Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Keekyoung Kim | + 2609 word(s) | 2609 | 2022-02-28 07:31:57 | | | |
| 2 | Camila Xu | Meta information modification | 2609 | 2022-03-07 05:00:43 | | | | |
| 3 | Camila Xu | Meta information modification | 2609 | 2022-03-07 05:01:17 | | | | |
| 4 | Catherine Yang | Meta information modification | 2609 | 2025-02-10 03:13:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kim, K. 3D Bioprinting Technology. Encyclopedia. Available online: https://encyclopedia.pub/entry/20228 (accessed on 07 February 2026).
Kim K. 3D Bioprinting Technology. Encyclopedia. Available at: https://encyclopedia.pub/entry/20228. Accessed February 07, 2026.
Kim, Keekyoung. "3D Bioprinting Technology" Encyclopedia, https://encyclopedia.pub/entry/20228 (accessed February 07, 2026).
Kim, K. (2022, March 04). 3D Bioprinting Technology. In Encyclopedia. https://encyclopedia.pub/entry/20228
Kim, Keekyoung. "3D Bioprinting Technology." Encyclopedia. Web. 04 March, 2022.
Copy Citation
3D bioprinting, an additive manufacturing process, is a pioneering technology that prints 3D structures with biocompatible materials including living cells (i.e., bioinks).
3D bioprinting
regenerative medicine
optimization
1. Introduction
Tissue engineering has evolved from biomaterials science by integrating scaffolds, cells, and biomolecules to fabricate functional tissues. The main objective of tissue engineering is to develop three-dimensional (3D) artificial tissues and organs which can be used to augment, repair, and replace damaged or diseased tissue. Regenerative medicine is a broad field that includes tissue engineering and also involves research on the self-healing of the body by using its own or foreign materials to regenerate cells, tissues, or organs [1]. However, the terms “tissue engineering” and “regenerative medicine” have been used interchangeably, as the research area focuses on restoring impaired functions resulting from any cause, including congenital disabilities, disease, trauma, and aging.
On the other hand, personalized medicine is a novel field of treating individuals with optimized treatment [2]. Since everyone has different family medical histories and disease specificity, treating them with a homogeneous medication is not recommended [3]. People opt for personalized medicine over time, which has a low rate of immune rejection. Personalized medicine is different from precision medicine, commonly known as patient-specific therapy [4]. Precision medicine and patient-specific therapy are the creation of treatments that can be applied to groups of individuals with specific characteristics. Personalized therapy refers to patient-oriented treatment based on the clinical features of each patient by comprehensively considering the diversity, severity, and genetic characteristics of the symptoms.
Driven by tissue engineering, 3D bioprinting, an additive manufacturing process, is a pioneering technology that prints 3D structures with biocompatible materials including living cells (i.e., bioinks) [5]. While general 3D printing extrudes molten plastic that hardens to become a 3D object, 3D bioprinting is designed to print biological materials or bioink. A bioink is a combination of biomaterial, living cells, and hydrogel, and is the most widely used due to a highly hydrated environment for cell proliferation. Most matrix bioinks are naturally derived hydrogels, such as collagen, hyaluronic acid, or alginate. Moreover, materials widely used in bioink are gelatin methacrylate, collagen, poly(ethylene glycol), pluronic, alginate, and decellularized extracellular matrix-based materials. With 3D bioprinting, complex structures that cannot be fabricated using traditional manufacturing methods can be made and can alleviate the organ shortage problem in transplantation [6]. The bioprinted scaffolds can be turned into functional tissues or organs by encouraging cellular activities with various growth factors for regeneration. In addition, the bioprinted tissues or organs could be vital for surgeons to simulate complicated surgery. Three-dimensional bioprinting technology has the potential to replicate a variety of complex human tissues, leading surgeons and medical device companies to turn to it for their surgical training and testing needs. With more realistic surgical simulation models printed by the bioprinter, surgeons can improve their surgical techniques and reduce the chance of making mistakes in the surgery process.
