| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dietrich Büsselberg | + 2969 word(s) | 2969 | 2020-09-07 04:30:58 | | | |
| 2 | Bruce Ren | Meta information modification | 2969 | 2020-09-15 02:35:27 | | | | |
| 3 | Bruce Ren | Meta information modification | 2969 | 2020-09-15 02:45:09 | | |
Video Upload Options
Despite the leaps and bounds in achieving success in the management and treatment of breast cancers through surgery, chemotherapy, and radiotherapy, breast cancer remains the most frequently occurring cancer in women and the most common cause of cancer-related deaths among women. Systemic therapeutic approaches, such as chemotherapy, although beneficial in treating and curing breast cancer subjects with localized breast tumors, tend to fail in metastatic cases of the disease due to (a) an acquired resistance to the chemotherapeutic drug and (b) the development of intrinsic resistance to therapy. The existence of cancer stem cells (CSCs) plays a crucial role in both acquired and intrinsic chemoresistance. CSCs are less abundant than terminally differentiated cancer cells and confer chemoresistance through a unique altered metabolism and capability to evade the immune response system. Furthermore, CSCs possess active DNA repair systems, transporters that support multidrug resistance (MDR), advanced detoxification processes, and the ability to self-renew and differentiate into tumor progenitor cells, thereby supporting cancer invasion, metastasis, and recurrence/relapse. Hence, current research is focusing on targeting CSCs to overcome resistance and improve the efficacy of the treatment and management of breast cancer. Studies revealed that metformin (1, 1-dimethylbiguanide), a widely used anti-hyperglycemic agent, sensitizes tumor response to various chemotherapeutic drugs. Metformin selectively targets CSCs and improves the hypoxic microenvironment, suppresses the tumor metastasis and inflammation, as well as regulates the metabolic programming, induces apoptosis, and reverses epithelial–mesenchymal transition and MDR. Here, we discuss cancer (breast cancer) and chemoresistance, the molecular mechanisms of chemoresistance in breast cancers, and metformin as a chemo-sensitizing/re-sensitizing agent, with a particular focus on breast CSCs as a critical contributing factor to acquired and intrinsic chemoresistance. The review outlines the prospects and directions for a better understanding and re-purposing of metformin as an anti-cancer/chemo-sensitizing drug in the treatment of breast cancer. It intends to provide a rationale for the use of metformin as a combinatory therapy in a clinical setting.
1. Introduction - Cancer and Chemotherapy
Cancer remains the second leading cause of death worldwide, accounting for nearly 10 million deaths (one in six deaths is cancer-related) in 2018 alone and continues to impose an ever-growing physical, emotional, and financial burden on individuals, families, communities, and healthcare systems [1]. Nevertheless, the survival rates of several types of cancers have significantly improved due to early detection, better treatment options, and high-quality post-treatment care [1].
The foundation for ‘scientific oncology’ was laid by Dr. Giovanni Morgagni of Padua, as early as the 18th century, when the post-mortem pathologic findings from autopsies that he performed were used to relate the cause of death to the clinical course of the patient’s illness [2]. During the same period (the 1760s), almost a century before the development of anesthesia (1846), Dr. John Hunter’s (a Scottish surgeon) observation, “If the tumor had not invaded nearby tissue and was moveable, there is no impropriety in removing it,” paved the way for the surgical removal of solid tumors [2][3]. Over the decades and centuries, cancer treatment has drastically improved to include advanced strategies such as immunotherapy, hormone therapy, gene therapy, and stem-cell therapy in addition to precision medicine that is tailored by oncologists for patients on a case-by-case basis. However, surgical removal of the solid tumor, chemotherapy, and radiation therapy (either one, but most of the time in combinations) remains the mainstay standard in the treatment of cancer.
