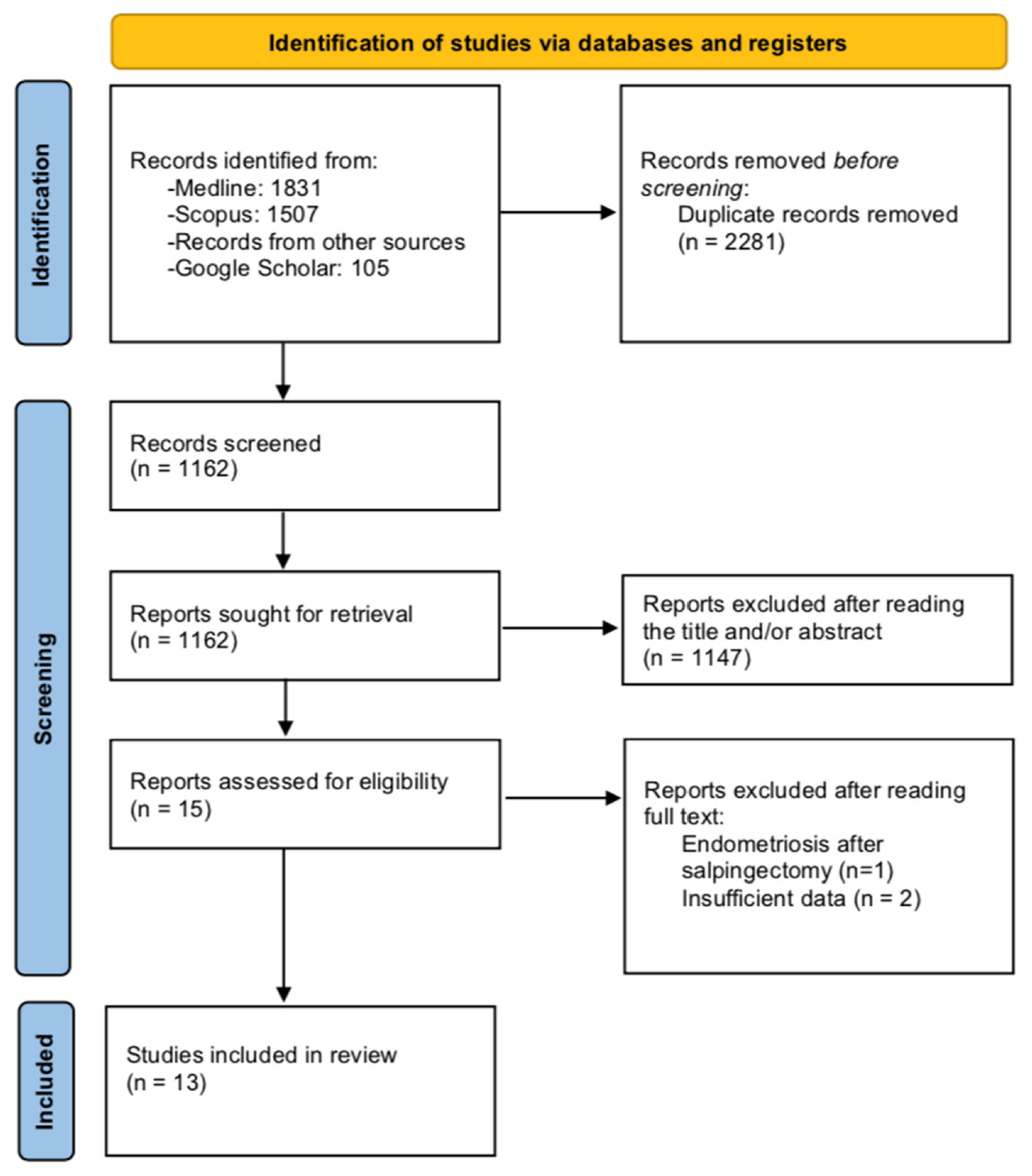
Figure 1. Search flow diagram.
4. Prevalence and Disease Characteristics
Table 1 depicts the main study and patient characteristics derived from the included observational studies.