There are various types of 3D bioprinting technologies. Extrusion-based bioprinting is the most widely used. It can produce scaffolds that do not contain organic solvents harmful to the human body and fabricate them precisely by applying pressure to the biomaterials [7]. Inkjet bioprinting has the advantage of printing various cells in a particular location precisely [8]. Stereolithography bioprinting uses a liquid photocurable resin that can be crosslinked with ultraviolet light [9]. The bioprinting process is broadly divided into three steps: pre-bioprinting, bioprinting, and post-bioprinting [10]. In the pre-printing stage, computer-aided design (CAD) is utilized with different types of software. Once the design is created in CAD, it will move to the slicing step for transitioning to the standard tessellation language (STL) file by generating G-code. G-code is a computer language that can tell a command to the printer. Appropriate design is related to how many tissues or organs can get the nutrients and oxygen. The initial design would provide valuable data to compare and analyze with post-printing results. Furthermore, the selection of proper biomaterials and bioprinters is important since these affect to rheological, biological, and degradation properties in the outcomes. Biocompatibility is an important factor when selecting the biomaterial, as it works with living tissues in the body or body fluids and the production of a toxic or immunological response can be lethal. In the actual printing phase, the defect detection of printed patterns in real-time would be investigated. In the post-printing step, suitable storage and continuous quality monitoring methods are investigated to maintain the best environment for cell growth. In this way, there are many challenges in 3D bioprinting and 3D printing when finding a suitable printing setup.
As a solution, machine learning (ML) techniques first appeared in the 3D printing field [11]. ML is a sub-branch of artificial intelligence (AI), and it is a technology that aims to realize functions such as the human learning ability in computers. ML algorithms can learn based on empirical data, make predictions, and improve their performance by themselves. These algorithms do not execute static program instructions but rather build an ideal model for making predictions or decisions based on input data. These computing technologies can be combined with cutting-edge medical technologies to create synergy. The ML-based model can achieve better performance compared to the physics-based model [12]. The traditional physics-based model has a challenge of non-linearities, varying parameters, and uncertainties. However, ML is highly flexible, has adaptable methods, and is excellent in prediction. A key question is how to choose between a physics-based model and a data-driven ML model. The answer depends on the problems to be solved. If there is no direct knowledge about the behaviour of a system, then mathematical models cannot be formulated and are unable to make an accurate prediction. However, if there are many example outcomes, the ML-based model can be used with training, validation, and testing datasets. In ML, the cost function can help analyze how well an ML model performs by comparing the predicted values with the actual values. The cost function is defined on an entire data instance, while the loss function is defined on a single data instance. The optimization algorithms in the cost function are used to find the optimal values for the model parameters by finding the minima of cost functions. As a nature of optimization, many printing parameters can be optimized to achieve the best design and printing environment relative to a set of prioritized criteria or constraints.
2. Machine-Learning Principles Used in 3D Bioprinting
The 3D printing technology significantly impacts various industries and research fields (e.g., manufacturing, medical, robotics, automotive, aerospace, and education) [13]. In tissue engineering, 3D bioprinters can print human tissues and organs with bioinks consisting of biomaterials and cells [14]. They can create complete substitutes for damaged tissues and fabricate microscale tissues or organoids for disease study and drug discovery [15][16].
2.1. Materials and Modalities of 3D Bioprinting
Regardless of specific 3D bioprinters, bioinks in the bioprinting process are indispensable components [17]. Bioinks consist of cells and hydrogel pre-polymers for the formation of tissue scaffolds. The cellular component should be selected as the essential consideration. According to the methods and applications of printing, the selected cells for bioink should mimic the physiological state in vivo [18]. Then, the cells can maintain their functions in biomaterials after they are printed, as shown in Figure 1.