The depletion of the lymph nodes, bone marrow aplasia, and neutropenia observed in the autopsies performed on individuals exposed to sulfur mustard gas during first world war spurred years of research with sulfur mustard agents as a possible cure for lymphoma [4]. However, it was in the 1940s when pharmacologists Dr. Louis S. Goodman and Dr. Alfred Gilman (Yale School of Medicine, CT, USA) with the help of Dr. Gustaf Lindskog, a thoracic surgeon, injected cytotoxic ‘nitrogen mustard’ (a modified, less toxic/potent version of the mustard sulfur gas in which the sulfur was replaced by nitrogen) into a patient suffering from non-Hodgkin’s lymphoma. Later, they realized that the patient’s tumor mass significantly reduced for a few weeks after the treatment, thus thrusting into the limelight the possibility and the realization that cancer could be treated using certain chemicals/pharmacological agents [4][5]. Later in 1948, Dr. Sidney Farber (a pediatric pathologist from Harvard Medical School, MA, USA, regarded as the father of modern chemotherapy) identified that folate analogs (aminopterin and amethopterin) antagonistic to folic acid, inhibited folate requiring enzymes and caused a remission of acute lymphoblastic leukemia (ALL) in children [4][5]. Since then, either by a clear biological insight or by a pure stroke of luck, several synthetic and naturally occurring agents were identified, studied, and used in clinics for their cytotoxic and chemotherapeutic effects in the treatment of several cancers.
2. Chemoresistance
The patient responses to the chemotherapeutic drugs are often hugely variable in the clinical scenario. While some cancer subjects respond positively to drug treatment and are effectively cured of the disease, other patients show a ‘resistance’ to the drug treatment, leading to a decreased drug response and recurrence/relapse of the disease, thus contributing to over 90% of the mortality among patients with malignant tumors [6]. In some instances, cancer cells possess an inherent capacity (intrinsic/innate resistance) to survive clinically relevant dosages of the drug, resulting in inadequate initial patient response to the administered treatment [7]. Cases of ‘acquired’ resistance in which the patient shows an initial positive response to the therapeutic intervention (chemotherapy, molecular-targeted anti-cancer drugs, and cancer immunotherapy) and then gradually becomes unresponsive to the treatment as a result of acquired genetic, epigenetic, and protein alterations, leading to cancer relapse is a significant hurdle in a clinical setting [7][8]. In neoplasms, one or more complex mechanisms of tumor evolution and heterogeneity, metabolic adaptations, acquired secondary genetic and epigenetic alterations, signaling pathway feedback loops and bypass mechanisms, tumor-induced modifications of the microenvironment, and the presence of cancer stem cells (CSCs), contribute to drug resistance depending on the class of drugs and treatment strategies being employed [8][9][10]. Although our knowledge of many of the individual drug-resistance mechanisms has expanded significantly, a comprehensive understanding and means of addressing multifactorial drug-resistance mechanisms have yet to be achieved.
Among women, breast cancer remains the most prevalent type of cancer and the second leading cause of death due to cancers worldwide [11]. Breast cancers are highly heterogenous (inter-tumor and intra-tumor) in nature, and the identification of their molecular subtypes holds the key for clinicians toward the decision making with regard to the choice of the treatment strategy and prediction of the treatment outcome and prognosis [12][13][14]. In terms of the absence or presence of one or more of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) and the Ki-67 status, breast cancers are classified into triple-negative breast cancers (ER−, PR−, HER2−), luminal A (ER+, PR+, HER2−, Ki-67low), luminal B (ER+, PRlow, HER2−, Ki-67high) and HER2+ (ER−, PR−, HER2high/overexpressed) subtypes [13]. While the breast cancer subtypes positive for one or more of the receptors (ER, PR, or HER2) are treated by hormonal intervention, the triple-negative breast cancers (TNBC) will not benefit from the hormone-based therapy and treatment. Using chemotherapeutic agents remains the mainstay standard for the treatment for TNBCs with very few other targeted options to control and irradicate the growth of the TNBC tumors [12][13]. The complexity among TNBCS further deepens since based on gene expression analysis and gene ontologies, TNBCs are further classified into basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal (M), mesenchymal stem-like (MSL), immunomodulatory (IM), and luminal androgen receptor (LAR) subtypes [12][13][15]. In a majority of cases of breast cancer (stages I, II, and III) where the primary solid tumor is localized, the therapeutic intervention requires surgical elimination of the tumor and radiation therapy, while advanced metastatic (stage IV) breast cancers are treated using systemic drug interventions [16][17]. Furthermore, neoadjuvant/adjuvant therapy using drugs is usually administered before or after surgical removal of the tumor, respectively [16][17].
