Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Davide Panzeri | + 2716 word(s) | 2716 | 2022-02-22 09:52:43 | | | |
| 2 | Jason Zhu | -6 word(s) | 2710 | 2022-03-04 02:53:44 | | | | |
| 3 | Jason Zhu | -6 word(s) | 2710 | 2022-03-04 02:55:33 | | | | |
| 4 | Jason Zhu | -6 word(s) | 2710 | 2022-03-04 02:58:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Panzeri, D. Domestication Process of African Vigna Species. Encyclopedia. Available online: https://encyclopedia.pub/entry/20155 (accessed on 07 February 2026).
Panzeri D. Domestication Process of African Vigna Species. Encyclopedia. Available at: https://encyclopedia.pub/entry/20155. Accessed February 07, 2026.
Panzeri, Davide. "Domestication Process of African Vigna Species" Encyclopedia, https://encyclopedia.pub/entry/20155 (accessed February 07, 2026).
Panzeri, D. (2022, March 03). Domestication Process of African Vigna Species. In Encyclopedia. https://encyclopedia.pub/entry/20155
Panzeri, Davide. "Domestication Process of African Vigna Species." Encyclopedia. Web. 03 March, 2022.
Copy Citation
Legumes are one of the most economically important and biodiverse families in plants recognised as the basis to develop functional foods. Among these, the Vigna genus stands out as a good representative because of its relatively recent African origin as well as its outstanding potential. Africa is a great biodiversity centre in which a great number of species are spread, but only three of them, Vigna unguiculata, Vigna subterranea and Vigna vexillata, were successfully domesticated.
Vigna genus
Introgression
Hybridisation
Domestication
1. Introduction
Legumes (Fabaceae) are considered one of the most important families of plants for human nutrition, especially considering the rapid growth rate of the world population [1]. However, almost all the efforts and resources invested in agriculture during the last century were focused on improving the yield, resistance and quality of a few specific staple crops. Neglected landraces are regarded as having interesting potential, and recent studies have demonstrated that some wild legumes can be an important target to develop modern functional foods because they possess various bioactive molecules that interact positively with human health [2][3][4][5]. Among these, members of the Vigna genus show a growing social and economic importance in several African regions, especially where the local population is not able to afford animal proteins [6][7][8]. Their seeds are rich in essential amino acids and contain a high concentration of minerals, lipids and vitamins [9][10].
The genus Vigna (Savi, 1824), which belongs to the tribe Phaseoleae of the family Fabaceae, includes over 100 species [11] distributed in the tropical and subtropical areas of the world [12] grouped in five subgenera: Vigna, Ceratotropis, Plectotropis, Lasiosporon and Haydonia [13][14][15]. Phylogenetic findings propose the age of split between Phaseolus and Vigna genera at about 8–10 million years (Mya) and the age of split between Ceratotropis and Vigna subgenera at about 3–4 Mya [13][14][15][16][17], but the genetic relationships between subgenera are particularly complex and far from being completely solved. Although most domesticated or semi-domesticated species are distributed in Asia, the greatest diversity of the Vigna genus is located in Sub-Saharan Africa [14][18]. Vigna subgenus, distributed in Africa, includes about 40 wild and 2 domesticated species, namely cowpea (also called black-eyed peas, chawli and kunde) (Vigna unguiculata L.) and Bambara groundnut (V. subterranea L.) [19] while Ceratotropis (Piper) Verdc., distributed in Asia, contains 21 wild and 7 domesticated species used widely for food and forage, namely mungbean or green gram (V. radiata L. Wilczek), black gram (V. mungo L. Hepper), moth bean (V. aconitifolia Jacq. Maréchal), rice bean (V. umbellata Thunb. Ohwi and Ohashi), adzuki bean (V. angularis L. Ohwi and Ohashi), creole bean (V. reflexo-pilosa Hayata), jungli bean (V. trilobata L. Verdc.). [15][20][21][22]. Moreover, three species belonging to Plectrotropis (Schumach.) are distributed in Africa, including tuber cowpea (V. vexillata L.) [23]. Most of the African Vigna germplasm is based on wild plants and neglected or underutilized landraces, and many of these lineages are declining with a high risk of extinction. The recovery of wild accessions and research devoted to the phylogeny of the genus is therefore essential to prevent genetic erosion and the loss of Vigna diversity.