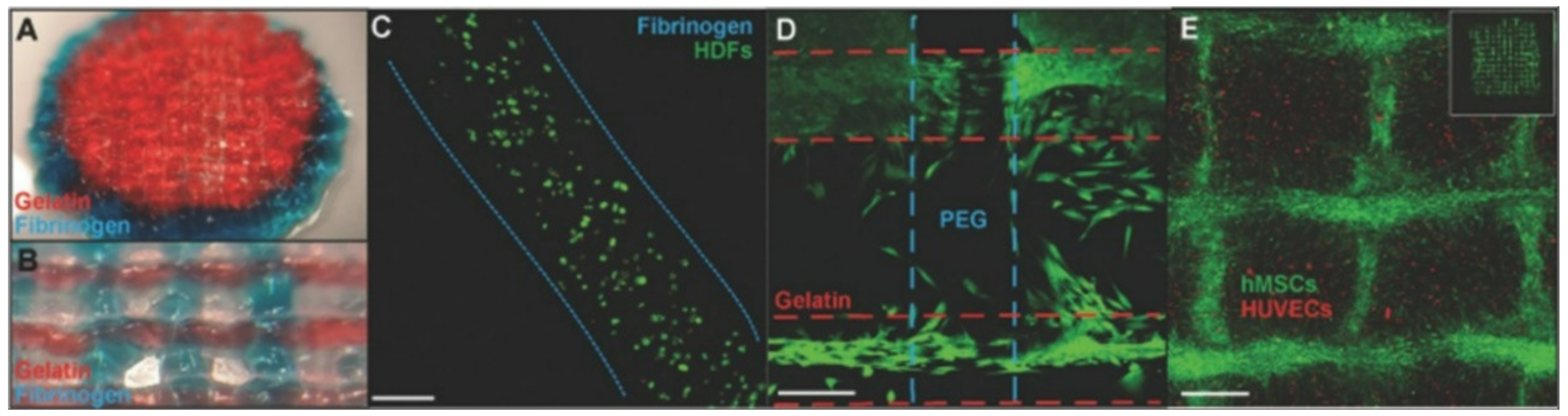
Figure 1. Cell-laden bioprinting. (A) Polyethylene glycol ending in two reactive groups (PEGX)-gelatin (red) and PEGX-fibrinogen (blue) coprinted cylinder, 15 mm diameter. (B) Coprinting with inner structure pattern ≈650 μm diameter. (C) Stained with Live/Dead assay at one day to check cell viability. (D) Coprinted structure with human dermal fibroblasts cell-laden printing. (E) human umbilical vein endothelial cell (HUVEC)-laden printing and human mesenchymal stem cells (hMSC) (cell tracker green) spread into open spaces of construct and onto printed bioink strands at day 4 (adapted from [19] with permission).
The cell source includes primary cell lines from human and animal tissues, stem cells, etc. [20]. Primary cells are directly taken from human or animal tissue using enzymatic or mechanical methods [21]. Stem cells are the proper cell sources for bioprinting since they increase the proliferative capacity and sufficiently mimic the function of the original cells. Even though most of these cells can be purchased easily, specific cells are challenging to cultivate due to the limitations of the current bioprinting systems. Thus, the labs that mainly focus on bioprinting develop advanced cells for commercial use or research.
Biomaterials: The viscosity and rheological behaviour of biomaterials influence the quality and shape of printed constructs. Therefore, the selection of appropriate biomaterials before printing is essential. Hydrogels have become a popular material to pattern cells in 3D bioprinting due to their high biocompatibility and processability. Hydrogels are necessary material, especially in bioprinting [22]. The terminology hydrogels is composed of “hydro” (water) and “gel” and refers to aqueous (water-containing) gels [23]. They are polymer networks that are insoluble in water and swell to an equilibrium volume but retain their shape. Hence, hydrogels directly contact cells to provide a growing environment like an extracellular matrix and dominate the mechanical and biocompatible properties of the printed object. The extracellular matrix forms an integral part of the scaffold to induce cell proliferation, thereby leading to new tissue formation. Printable and crosslinkable properties should normally characterize a hydrogel pre-polymer solution for bioprinting. This means the pre-polymers are usually required to have shear thinning properties or the carbon–carbon double bond structures, as shown in Figure 2. In addition to the printability, the pre-polymer solution should be a material compatible with living tissues as a biocompatible property. In terms of crosslinking, the strategies to transfer pre-polymer solution to hydrogels include physical crosslinking, chemical crosslinking, and enzymatic crosslinking. Physical crosslinking uses interactions other than a covalent bond, such as coordination bonding, hydrogen bonding, ionic interaction, or van der Waals interaction. Thermoplastic elastomers are the representative material that undergo physical crosslinking, and thermosetting polymers are made from chemical crosslinking. Chemical crosslinking refers to the intermolecular or intramolecular combining of two or more molecules by a covalent bond. The mechanism of different crosslinking methods comprises the non-covalent/covalent interactions to combine polymer chains and make them form a stronger network. According to the crosslinking techniques, different bioprinters are recommended, as shown in Figure 2.