While modern-day diagnostics and awareness programs have increased the chances for the early detection of the disease, it is still more likely that the disease comes to light only during its later stages, since the early stages remain asymptomatic, and often, in addition to the cultural stigma that limits potential patients from seeking medical care and advice, most of them have limited access to medical care or screening programs and pose a financial burden in the absence of health insurance coverage [18][19][20]. In some instances, the efficacy of the therapeutic intervention is compromised when the patients undergoing therapy are unable to absorb the drug adequately or at times rapidly metabolize and excrete the drug, leading to less than adequate levels of the drug in the body that are required for its anti-tumor effect [21]. Additionally, especially in the elderly, the occurrence of debilitating side effects and reduced tolerance to the drug may require a reduction in the dosage to sub-optimal levels, further reducing the effect of the drug on the tumor [21]. In turn, this would require that multiple low-dose therapeutic interventions be performed to achieve maximum efficacy in the treatment, which is when the tumor cells develop resistance to the treatment. However, the most gripping concern is the fear of relapse/recurrence of the disease in patients (most of them leading ultimately to death) that adversely impact the mental health, quality of life, and adherence to post-treatment surveillance among cancer survivors who had otherwise positively responded to the various therapeutic interventions and had shown significant recovery from the disease [22][23].
A study that analyzed the 10-year breast cancer recurrence rates among 8062 women from the Netherlands reported that women with the HER2+ type of tumor had the highest rates of local recurrences (7.5%) and distant metastases (25.6%), while the luminal A subtype showed the least chance of local recurrence (3.7%) and distant metastases (9.5%) [24]. TNBC tumors, on the other hand, were characterized by high rates of regional recurrences (5.2%), while the luminal A tumors showed the lowest rates (1.7%) of regional recurrences [24]. TNBC tumors had the worst 10-year overall survival (OS) rates, while HER2+ tumors had the lowest 10-year recurrence-free survival (RFS) [24]. Several other studies have shown similar patterns of OS and RFS, among other populations [25][26]. More than 50% of the disease relapse/recurrence in hormone receptor-positive breast cancers occurs nearly 6+ years (recurrence rate is twice as high when compared to hormone receptor-negative tumors) after initial diagnosis and following 5 years of adjuvant endocrine therapy, posing a significant clinical challenge [27]. ER+ tumors have shown late recurrences, while ER-negative tumors tend to recur during the first 5 years [28]. TNBCs, on the other hand, show a high risk for distant recurrences when compared to hormone receptor-positive tumors [28]. The cancer treatment administered after initial diagnosis also significantly influences the risk of recurrence among breast cancer patients [28]. In tamoxifen-treated breast cancer patients (5-year treatment), more than 50% of all recurrence occurred between 6 and 15 years after initial diagnosis [27]. The relapse/recurrence of the disease could be mainly attributed to dormant cancer cells (undetected sub-clinical residual disease), which gain the capacity of active re-growth locally or at distant metastatic sites and the existence of ‘innate/intrinsic’ or ‘acquired’ resistance to therapeutic interventions in these cancers, where the neoplastic cells survive the primary treatment(s), resulting in the enrichment and growth of drug-resistant variant population, leading to cancer progression and relapse of the disease [27].