Plant domestication is widely recognised as an accelerated evolutionary process driven by a synergistic impact of human and natural selection, occurring in geographically restricted areas from wild progenitors. In legumes, the main modification is the loss of seed pod dehiscence or shattering [24][25]. The split at the dorsal and ventral sutures of the dry pod and successive release of the seeds occurs due to the desiccation of lignified cells in the pods [26]. The shattering habit is related to environmental aridity and persists in many varieties of domesticated Vigna species, thereby determining severe yield losses [27][28]. Additional implementations in Vigna domesticated species include an increase in seed or fruit size, change in seed colour, loss of seed dormancy, apical dominance and change in flowering timing [29][30][31][32][33]. These modifications were inherited more or less effectively in the various vine species currently cultivated, and this is the basis of the agrobiodiversity of this genus.
Generally, the current existing crops show lower resistance to biotic and abiotic stress compared to wild relatives, and often they have reached their full yield. The selection of desirable traits and breeding processes to improve crop productivity have caused the depletion of diversity and the increase in the frequency of deleterious genetic variants that are fixed in the genomes of crops [34][35][36]. These constraints have a serious impact on agriculture, limiting the possibility to grow such crops under more extreme environmental conditions. Thanks to this residual genetic diversity and also to studies performed on Vigna species, most of the accessions are well adapted to a wide range of extreme environmental conditions, such as sandy beaches, arid lands and wetlands, harbouring tolerance and resistance genes towards biotic and abiotic stresses. These genetic traits are used for developing new stress-tolerant crops [37][38][39][40][41][42][43]. By contrast, less is known about the effects of domestication on the nutritional value of seeds [7] even if recent studies have reported that cultivated legumes show a lower carotenoid and protein content in seeds compared with the wild relatives [44][45]. Where, when and how many times the domestication process of African Vigna crops occurred continues to be debated among researchers. Although archaeological remains of Vigna indicate that the domestication process in Africa was started recently compared to other field crops [46][47]. Modern evolutionary models proposed for other crops suggest that the predomestication phase may have lasted several thousands of years [48][49]. Generally, the centres of origin are also recognized as centres of diversity, and thus these areas require special precautionary measures of conservation [50]. Although for many crops the single-origin model is usually the most parsimonious, the hypothesis that provides multiple origins starting from independent founder lineages seems well suited for the crops of Vigna originated in Africa [51][52]. Moreover, despite whether and to what extent introgression influences the domestication process is still underexplored, some studies already show that gene flow between cowpea and its wild relatives may occur. Pervasive introgression can also intensify the feralisation process, promoting the crops to return to a wild environment and causing serious problems for the conservation of biodiversity [53].
2. Vigna unguiculata (L.) Walp.
V. unguiculata, which was considered an orphan crop for several decades, has recently become one of the most important legumes in the world. Its name derives from Latin and means “with a small claw”, referring to the size of the claw of the petals [54] or commonly named as “cowpea” because of its use as fodder for cows [55] and black-eyed pea/bean for the black hilum. This crop is largely cultivated, especially in semiarid regions of Africa and Asia where other crops fail to grow [10]. Currently, on a global scale, about 15 million hectares are dedicated to V. unguiculata, with an annual production of 7 million Mg and an average yield of 0.6 Mg ha−1 [56]. The most interesting environmental traits of this species are represented by the generalized low agrochemical input requirements. In fact, this crop shows relatively high adaptation to drought, especially in comparison to other legumes [57] and can fix up to 200 kg N ha−1 [58] with a positive soil N balance of up to 92 kg ha−1 [59]. Nevertheless, several abiotic and biotic constraints (i.e., low soil fertility, pests, diseases, parasitic weeds, and nematodes) limit the yield [43][60][61]. Moreover, low productivity is often associated with the use of traditional and unimproved varieties, still widely cultivated in Africa [62]. This crop has a fundamental role in human nutrition, showing seeds rich in proteins and essential amino acids (i.e., tryptophan and lysine), carbohydrates, folic acid and minerals. Recent studies carried on a large sampling have shown high variability in protein and mineral concentrations, suggesting that some lineages could be potential sources of genes useful to produce new varieties [63][64][65][66].