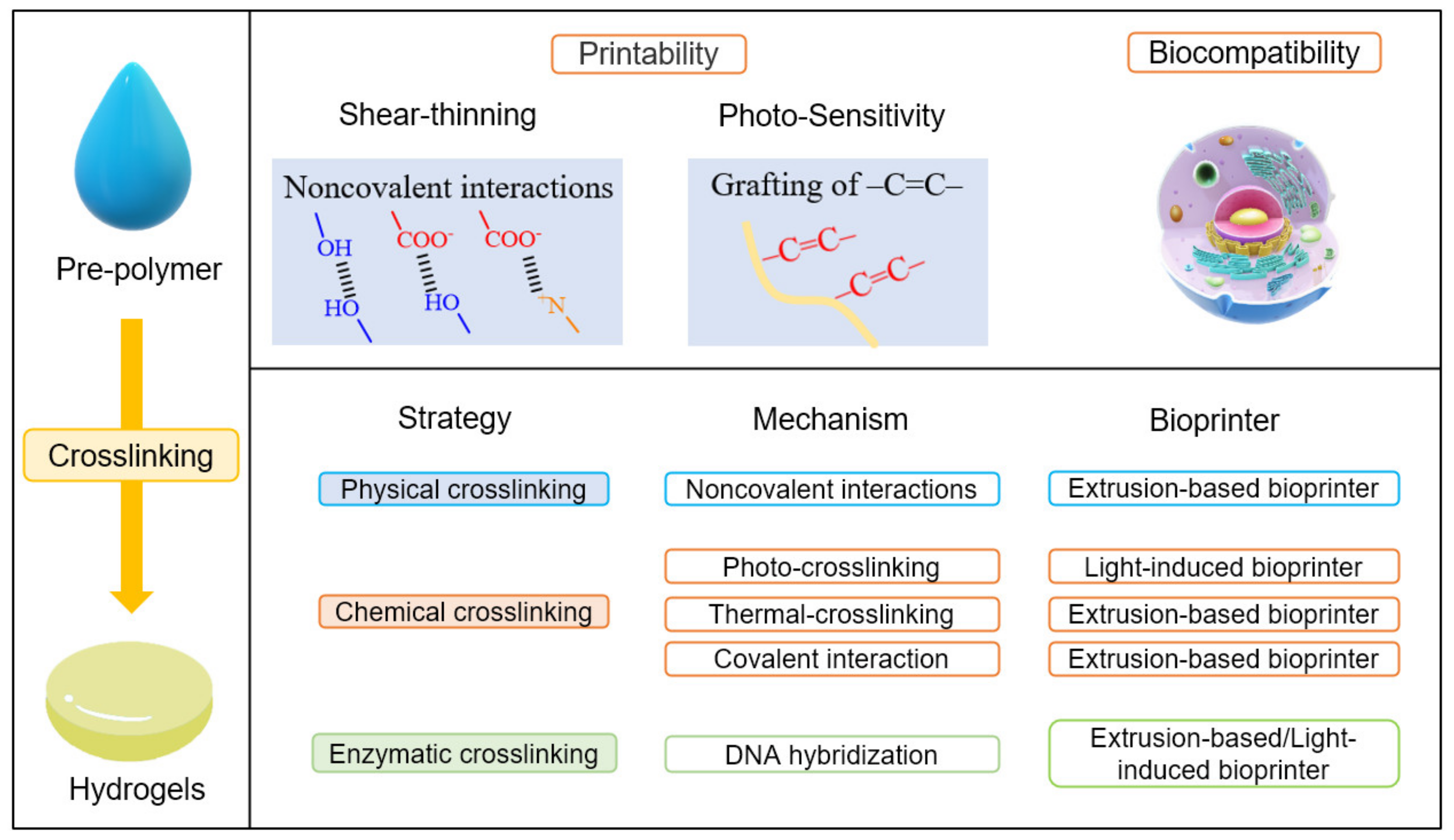
Figure 2. The crosslinking methods in 3D bioprinting of polymeric hydrogels.