3. Mechanisms of Chemoresistance in Breast Cancers
Cancer, once considered as a pathological condition stemming from the dysregulation of proliferation and apoptosis caused by gene mutations, has gained importance as a metabolic, inflammatory, and lifestyle disease, which can also be caused by infectious agents (bacteria and viruses) [29][30][31][32][33]. The boom in knowledge regarding the multifaceted aspects of the etiology of cancer has also revealed potential targets that can be therapeutically modified using new/re-purposed anti-cancer agents and/or radiation therapy to treat the disease efficiently. However, one of the most significant challenges faced by oncologists worldwide is the emergence of resistance to therapy, metastasis, and relapse of the disease [21]. The ‘intrinsic/innate’ resistance and, more importantly, the ‘acquired’ resistance to chemo- and radiotherapeutic interventions is a major contributor to tumor relapse/recurrence and an increase in the mortality among cancer patients [34].
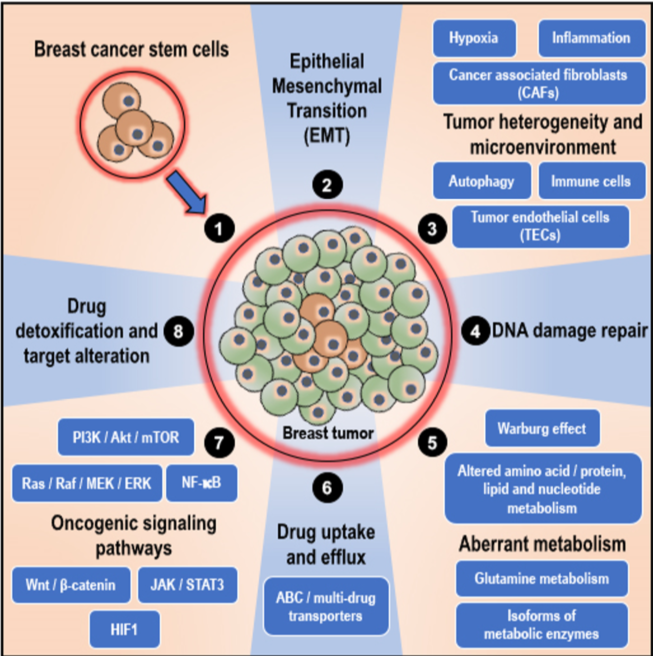
As mentioned earlier, at times, the positive effect of the therapeutic intervention is largely compromised when the adequate therapeutic concentration is not achieved by the patient due to reduced absorption of the drug or when the drug is metabolized and eliminated from the body [21]. Intolerance and severe side effects to therapeutic drugs also may require the administration of a reduced dosage of the drug, thus decreasing the response to the drug and overall efficacy of the treatment [21]. However, in a majority of the cases, the tumor/cancer cells themselves are genetically/epigenetically equipped to fight off/resist the cytotoxic effects of the therapeutic interventions through various resistance mechanisms that are unique, owing to the ‘intra-tumor’ and ‘inter-tumor’ heterogeneity and variability [21][35][36][37][38][39][40]. Furthermore, random variations in the expression of oncogenes and tumor suppressor genes in response to exposure to anti-cancer therapeutic interventions result in the enrichment of therapy-resistant variant cells in the tumor and the development of ‘acquired’ resistance to therapeutic intervention [21][34]. In cancers, including breast cancers, the factors that influence therapeutic resistance mainly include (see Figure 1; in published article): (1) the presence of breast cancer stem cells (BCSCs), (2) epithelial–mesenchymal transition, (3) tumor heterogeneity and microenvironment, (4) active DNA damage repair mechanisms, (5) altered/adaptive/aberrant metabolism, (6) variations in drug uptake and active drug efflux systems, (7) activation of oncogenic, pro-survival, and anti-apoptotic signaling pathways, and (8) active drug detoxification and target alteration systems [21][34]. Various authoritative reviews explained these aspects in detail. As these reviews are widely available online and the topic is beyond the scope of this article, this review will not focus on these aspects [37][38][39][40][41][42][43][44][45][46]. However, similar mechanisms, in the perspective of BCSCs, which confer resistance to breast cancer against therapeutic intervention, are explained in section 4 (role of BCSCs in chemoresistance in breast cancers) below.