The high number of wild subspecies found exclusively in Africa strongly supports the idea of an African origin. However, intraspecies phylogeny remains far from being completely elucidated [67]. The centre of origin of the species is probably located in the southernmost regions of Africa, where most subspecies are found and where most genetic diversity could be still hidden [68]. Several taxonomic revisions based on morphological and molecular traits permitted to identify 10 perennial and 1 annual subspecies, the latter split into two varieties: ssp. unguiculata var. unguiculata (domesticated cowpea) and ssp. unguiculata var. spontanea (Schweinf.) Pasquet., also known as subsp. dekindtiana sensu Verdcourt non Harms [69][70][71][72][73][74][75][76]. Besides the domesticated cowpea, the dekindtiana group includes some obligate short-day wild forms, well adapted to arid environments. While the var. spontanea grows especially around cultivated fields and roadsides, and it is recognized as the progenitor of domesticated cowpea [75][77][78][79], the subspecies alba, pubescens, tenuis, stenophylla and dekindtiana are perennial plants [75][76]. The development of new molecular tools to discriminate among wild, weedy, and cultivated accessions is considered a modern and fundamental target, particularly needed for disentangling the complex taxonomic relationships among subspecies and to discriminate between true wild plants and ferals.
Although little is known about the domestication process, some scientists have hypothesized that ancient cowpea progenitors, such as the modern wild forms, were adapted to dry habitats and grew spontaneously south of the Sahara Desert [80]. These plants were gathered, cultivated and dispersed by men near the villages, but they were unsuited for cultivation. Although they did not show high yield, the wild lineages were spread in the humid zones thanks to their pods that remained closed for the humid atmosphere. Through several generations of cultivation, new mutants have arisen, showing interesting domestic traits, including resistance to shattering. Subsequently, humans have selected and helped spread these landraces by exchange and trade activities. Since the oldest archaeological records of domesticated forms found in central Ghana are dated around 1500 BC, the domestication process likely started before that period (Figure 1) [47][81]. However, the precise origin is widely debated, and two independent domestication centres in West and East Africa are proposed by different researchers [68][74][79][82][83][84][85][86].