The prevalence of tubal EM ranged from 9% to 68.6% among the included studies. According to the prospective study by Xia et al., the prevalence of tubal EM in a group of 35 patients with pelvic EM was 68.6% (n = 24) [15]. In the study by Qi et al., 1112 premenopausal women who underwent salpingectomy due to various gynecological indications were grouped to those with and without tubal EM and analyzed [16]. In their study, the prevalence of tubal EM was 14.48% (n = 161/1112) [16]. The retrospective study by Xue et al. separated patients with tubal EM into three groups: those with EM (n = 178), those with other benign diseases (n = 65) and 18 others with malignant gynecologic diseases [17]. The prevalence of tubal EM was highest in the EM group. McGuinness et al. assessed the incidence of tubal EM among women who underwent operative laparoscopy due to EM, pelvic pain, infertility or adnexal cystic masses [18]. Ninety-seven patients underwent salpingectomy, whereas in 88 others, the macroscopic recognition of fallopian tube endometriotic lesions were ablated with CO2 laser, or electrosurgery (non-salpingectomy group). Tubal EM was detected in 35% (n = 34/97) and in 9% (n = 8/88) in the salpingectomy and non-salpingectomy groups, respectively, while the respective proportions in the subgroup of 153 patients with EM, was 42.5% for histologically proved tubal EM, and 11–12% for macroscopic tubal disease [18].
According to McGuinness et al., tubal EM was significantly related to severe disease when compared to mild or moderate (p = 0.0196) [18]. The same was also observed in the study by Qi et al., who reported an increment in tubal EM prevalence as the severity of pelvic EM increased (r = 0.26, p < 10−4) [16]. Regarding the factors that were related to elevated tubal EM rates, tubal ligation, abnormal uterine bleeding and previous surgery for EM were found significant in both uni- and multivariate analysis [16]. Additionally, patients with multi-organ EM presented an increased incidence of tubal EM compared to those with single-organ (43.94% vs. 24.24%, p < 0.05) [16].
Left side tubal EM was more prevalent than right side as proved by Qi et al. and Xue et al. (52.17% vs. 40.37%, p < 0.05 and n = 168/261, 64.37% vs. n = 93/ 261, 35.63%, p < 0.001, respectively) [16][17]. This was also observed when patients who were operated due to EM and malignant diseases were separately analyzed (p < 0.001 and p < 0.05, respectively) [17].
The literature search revealed a total of 7 case reports during the study period [21][22][23][24][25][26][27]. Table 2 depicts the main patients’ and disease-related characteristics from case reports. Median patients’ age was 33 years (range: 18–60), while five out of six patients were nulliparous. All patients were premenopausal except a case of detection of tubal EM in a 60-year-old postmenopausal woman who was diagnosed with clear-cell stage IIIC fallopian tube carcinoma associated with an endometriotic tubal wall cyst. Only one patient reported a history of EM prior to surgery.
5. Histopathological Findings
The analysis of patients by Xia et al. revealed significantly decreased ciliary beat frequency (CBF) in both ampulla and isthmus when compared to either 20 control patients who underwent surgery for uterine leiomyoma or the remaining 11 without EM (non-tubal EM group) [15]. The same was also observed in the percentages of ciliated cells. Finally, tubal EM group presented significantly lower contraction frequencies and weaker muscular contractility [15]. Concerning the histopathological findings reported by Qi et al., mucosa and serosa were the most common layers of tubal EM detection with more than 80% of the proximal tubal lesions detected in the mucosa, whereas 53.85% of lesions in the distal tube were found in the serosa [16]. Finally, serosal lesions presented a more prominent inflammatory reaction and fibroblasts and collagenous proliferation near the lesion than mucosal ones [16].
6. Genetic Background
The study group by Qi et al. recently published two studies on the analysis of the genetic profile of tubal EM [19][20]. More specifically, a study published in 2019 compared the miRNA-microarray expression among four patients with tubal EM and five controls [19]. The authors identified a total of 17 miRNAs in the tubal epithelium that were expressed different in the tubal EM group (four upregulated and 13 downregulated) [19]. Bioinformatic analysis revealed that some of the detected miRNAs play a significant role in the mTOR signaling pathway, SNARE interactions and endocytosis, thus participating in the pathogenesis of EM [19]. Accordingly, a study published in 2020 by the same study group found a total of 50 significantly dysregulated genes in the tubal epithelial analysis of four women with tubal EM compared to specimens of four controls without tubal EM, while a respective proteomic analysis of tubal fluid showed 33 over-expressed proteins and 19 under-expressed ones in patients with tubal EM [20]. Among them, IL-6, TNFA, C2, C4B, MMP7 and AHSG are common proteins that were found to be preferentially expressed in patients with tubal EM both in epithelium and tubal fluid [20]. Additionally, ORM2, SAA4, CP HP and MAP2K6 are some further innovative proteins that have also been identified [20]. IL-6, C4B, CP, C2, HP, TNFA and ORM2 were among the up-regulated proteins while AHSG and MAP2K6 were the down-regulated ones [20]. The commonly expressed genes and proteins participated in the inflammatory response, cellular movement and immune cell trafficking, which can all explain a part of the molecular mechanisms of EM formation [20].