Pre-polymers that form hydrogels in bioprinting can be classified into biopolymers and synthetic polymers. Biopolymers are natural polymers produced by the cells of living organisms. They are derived from humans, animals, and plants with the examples of cellulose, alginate, collagen, and chitosan [24]. Usually, these natural biopolymers are suitable for extrusion printing due to non-covalent interactions. This results in excellent rheological properties such as shearing shining and thixotropy. The advantage of the biopolymers is they can naturally cover the surface of eukaryotic cells and combine with proteins. These characteristics can form a natural extracellular matrix, resulting in them having high biocompatibility and cell affinity.
On the other hand, synthetic materials are derived from petroleum oil and are made by scientists and engineers [25]. The chemical and physical properties as crosslinking rates and mechanical properties can be precisely designed. Polyethene glycol and polyacrylamide, polyacrylamide, and poly (N-isopropyl acrylamide) are commonly used in 3D printing. The ink containing polymer or monomers for hydrogel printing should have suitable rheological or light-curing properties [19]. The printable hydrogels must have good biocompatibility and provide an appropriate environment for the survival and growth of cells. In addition, hydrogels should have excellent mechanical properties. Some biomaterials need to be mixed with cellulose, polyvinyl alcohol, cellulose fibrils, and chitosan to improve the viscosity and shear-shining properties via molecular interactions. There is another method to refine the light-curing property of biomaterials [26]. Gelatin methacrylate and hyaluronic acid methacrylate modify biopolymers by grafting carbon–carbon double bonds on the polymer chains. Unlike natural biopolymers, these modified polymers are more suitable photopolymerization print modalities due to the existence of carbon–carbon double bonds. These polymers simultaneously have the advantages of biopolymers and synthetic polymers and can be crosslinked under ultraviolet or visible light. However, these materials are not enough to fabricate sophisticated and complex human tissue/organs due to the rapid development of printing technology. Seeking a new hydrogel for 3D bioprinting remains an ongoing and exciting task.
Modalities: Four primary 3D bioprinters were categorized and explored in this section [8][27][28][29][30]. As shown in Figure 3, there are extrusion-based bioprinting, scaffold-free bioprinting, inkjet bioprinting, and laser-induced forward transfer (LIFT) bioprinting. Extrusion-based bioprinting is a relatively simple 3D bioprinting method, which uses shear stress to extrude bioinks to deposition 3D structures [31][32]. Bioinks for extrusion-based bioprinting usually consist of alginate or fibrin polymers. They are flexible enough after shearing smoothly extruded from the nozzle. Furthermore, they are integrated with cellular adhesion molecules, which support the physical attachment of cells and maintain structural stability rigidly after printing.