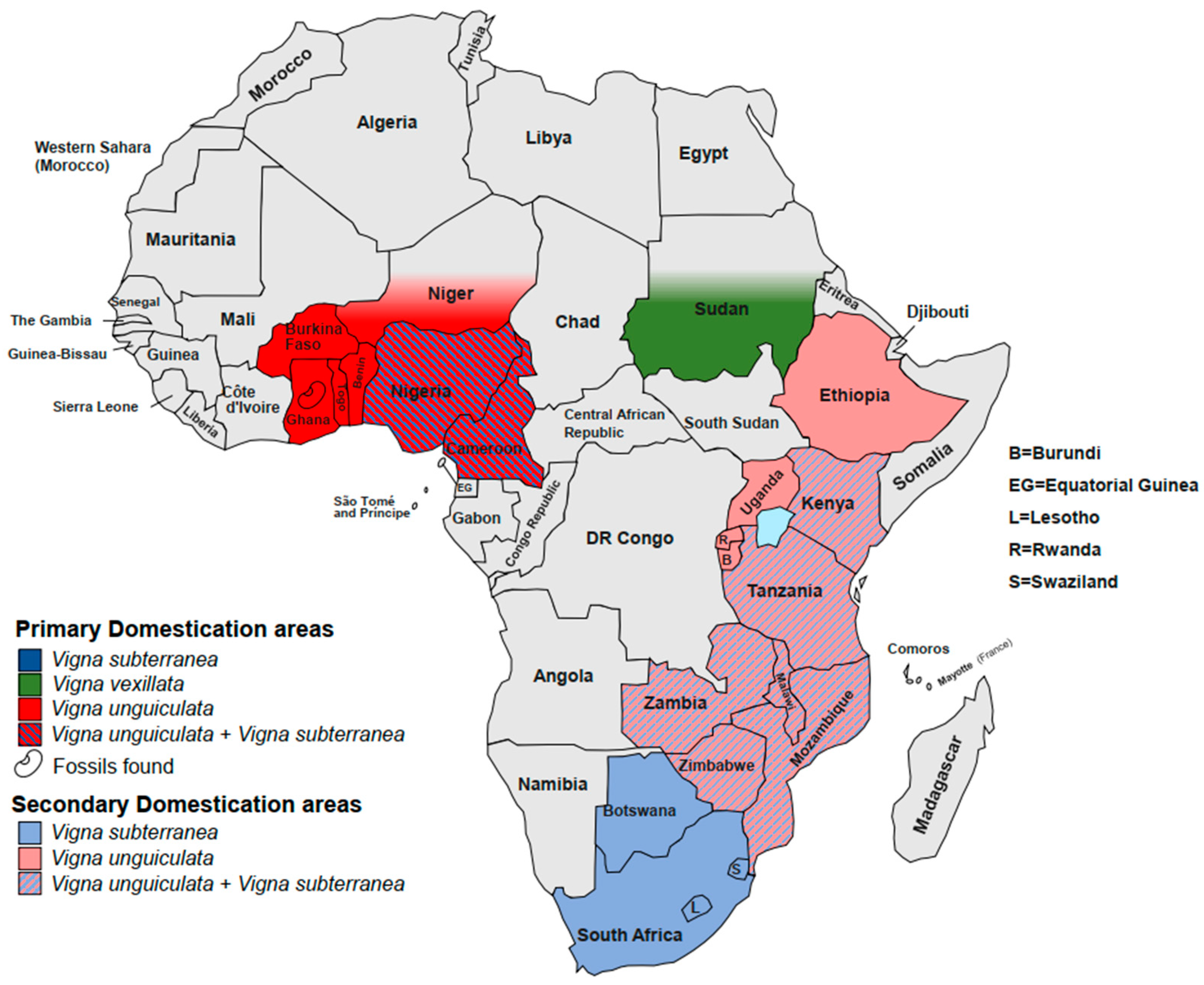
Figure 1. Primary and secondary domestication sites in Africa.
Morphological and DNA markers support the idea that domestication occurred only once, but analyses on whole genomes provide evidence for more independent domestication events in Africa and diversification events out of Africa [51][87]. Analyses of genetic variability are generally applied to identify the origin of species and the groups of accessions that show high variability in certain geographic areas and are interpreted as the most ancient populations. Although cowpea from West Africa showed a high genetic variability [88], cultivated accessions grown in East and West Africa were shown to be most closely related to the respective local wild lineages [52][89], thereby indicating that domestication could have occurred in both regions. Outside Africa, cultivated cowpea was exposed to different ecological conditions, including new biotic and abiotic stresses that probably have contributed to shaping the genetic structure of landraces. When cowpea moved through Asian regions (especially in Thailand, China, the Philippines and India), it encountered environments with more humidity and less brightness where the drying of pots and grains was hindered. Some accessions were selected for the use of the immature pods to produce a peculiar form of vegetable called yardlong bean (V. unguiculata ssp. unguiculata cv. sesquipedalis) [51][90][91]. Although Chinese accessions show lower genetic diversity compared to African cowpea, signals of genetic bottlenecks lead to the conclusion that a limited number of relatively recent selection events occurred;however, where the selection process arose is still unknown [92]. Moreover, other cultivar groups (e.g., ‘Textilis’, ‘Biflora’ or ‘Cylindrica’, ‘Melanophthalmus’) are classified by morphological traits [75][93]. Still, additional genomic analyses should be performed to confirm the genetic relationships and understand how and where these accessions originated [67][85][88][94][95].