Figure 1. Mechanisms of chemoresistance in breast cancers: In breast cancers, the factors that influence therapeutic resistance mainly include (illustrated clockwise); (1) the presence and influence of breast cancer stem cells (BCSCs) that can initiate and re-populate tumors, (2) epithelial–mesenchymal transition (EMT), (3) tumor heterogeneity and microenvironment (characterized by hypoxia, inflammation, autophagy, and presence of cancer-associated fibroblasts, immune cells such as tumor-associated macrophages, and tumor endothelial cells), (4) active DNA damage repair mechanisms, (5) altered/adaptive/aberrant metabolism (characterized by the Warburg effect, altered amino acid/protein/lipid and nucleotide metabolism, utilization of glutamine, and isoforms of metabolic enzymes that support cancer initiation, progression, and resistance to therapy), (6) variations in drug uptake and active drug efflux systems (ATP binding cassette; ABC/multidrug transporters), (7) activation of oncogenic, pro-survival and anti-apoptotic signaling pathways (the phosphatidylinositol-3-kinase; PI3K/protein kinase B; Akt/ mammalian target of rapamycin; mTOR, mitogen activated protein kinase; MAPK, nuclear factor-kappa B; NF-kB, Wnt/b-catenin, janus kinase; JAK/signal transducer and activator of transcription 3; STAT3 and hypoxia inducible factor 1; HIF1 pathways), and (8) active drug detoxification and target alteration systems.
The occurrence of intrinsic and acquired therapeutic resistance remains a major hurdle faced by the clinician during the course of the treatment of cancer. From a cancer patient’s point of view, apart from the debilitating side-effects that one suffers during the course of the treatment, there is nothing more depressing than the fear of relapse/recurrence of the disease due to the ineffectiveness of the treatment. Therefore, counteracting therapeutic resistance remains a key challenge that determines the efficacy of cancer treatment and the overall outcome and impact of the disease in the lives of affected individuals.
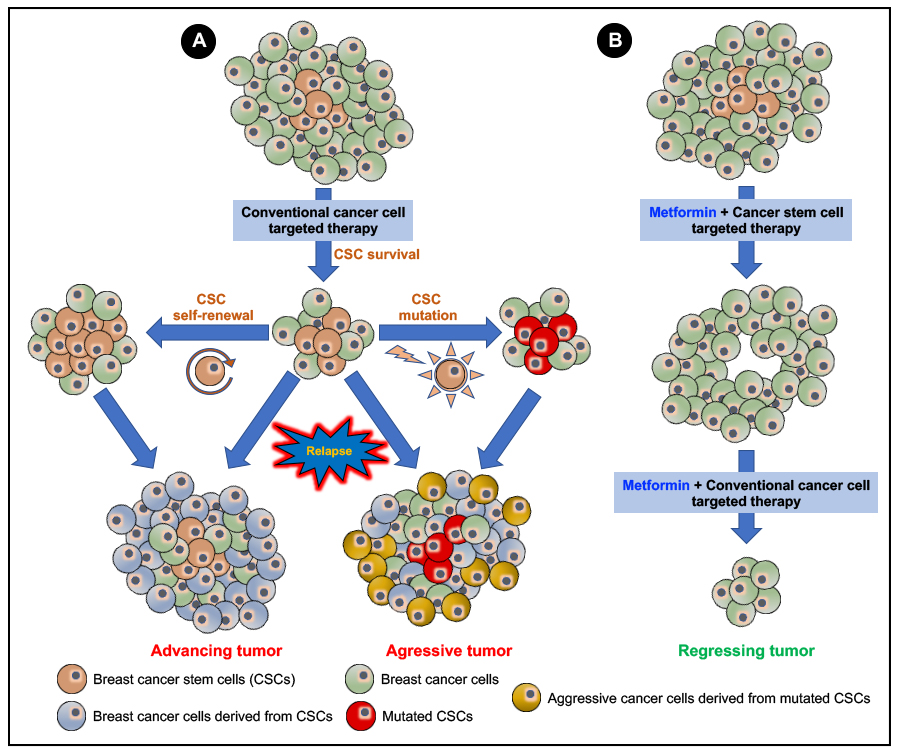
In our article we have detailed how breast cancer stem cells (BCSCs) contribute to drug/therapeutic resistance in breast cancers and discussed how targeting the various aspects of BCSC conferred drug/therapeutic resistance could in turn sensitize breast cancers to therapeutic intervention and prevent relapse/recurrence of the disease. Furthermore, the current data available on the anti-neoplastic effects of metformin (the most widely prescribed anti-diabetic drug; see Figure 5 in our published article; also given below) makes it an interesting candidate for drug re-purposing for the treatment of cancers. In this regard, we have examined and discussed the available data (in vitro, in vivo and clinical data) on how targeting BCSCs using metformin can counteract BCSC-related therapeutic resistance, which when followed by conventional anti-cancer therapy could prove to be more efficient in the treatment of breast cancers. However, the possibility of the development of an ‘acquired’ resistance to metformin cannot be ignored and must