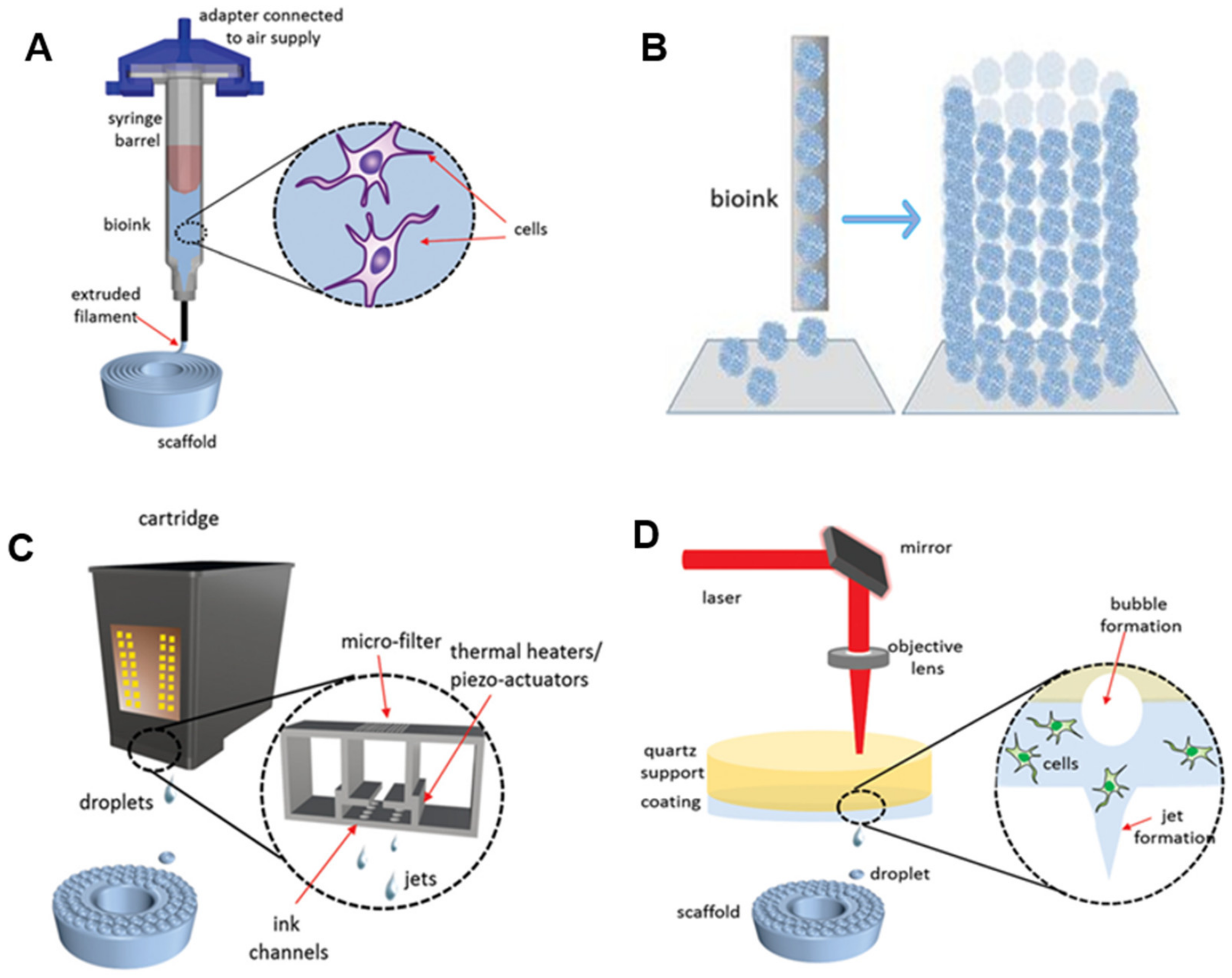
Figure 3. Modalities for bioprinting technologies. (A) Extrusion-based bioprinting. (B) Scaffold-free bioprinting. (C) Inkjet bioprinting. (D) Laser-induced forward transfer bioprinting (adapted from [30] with permission).
As shown in Figure 3A, the bioink-containing cells are put into the syringe. The bioinks are extruded through nozzles by shear force to create 3D structures. The extruded bioink forms the specific shape that was expected to be printed through a CAD program. Although there are many 3D printings processes, most of them rely on CAD [33]. CAD is the process of designing an object using computer software and is an essential component of 3D printing. Slicing a 3D model means taking the design in STL format and slicing it into individual layers. The software then generates the tool path (G-code). This process is important for translating the 3D drawing into a language that a 3D printer can understand and print. It commands the 3D printer how much material it needs to deposit and where the material should be deposited.