7. Diagnosis
According to the data derived from case reports, the primary indication for surgery was infertility in three patients. Among them, two had primary infertility and one was a primiparous patient with secondary infertility. The case reported by Kahyaoglou et al., suffered from 18-year infertility and presented with acute pelvic pain and vaginal bleeding 20 days after embryo transfer, and thus referred to emergent laparoscopy with the suspicion of ectopic pregnancy [23]. Acute abdominal pain was also the predominant symptom in two patients who underwent emergent surgery, whereas two other patients reported dull abdominal pain and distention. The preoperative imaging findings and the reported histopathological findings are shown in Table 2.
8. Treatment-Follow-Up
All seven patients from the cases reports underwent surgery for the management of their disease. Intraoperative findings revealed that among the five patients with no previous EM history, EM implants were identified during surgery in four of them, while in one patient no intraoperative EM lesions were macroscopically detected. The last patient underwent surgery for suspected hydrosalpinx, and no EM signs were present at macroscopic examination during diagnostic laparoscopy, while histological examination of the excised right tube revealed intraluminal tubal EM. Five patients had laparoscopic approach and the remaining two underwent laparotomy. In four patients the tubal EM lesion was right-sided, in one a left tubal EM was detected and two other had bilateral EM tubal lesions. Five patients aged from 18 to 34 years underwent salpingectomy to manage their disease, whereas two patients aged 49 and 60 years underwent total abdominal hysterectomy (TAH) with right salpingo-ophorectomy, and (TAH) with bilateral salpingo-ophorectomy, pelvic lympadenectomy and para-aortic lympadenectomy, respectively. From the five patients that had salpingectomy, follow-up was available for two of them with both being disease-free with no evidence of EM recurrence at follow up [21][22].
References
- Gruber, T.M.; Mechsner, S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells 2021, 10, 1381.
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852.
- Lee, H.J.; Park, Y.M.; Jee, B.C.; Kim, Y.B.; Suh, C.S. Various anatomic locations of surgically proven endometriosis: A single-center experience. Obstet. Gynecol. Sci. 2015, 58, 53–58.
- Chapron, C.; Fauconnier, A.; Vieira, M.; Barakat, H.; Dousset, B.; Pansini, V.; Vacher-Lavenu, M.C.; Dubuisson, J.B. Anatomical distribution of deeply infiltrating endometriosis: Surgical implications and proposition for a classification. Hum. Reprod. 2003, 18, 157–161.
- Ávalos Marfil, A.; Barranco Castillo, E.; Martos García, R.; Mendoza Ladrón de Guevara, N.; Mazheika, M. Epidemiology of Endometriosis in Spain and Its Autonomous Communities: A Large, Nationwide Study. Int. J. Environ. Res. Public Health 2021, 18, 7861.
- Gordts, S.; Koninckx, P.; Brosens, I. Pathogenesis of deep endometriosis. Fertil. Steril. 2017, 108, 872–885.e871.
- Arafah, M.; Rashid, S.; Akhtar, M. Endometriosis: A Comprehensive Review. Adv. Anat. Pathol. 2021, 28, 30–43.
- Giannella, L.; Marconi, C.; Di Giuseppe, J.; Delli Carpini, G.; Fichera, M.; Grelloni, C.; Giuliani, L.; Montanari, M.; Insinga, S.; Ciavattini, A. Malignant Transformation of Postmenopausal Endometriosis: A Systematic Review of the Literature. Cancers 2021, 13, 4026.
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case-control studies. Lancet. Oncol. 2012, 13, 385–394.
- de la Cuesta, R.S.; Eichhorn, J.H.; Rice, L.W.; Fuller, A.F., Jr.; Nikrui, N.; Goff, B.A. Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol. Oncol. 1996, 60, 238–244.