be subjected to detailed studies. While majority of the available data on the efficacy of metformin in targeting BCSCs is linked to in vitro and in vivo experiments the major setback is the lack of translational clinical trials and data that addresses the challenges faced in an actual clinical setting. More clinical studies are warranted to address the efficacy metformin in targeting BCSCs and to test the efficacy of targeted drug delivery systems for an improved therapeutic outcome. Additionally, the inter-tumor and intra-tumor heterogeneity and plasticity in cancers, including breast cancers, calls for specific focus on the various aspects of predictive, preventive and personalized medicine (PPPM/3PM) for a better treatment outcome on a case-by-case basis.
Figure 5. Efficacy of a combinatory metformin and breast cancer stem cell (BCSC) targeted therapy over conventional cancer cell-targeted therapy: A conventional anti-cancer therapy targeting terminally differentiated cancer cells (A) should efficiently inhibit cell proliferation and induce the apoptotic cell death of the cancer cells. However, the less abundant CSCs effectively evade therapeutic intervention. Then, the CSC through a process of self-renewal and generation of tumor progenitor cells and mutations mainly causes a relapse/recurrence of more aggressive, invasive, and metastatic forms of the neoplasm. Using metformin in combination with a cancer stem cell-targeted therapy (B) will effectively target and kill the CSCs, which then can be followed up with a combinatory treatment using metformin and the conventional anti-cancer therapy to cause tumor regression, effectively cure cancer, and avoid cancer relapse/recurrence.
References
- WHO. Cancer-Fact Sheets. 2018, Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 9 June 2020).
- Hawkes, N. A comprehensive history of cancer treatment. 2015, Available online: https://www.raconteur.net/healthcare/history-of-cancer-treatment (accessed on 9 June 2020).
- ACS. The History of Cancer-Cancer in the Sixteenth to Eighteenth Centuries. 2014, Available online: https://www.cancer.org/cancer/cancer-basics/history-of-cancer/sixteenth-to-eighteenth-centuries.html (accessed on 9 June 2020).
- Morrison, W.B. Cancer Chemotherapy: An Annotated History. J. Vet. Intern. Med. 2010, 24, 1249–1262, doi:10.1111/j.1939-1676.2010.0590.x.
- DeVita, V.T.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, e8643, doi:10.1158/0008-5472.CAN-07-6611.
- Schmidt, F.; Efferth, T. Tumor Heterogeneity, Single-Cell Sequencing, and Drug Resistance. Pharmaceuticals (Basel) 2016, 9, doi:10.3390/ph9020033.
- Chatterjee, N.; Bivona, T.G. Polytherapy and Targeted Cancer Drug Resistance. Trends Cancer 2019, 5, 170–182, doi:10.1016/j.trecan.2019.02.003.
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628, doi:10.1016/j.cell.2017.01.018.
- Konieczkowski, D.J.; Johannessen, C.M.; Garraway, L.A. A Convergence-Based Framework for Cancer Drug Resistance. Cancer Cell 2018, 33, 801–815, doi:10.1016/j.ccell.2018.03.025.
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 2019, 24, 65–78, doi:10.1016/j.stem.2018.11.011.