3. Vigna subterranea (L.) Verdc.
Vigna subterranea, also named Bambara groundnut, is an indigenous African grain legume. Its common name derives from the groundnut (Arachis hypogaea L.) due to the hypogean pods’ growth, whereas the “Bambara” name is derived from a Malian tribe [96]. Despite its potential in terms of nutritional value and resistance to biotic and abiotic stresses [97][98], Bambara is cultivated mainly in small farms or in families as a subsistence crop [99], and naturally grows in semi-arid regions in Africa. Regarding the origin of the species itself, the domesticated or semi-domesticated Vigna subterranea var. subterranea was most likely generated from its wild counterpart Vigna subterranea var. spontanea using both morphological and isozyme data [100][101].
The origin of this species is hypothesised to be in Mali, in the Timbuktu region [102], but the precise centre of origin is still unknown. In fact, there is no evidence of wild lineages in Mali [103]. Dalziel, Begemann and Goli [104][105][106] analysed a lot of morphologic traits such as seed morphology, seed pattern diversity and other diversity indices (number of days to maturity, pod length, number of stems per plant and internode length). They found that the most diversity is located in an area that spans from Jos Plateau and Yola Adamawa (Nigeria) to Garoua (Cameroon). Somta and Olukolu [107][108] evaluated the phylogeography of several accessions spread in Africa. The markers used (i.e., SSR and DaRT) showed a cluster with higher diversity in the area between Nigeria and Cameroon. The researchers confirm the area of origin while suggesting a possible subsequent introduction of Western domesticated accessions in East Africa (Figure 1). In contrast, Aliyu et al. [97], in an overview of the past two decades of genetic diversity analysis, also proposed that the Southern African region could constitute a divergent time-spaced domestication event. However, the researchers suggest that these hypotheses need further examination.
In terms of genetic diversity, Bambara has a peculiar behaviour. In fact, many researchers studied Bambara’s genetics with different techniques to clarify how wide the genetic pool is and how homogeneous the single landraces are. Molosiwa et al. [109] evaluated genetic distances between 24 landraces with phenotypic and genetic markers (i.e., SSR and DaRT). The main results report that landraces are different to each other, suggesting the existence of great allelic diversity among the various populations. At the same time, though, single landraces tend to be very homogeneous, and in three generations of inbreeding became pure lines. This is due mainly to its self-pollinating nature [110] but also small farmers, who, by breeding the same landraces, also acted as selection drivers [111][112]. Molosiwa [113] selected 12 SSR markers and 5 Bambara accessions to evaluate the potential for creating pure lines, finding that these accessions at the second cycle of selection completely have lost the heterozygosity.
All these findings suggest that Bambara has incredible genetic potential. The genetic screening through the different lineages and the consequent discovery of peculiar sites of interest could be the basis for an improvement of crop programs. Moreover, the use of pure lines in agriculture is fundamental not only for the optimisation and standardisation of agricultural practices but also for the development of breeding programs. Currently, there are no reports of ongoing improvement or breeding programs for this species. The extremely wide pool of wild and domesticated accessions can be used to create ideal crops that can better withstand climate change as well as being able to grow with low agronomic inputs.