Scaffold-free bioprinting has gained extensive attention due to the advantages of high cell content and shorter restructuring time than the scaffold-based technique [28]. Scaffold-free approaches have been used in clinical practices to restore damaged tissues such as bone, cartilage, and ligaments. A notable benefit of the scaffold-free strategy is that cells naturally create a tight intercellular connection [34]. This method is used to print tissues or organs with a high concentration of cells. Scaffold-free bioprinters generate aggregates or rods entirely composed of cells. These aggregates form larger constructs as time goes on based on tissue liquidity and fusion (Figure 3B) [28][34]. The self-assembly of cells resulting in more giant constructions depends on cell and molecular interactions.
Inkjet printing is a relatively low-cost technology since commercial printers (i.e., office inkjet printers) have been transformed into inkjet bioprinters [4]. The principle of 3D inkjet bioprinting is like traditional inkjet printing, in which the ink is applied using the piezoelectricity or heating strategy. Three-dimensional inkjet bioprinting creates biological structures by gradually depositing bioinks in a specific place (Figure 3C) [28][29]. The investigation of the combination of individual ink drops is the most crucial part of 3D inkjet bioprinting. As the dimension of printable droplets is tiny, the bioprinting of a large structure such as an organ might be a challenge; however, the small droplet size is of great merit to the resolution of printed designs.
LIFT bioprinting is similar to inkjet bioprinting [35]. The difference is that a laser beam is used as a driving force to project bioinks from a donor thin film composed of biopolymer and toward the receiving platform in LIFT bioprinting (Figure 3D) [25][35]. LIFT bioprinter does not have nozzles; hence, the clogging issues in nozzles can be naturally solved. Even though the setup of the LIFT printer is more expensive and is not fully automated, this printer can print a more accurate structure at a faster rate. Overall, these four bioprinters have advantages and disadvantages. It is greatly encouraged to select the most suitable printing method according to the characteristics of the tissue/organs, the cell, and biomaterials.
References
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452.
- Skardal, A.; Shupe, T.; Atala, A. Body-on-a-Chip: Regenerative Medicine for Personalized Medicine. Princ. Regen. Med. 2019, 769–786.
- Risse, G.B.; Warner, J.H. Reconstructing clinical activities: Patient records in medical history. Soc. Hist. Med. 1992, 5, 183–205.
- Hamburg, M.A.; Collins, F.S. The path to personalized medicine. N. Engl. J. Med. 2010, 363, 301–304.
- Jovic, T.H.; Combellack, E.J.; Jessop, Z.M.; Whitaker, I.S. 3D Bioprinting and the Future of Surgery. Front. Surg. 2020, 129.
- Mironov, V.; Kasyanov, V.; Markwald, R.R. Organ printing: From bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011, 22, 667–673.
- Ramesh, S.; Harrysson, O.L.A.; Rao, P.K.; Tamayol, A.; Cormier, D.R.; Zhang, Y.; Rivero, I.V. Extrusion bioprinting: Recent progress, challenges, and future opportunities. Bioprinting 2021, 21, e00116.
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833.
- Wang, Z.; Abdulla, R.; Parker, B.; Samanipour, R.; Ghosh, S.; Kim, K. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication 2015, 7, 045009.
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. 2009, 4, 1168–1177.
- Delli, U.; Chang, S. Automated Process Monitoring in 3D Printing Using Supervised Machine Learning. Procedia Manuf. 2018, 26, 865–870.
- Ji, C.; Mandania, R.; Liu, J.; Liret, A.; Kern, M. Incorporating Risk in Field Services Operational Planning Process. In International Conference on Innovative Techniques and Applications of Artificial Intelligence; Springer: Cham, Switzerland; Cambridge, UK, 2018; Volume 11311 LNAI, ISBN 9783030041908.