- Muley, H.; Fadó, R.; Rodríguez-Rodríguez, R.; Casals, N. Drug uptake-based chemoresistance in breast cancer treatment. Biochem. Pharmacol. 2020, 177, e113959, doi:10.1016/j.bcp.2020.113959.
- Varghese, E.; Samuel, S.M.; Abotaleb, M.; Cheema, S.; Mamtani, R.; Büsselberg, D. The “Yin and Yang” of Natural Compounds in Anticancer Therapy of Triple-Negative Breast Cancers. Cancers (Basel) 2018, 10, e346, doi:10.3390/cancers10100346.
- Varghese, E.; Samuel, S.M.; Líšková, A.; Samec, M.; Kubatka, P.; Büsselberg, D. Targeting Glucose Metabolism to Overcome Resistance to Anticancer Chemotherapy in Breast Cancer. Cancers (Basel) 2020, 12, e2252, doi:10.3390/cancers12082252.
- Subbiah, S.; Gopu, G.; Senthilkumar, P.; Muniasamy, P. Molecular subtypes as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. Indian J. Cancer 2017, 54, 652–657, doi:10.4103/ijc.IJC_238_17.
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767, doi:10.1172/JCI45014.
- ACS. Treatment of breast cancer by stage. 2019, Available online: https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-breast-cancer-by-stage.html (accessed on 9 June 2020).
- Moiseenko, F.; Volkov, N.; Bogdanov, A.; Dubina, M.; Moiseyenko, V. Resistance mechanisms to drug therapy in breast cancer and other solid tumors: An opinion [version 1; peer review: 2 approved, 1 approved with reservations]. F1000Research 2017, 6, doi:10.12688/f1000research.10992.1.
- Tripathi, L.; Datta, S.S.; Agrawal, S.K.; Chatterjee, S.; Ahmed, R. Stigma Perceived by Women Following Surgery for Breast Cancer. Indian J. Med. Paediatr Oncol. 2017, 38, 146–152, doi:10.4103/ijmpo.ijmpo_74_16.
- Vrinten, C.; Gallagher, A.; Waller, J.; Marlow, L.A.V. Cancer stigma and cancer screening attendance: A population based survey in England. BMC Cancer 2019, 19, e566, doi:10.1186/s12885-019-5787-x.
- Rivera-Franco, M.M.; Leon-Rodriguez, E. Delays in Breast Cancer Detection and Treatment in Developing Countries. Breast Cancer (Auckl) 2018, 12, e1178223417752677, doi:10.1177/1178223417752677.
- Gottesman, M.M. Mechanisms of Cancer Drug Resistance. Annu. Rev. Med. 2002, 53, 615–627, doi:10.1146/annurev.med.53.082901.103929.
- Ozakinci, G.; Sobota, A.; Humphris, G. Fear of Cancer Recurrence Among Breast Cancer Survivors. Curr. Breast Cancer Rep. 2014, 6, 219–225, doi:10.1007/s12609-014-0153-0.
- Ziner, K.W.; Sledge, G.W.; Bell, C.J.; Johns, S.; Miller, K.D.; Champion, V.L. Predicting fear of breast cancer recurrence and self-efficacy in survivors by age at diagnosis. Oncol. Nurs. Forum 2012, 39, 287–295, doi:10.1188/12.ONF.287-295.
- van Maaren, M.C.; de Munck, L.; Strobbe, L.J.A.; Sonke, G.S.; Westenend, P.J.; Smidt, M.L.; Poortmans, P.M.P.; Siesling, S. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: A large population-based study. Int. J. Cancer 2019, 144, 263–272, doi:10.1002/ijc.31914.
- Shim, H.J.; Kim, S.H.; Kang, B.J.; Choi, B.G.; Kim, H.S.; Cha, E.S.; Song, B.J. Breast cancer recurrence according to molecular subtype. Asian Pac. J. Cancer Prev. 2014, 15, 5539–5544, doi:10.7314/apjcp.2014.15.14.5539.