- Gaia-Oltean, A.I.; Braicu, C.; Gulei, D.; Ciortea, R.; Mihu, D.; Roman, H.; Irimie, A.; Berindan-Neagoe, I. Ovarian endometriosis, a precursor of ovarian cancer: Histological aspects, gene expression and microRNA alterations (Review). Exp. Ther. Med. 2021, 21, 243.
- Chakrabarti, I.; Ghosh, N. Post-salpingectomy endometriosis: An under-recognized entity. J. Mid-Life Health 2010, 1, 91–92.
- Audebert, A.; Petousis, S.; Margioula-Siarkou, C.; Ravanos, K.; Prapas, N.; Prapas, Y. Anatomic distribution of endometriosis: A reappraisal based on series of 1101 patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 36–40.
- Sinha, A.K.; Agarwal, A.; Lakhey, M.; Mishra, A.; Sah, S.P. Incidence of pelvic and extrapelvic endometriosis in Eastern region of Nepal. Indian J. Pathol. Microbiol. 2003, 46, 20–23.
- Xia, W.; Zhang, D.; Ouyang, J.; Liang, Y.; Zhang, H.; Huang, Z.; Liang, G.; Zhu, Q.; Guan, X.; Zhang, J. Effects of pelvic endometriosis and adenomyosis on ciliary beat frequency and muscular contractions in the human fallopian tube. Reprod. Biol. Endocrinol. RBE 2018, 16, 48.
- Qi, H.; Zhang, H.; Zhang, D.; Li, J.; Huang, Z.; Zhao, X.; Zhang, J. Reassessment of prevalence of tubal endometriosis, and its associated clinicopathologic features and risk factors in premenopausal women received salpingectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 4, 100074.
- Xue, R.H.; Li, J.; Huang, Z.; Li, Z.Z.; Chen, L.; Lin, Q.; Huang, H.F. Is tubal endometriosis an asymmetric disease? A 17-year retrospective study. Arch. Gynecol. Obstet. 2020, 301, 721–727.
- McGuinness, B.; Nezhat, F.; Ursillo, L.; Akerman, M.; Vintzileos, W.; White, M. Fallopian tube endometriosis in women undergoing operative video laparoscopy and its clinical implications. Fertil. Steril. 2020, 114, 1040–1048.
- Qi, H.; Liang, G.; Yu, J.; Wang, X.; Liang, Y.; He, X.; Feng, T.; Zhang, J. Genome-wide profiling of miRNA expression patterns in tubal endometriosis. Reproduction 2019, 157, 525–534.
- Qi, H.; Zhang, H.; Zhao, X.; Qin, Y.; Liang, G.; He, X.; Zhang, J. Integrated analysis of mRNA and protein expression profiling in tubal endometriosis. Reproduction 2020, 159, 601–614.
- Wenger, J.M.; Soave, I.; Lo Monte, G.; Petignat, P.; Marci, R. Tubal endometrioma within a twisted fallopian tube: A clinically complex diagnosis. J. Pediatric Adolesc. Gynecol. 2013, 26, e1–e4.
- Lim, S.Y.; Park, J.C.; Bae, J.G.; Kim, J.I.; Rhee, J.H. Isolated torsion of bilateral fallopian tubes combined with tubal endometriosis: A case report. Korean J. Obstet. Gynecol. 2012, 55, 55–58.
- Kahyaoğu, İ.; Kahyaoğlu, S.; Müftüoğlu, K.; Şengül, İ.; Şengül, D.; Önen, Ş.; Mollamahmutoğlu, L.; Batıoğlu, S. Tubal endometriosis as an insidious risk factor for tubal implantation in a procedure of assisted reproductive technique. Cumhur. Med. J. 2011, 33, 248–252.
- Ozturk, E.; Ugur, M.; Aydın, A.; Balat, O.; Kalaycı, H. Intraluminal tubal endometriosis mimicking hydrosalpinx: Report of an unusual case. IIUM Med. J. Malays. 2010, 9.
- Datta, S.; Priddy, A. Tubal endometriosis mimicking an ectopic pregnancy. Reproduction 2004, 5, 970.
- Ohara, N.; Narita, F.; Murao, S. Isolated torsion of haematosalpinx associated with tubal endometriosis. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2003, 23, 453–454.
- de la Torre, F.J.; Rojo, F.; García, A. Clear cells carcinoma of fallopian tubes associated with tubal endometriosis. Case report and review. Arch. Gynecol. Obstet. 2002, 266, 172–174.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
937
Revisions:
2 times
(View History)
Update Date:
08 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