4. Vigna vexillata (L.) A. Rich.
Widely distributed in Africa, Asia, America and Australia, V. vexillata (Zombi pea) is one the least known and underutilized Vigna crops. Likewise, V. unguiculata, Zombi pea shows a high morphological diversity probably determined by geological, ecological, climatic and anthropomorphic constraints that also determined exceptional patterns of genetic variability [19][71]. Eight varieties including vexillata, angustifolia, ovata, dolichomena, yunnanensis, plurifora, lobatifloria and macrosperma are recognized [12][19][23][114][115]. Var. macrosperma shows typical traits associated with domestication syndrome such as bush-like habit, early flowering and higher seed yield [116][117]. Moreover, loss of seed dormancy and various degrees of pod shattering were detected in different crop accessions while the wild seeds remained intrinsically dormant [118][119]. Several researchers reported that two forms were domesticated independently (i.e., seed type and tuber type), and some evidence lines suggest that the seed type was domesticated in Sudan, whereas the tuber type was domesticated in India (Figure 1) [120][121][122][123][124]. However, molecular analyses were performed on a limited number of accessions and loci [124], and the phylogenetic intra-specific delimitation has resulted in much more complexity than that of other Vigna crops [125]. Thus, modern genomic analyses are needed to resolve the genetic relationships and confirm the origin of the two forms.
Several studies have also shown that the Zombi pea is the result of a long adaptation process to different environmental stress, including acid, alkaline, saline, drought and wet soils [115][117][126][127][128]. Moreover, since some accessions were found to be resistant to different viral diseases and parasite insects, widely recognized as major pests of cowpea, this species is an important harbour of resistances to various biotic stresses, particularly useful to improve modern Vigna crops [129][130][131][132][133][134].
References
- Kahane, R.; Hodgkin, T.; Jaenicke, H.; Hoogendoorn, C.; Hermann, M.; d’Arros Hughes, J.; Padulosi, S.; Looney, N. Agrobiodiversity for Food Security, Health and Income. Agron. Sustain. Dev. 2013, 33, 671–693.
- Hu, X.-R.; Chou, G.-X.; Zhang, C.-G. Flavonoids, Alkaloids from the Seeds of Crotalaria Pallida and Their Cytotoxicity and Anti-Inflammatory Activities. Phytochemistry 2017, 143, 64–71.
- Lam, S.-H.; Li, Y.-C.; Kuo, P.-C.; Hwang, T.-L.; Yang, M.-L.; Wang, C.-C.; Tzen, J.T. Chemical Constituents of Vigna luteola and Their Anti-Inflammatory Bioactivity. Molecules 2019, 24, 1371.
- Takahashi, Y.; Sakai, H.; Yoshitsu, Y.; Muto, C.; Anai, T.; Pandiyan, M.; Senthil, N.; Tomooka, N.; Naito, K. Domesticating Vigna stipulacea: A Potential Legume Crop with Broad Resistance to Biotic Stresses. Front. Plant Sci. 2019, 10, 1607.
- Ku, Y.-S.; Contador, C.A.; Ng, M.-S.; Yu, J.; Chung, G.; Lam, H.-M. The Effects of Domestication on Secondary Metabolite Composition in Legumes. Front. Genet. 2020, 11, 581357.
- Marconi, E.; Ruggeri, S.; Carnovale, E. Chemical Evaluation of Wild Under-Exploited Vigna spp. Seeds. Food Chem. 1997, 59, 203–212.
- Duranti, M. Grain Legume Proteins and Nutraceutical Properties. Fitoterapia 2006, 77, 67–82.
- Tomooka, N.; Naito, K.; Kaga, A.; Sakai, H.; Isemura, T.; Ogiso-Tanaka, E.; Iseki, K.; Takahashi, Y. Evolution, Domestication and Neo-Domestication of the Genus Vigna. Plant Genet. Resour. 2014, 12, S168–S171.