- Ruberu, K.; Senadeera, M.; Rana, S.; Gupta, S.; Chung, J.; Yue, Z.; Venkatesh, S.; Wallace, G. Coupling machine learning with 3D bioprinting to fast track optimisation of extrusion printing. Appl. Mater. Today 2021, 22, 100914.
- Visconti, R.P.; Kasyanov, V.; Gentile, C.; Zhang, J.; Markwald, R.R.; Mironov, V. Towards organ printing: Engineering an intra-organ branched vascular tree. Expert Opin. Biol. Ther. 2010, 10, 409–420.
- Fedorovich, N.E.; Alblas, J.; De Wijn, J.R.; Hennink, W.E.; Verbout, A.B.J.; Dhert, W.J.A. Hydrogels as extracellular matrices for skeletal tissue engineering: State-of-the-art and novel application in organ printing. Tissue Eng. 2007, 13, 1905–1925.
- Mironov, V.; Boland, T.; Trusk, T.; Forgacs, G.; Markwald, R.R. Organ printing: Computer-aided jet-based 3D tissue engineering. Trends Biotechnol. 2003, 21, 157–161.
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 1–15.
- Kim, H.; Park, M.N.; Kim, J.; Jang, J.; Kim, H.K.; Cho, D.W. Characterization of cornea-specific bioink: High transparency, improved in vivo safety. J. Tissue Eng. 2019, 10, 2041731418823382.
- Rutz, A.L.; Hyland, K.E.; Jakus, A.E.; Burghardt, W.R.; Shah, R.N. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015, 27, 1607–1614.
- Tasoglu, S.; Demirci, U. Bioprinting for stem cell research. Trends Biotechnol. 2013, 31, 10–19.
- Reimann, F.; Habib, A.M.; Tolhurst, G.; Parker, H.E.; Rogers, G.J.; Gribble, F.M. Glucose Sensing in L Cells: A Primary Cell Study. Cell Metab. 2008, 8, 532–539.
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434.
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23.
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543.
- Skardal, A.; Atala, A. Biomaterials for Integration with 3-D Bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746.
- Mei, Q.; Rao, J.; Bei, H.P.; Liu, Y.; Zhao, X. 3D Bioprinting Photo-Crosslinkable Hydrogels for Bone and Cartilage Repair. Int. J. Bioprinting 2021, 7, 367.
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343.
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917.
- Duocastella, M.; Colina, M.; Fernández-Pradas, J.M.; Serra, P.; Morenza, J.L. Study of the laser-induced forward transfer of liquids for laser bioprinting. Appl. Surf. Sci. 2007, 253, 7855–7859.
- Peng, W.; Datta, P.; Ayan, B.; Ozbolat, V.; Sosnoski, D.; Ozbolat, I.T. 3D bioprinting for drug discovery and development in pharmaceutics. Acta Biomater. 2017, 57, 26–46.
- Kirchmajer, D.M.; Gorkin, R., III; in het Panhuis, M. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. J. Mater. Chem. B 2015, 3, 4105–4117.
- Pescosolido, L.; Schuurman, W.; Malda, J.; Matricardi, P.; Alhaique, F.; Coviello, T.; Van Weeren, P.R.; Dhert, W.J.A.; Hennink, W.E.; Vermonden, T. Hyaluronic acid and dextran-based semi-IPN hydrogels as biomaterials for bioprinting. Biomacromolecules 2011, 12, 1831–1838.
- Jung, J.W.; Lee, J.S.; Cho, D.W. Computer-Aided multiple-head 3D printing system for printing of heterogeneous organ/tissue constructs. Sci. Rep. 2016, 6, 1–9.
- Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-free 3-D cell sheet technique bridges the gap between 2-D cell culture and animal models. Int. J. Mol. Sci. 2019, 20, 4926.
- Serra, P.; Piqué, A. Laser-Induced Forward Transfer: Fundamentals and Applications. Adv. Mater. Technol. 2019, 4, 1–33.
More
Information
Subjects:
Engineering, Biomedical
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.4K
Revisions:
4 times
(View History)
Update Date:
10 Feb 2025
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