- Montagna, E.; Bagnardi, V.; Rotmensz, N.; Viale, G.; Renne, G.; Cancello, G.; Balduzzi, A.; Scarano, E.; Veronesi, P.; Luini, A.; et al. Breast cancer subtypes and outcome after local and regional relapse. Ann. Oncol. 2012, 23, 324–331, doi:10.1093/annonc/mdr129.
- Lim, E.; Metzger-Filho, O.; Winer, E.P. The natural history of hormone receptor-positive breast cancer. Oncol. (Williston Park N.Y.) 2012, 26, 688–694.
- Zimmerman, M.P.; Mehr, S.R. Recurrence of Breast Cancer Years After the Initial Tumor. Am. J. Manag. Care 2014, 20, SP409–SP413.
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867, doi:10.1038/nature01322.
- Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D'Agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527, doi:10.1093/carcin/bgt480.
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615, doi:10.1016/S1470-2045(12)70137-7.
- van Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep. 2018, 19, e46632, doi:10.15252/embr.201846632.
- Morales-Sánchez, A.; Fuentes-Pananá, E.M. Human viruses and cancer. Viruses 2014, 6, 4047–4079, doi:10.3390/v6104047.
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160, doi:10.20517/cdr.2019.10.
- Lovly, C.M.; Salama, A.K.S.; Salgia, R. Tumor Heterogeneity and Therapeutic Resistance. Am. Soc. Clin. Oncol. Educ. Book 2016, e585–e593, doi:10.1200/EDBK_158808.
- Dzobo, K.; Senthebane, D.A.; Thomford, N.E.; Rowe, A.; Dandara, C.; Parker, M.I. Not Everyone Fits the Mold: Intratumor and Intertumor Heterogeneity and Innovative Cancer Drug Design and Development. Omics: A J. Integr. Biol. 2018, 22, 17–34, doi:10.1089/omi.2017.0174.
- Chen, W.; Qin, Y.; Liu, S. Cytokines, breast cancer stem cells (BCSCs) and chemoresistance. Clin. Transl Med. 2018, 7, 27–27, doi:10.1186/s40169-018-0205-6.
- Chuthapisith, S.; Eremin, J.; El-Sheemey, M.; Eremin, O. Breast cancer chemoresistance: Emerging importance of cancer stem cells. Surg. Oncol. 2010, 19, 27–32, doi:10.1016/j.suronc.2009.01.004.
- De Angelis, M.L.; Francescangeli, F.; Zeuner, A. Breast Cancer Stem Cells as Drivers of Tumor Chemoresistance, Dormancy and Relapse: New Challenges and Therapeutic Opportunities. Cancers (Basel) 2019, 11, e1569, doi:10.3390/cancers11101569.
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, e108800, doi:10.1016/j.biopha.2019.108800.
- Ma, L.; Zong, X. Metabolic Symbiosis in Chemoresistance: Refocusing the Role of Aerobic Glycolysis. Front Oncol. 2020, 10, 5–5, doi:10.3389/fonc.2020.00005.
- Nunes, T.; Hamdan, D.; Leboeuf, C.; El Bouchtaoui, M.; Gapihan, G.; Nguyen, T.T.; Meles, S.; Angeli, E.; Ratajczak, P.; Lu, H.; et al. Targeting Cancer Stem Cells to Overcome Chemoresistance. Int. J. Mol. Sci. 2018, 19, e4036, doi:10.3390/ijms19124036.
- Velaei, K.; Samadi, N.; Barazvan, B.; Soleimani Rad, J. Tumor microenvironment-mediated chemoresistance in breast cancer. The Breast 2016, 30, 92–100, doi:10.1016/j.breast.2016.09.002.
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers (Basel) 2014, 6, 1769–1792, doi:10.3390/cancers6031769.
- Hu, W.; Tan, C.; He, Y.; Zhang, G.; Xu, Y.; Tang, J. Functional miRNAs in breast cancer drug resistance. Onco. Targets Ther. 2018, 11, 1529–1541, doi:10.2147/OTT.S152462.
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, e957, doi:10.3390/cells8090957.