- Harouna, D.V.; Venkataramana, P.B.; Ndakidemi, P.A.; Matemu, A.O. Under-Exploited Wild Vigna Species Potentials in Human and Animal Nutrition: A Review. Glob. Food Secur. 2018, 18, 1–11.
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O. Cowpea (Vigna unguiculata): Genetics, Genomics and Breeding. Plant Breed. 2019, 138, 415–424.
- Vigna Savi|Plants of the World Online|Kew Science. Available online: http://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:325971-2 (accessed on 17 January 2022).
- Maréchal, R. Etude Taxonomique d’un Groupe Complexe d’espèces Des Genres Phaseolus et Vigna (Papilionaceae) Sur La Base de Données Morphologiques et Polliniques, Traitées Par l’analyse Informatique. Boissiera 1978, 28, 1–273.
- Thulin, M.; Lavin, M.; Pasquet, R.; Delgado-Salinas, A. Phylogeny and Biogeography of Wajira (Leguminosae): A Monophyletic Segregate of Vigna Centered in the Horn of Africa Region. Syst. Bot. 2004, 29, 903–920.
- Javadi, F.; Tun, Y.T.; Kawase, M.; Guan, K.; Yamaguchi, H. Molecular Phylogeny of the Subgenus Ceratotropis (Genus Vigna, Leguminosae) Reveals Three Eco-Geographical Groups and Late Pliocene–Pleistocene Diversification: Evidence from Four Plastid DNA Region Sequences. Ann. Bot. 2011, 108, 367–380.
- Delgado-Salinas, A.; Thulin, M.; Pasquet, R.; Weeden, N.; Lavin, M. Vigna (Leguminosae) Sensu Lato: The Names and Identities of the American Segregate Genera. Am. J. Bot. 2011, 98, 1694–1715.
- Lavin, M.; Herendeen, P.S.; Wojciechowski, M.F. Evolutionary Rates Analysis of Leguminosae Implicates a Rapid Diversification of Lineages during the Tertiary. Syst. Biol. 2005, 54, 575–594.
- Li, H.; Wang, W.; Lin, L.; Zhu, X.; Zhu, X.; Li, J.; Chen, Z. Diversification of the Phaseoloid Legumes: Effects of Climate Change, Range Expansion and Habit Shift. Front. Plant Sci. 2013, 4, 386.
- Singh, B. Cowpea: The Food Legume of the 21st Century; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 164.
- Maxted, N.; Mabuza-Diamini, P.; Moss, H.; Padulosi, S.; Jarvis, A.; Guarino, L. An Ecogeographic Study African Vigna; International Plant Genetic Resources Institute (IPGRI): Rome, Italy, 2004; ISBN 978-92-9043-637-9.
- Tateishi, Y. Systematics of the Species of Vigna Subgenus Ceratotropis. Mungbean Germplasm Collect. Util. Breed. Program. 1996, 9–24.
- Tomooka, N.; Maxted, N.; Thavarasook, C.; Jayasuriya, A.H.M. Two New Species, Sectional Designations and New Combinations in Vigna Subgenus Ceratotropis (Piper) Verdc. (Leguminosae, Phaseoleae). Kew Bull. 2002, 57, 613–624.
- Tomooka, N.; Yoon, M.S.; Doi, K.; Kaga, A.; Vaughan, D. AFLP Analysis of Diploid Species in the Genus Vigna Subgenus Ceratotropis. Genet. Resour. Crop. Evol. 2002, 49, 521–530.
- Pienaar, B.J. The Vigna vexillata Complex (Fabaceae) in Southern Africa. South Afr. J. Bot. 1991, 57, 236–245.
- Ladizinsky, G. Seed Dispersal in Relation to the Domestication of Middle East Legumes. Econ. Bot. 1979, 33, 284–289.
- Takahashi, Y.; Kongjaimun, A.; Muto, C.; Kobayashi, Y.; Kumagai, M.; Sakai, H.; Satou, K.; Teruya, K.; Shiroma, A.; Shimoji, M. Same Locus for Non-Shattering Seed Pod in Two Independently Domesticated Legumes, Vigna angularis and Vigna unguiculata. Front. Genet. 2020, 11, 748.
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, Dehiscence, and Other Cell Separation Processes. Annu. Rev. Plant Biol. 2002, 53, 131–158.
- Lo, S.; Parker, T.; Muñoz-Amatriaín, M.; Berny-Mier y Teran, J.C.; Jernstedt, J.; Close, T.J.; Gepts, P. Genetic, Anatomical, and Environmental Patterns Related to Pod Shattering Resistance in Domesticated Cowpea (Vigna unguiculata Walp.). J. Exp. Bot. 2021, 72, 6219–6229.
- Parker, T.A.; Berny Mier y Teran, J.C.; Palkovic, A.; Jernstedt, J.; Gepts, P. Pod Indehiscence Is a Domestication and Aridity Resilience Trait in Common Bean. New Phytol. 2020, 225, 558–570.
- Meyer, R.S.; Purugganan, M.D. Evolution of Crop Species: Genetics of Domestication and Diversification. Nat. Rev. Genet. 2013, 14, 840–852.
- Herniter, I.A.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.-N.; Close, T.J. Identification of Candidate Genes Controlling Black Seed Coat and Pod Tip Color in Cowpea (Vigna unguiculata Walp.). G3 Genes Genomes Genet. 2018, 8, 3347–3355.
- Lo, S.; Muñoz-Amatriaín, M.; Hokin, S.A.; Cisse, N.; Roberts, P.A.; Farmer, A.D.; Xu, S.; Close, T.J. A Genome-Wide Association and Meta-Analysis Reveal Regions Associated with Seed Size in Cowpea . Theor. Appl. Genet. 2019, 132, 3079–3087.
- Seo, E.; Kim, K.; Kang, R.; Kim, G.; Park, A.; Kim, W.J.; Sun, H.; Ha, B.-K. Genome-Wide Association Study for Flowering Time in Korean Cowpea Germplasm. Plant Breed. Biotechnol. 2020, 8, 413–425.
- Amkul, K.; Somta, P.; Laosatit, K.; Wang, L. Identification of QTLs for Domestication-Related Traits in Zombi Pea , a Lost Crop of Africa. Front. Genet. 2020, 11, 803.
- Lu, J.; Tang, T.; Tang, H.; Huang, J.; Shi, S.; Wu, C.-I. The Accumulation of Deleterious Mutations in Rice Genomes: A Hypothesis on the Cost of Domestication. Trends Genet. 2006, 22, 126–131.
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.-H. Back into the Wild—Apply Untapped Genetic Diversity of Wild Relatives for Crop Improvement. Evol. Appl. 2017, 10, 5–24.
- Liu, Q.; Zhou, Y.; Morrell, P.L.; Gaut, B.S. Deleterious Variants in Asian Rice and the Potential Cost of Domestication. Mol. Biol. Evol. 2017, 34, 908–924.
- Yokoyama, T.; Tomooka, N.; Okabayashi, M.; Kaga, A.; Boonkerd, N.; Vaughan, D.A. Variation in the Nod Gene RFLPs, Nucleotide Sequences of 16S RRNA Genes, Nod Factors, and Nodulation Abilities of Bradyrhizobium Strains Isolated from Thai Vigna Plants. Can. J. Microbiol. 2006, 52, 31–46.
- Chankaew, S.; Isemura, T.; Naito, K.; Ogiso-Tanaka, E.; Tomooka, N.; Somta, P.; Kaga, A.; Vaughan, D.A.; Srinives, P. QTL Mapping for Salt Tolerance and Domestication-Related Traits in Vigna marina subsp. oblonga, a Halophytic Species. Theor. Appl. Genet. 2014, 127, 691–702.
- Tomooka, N.; Kaga, A.; Isemura, T.; Vaughan, D.V. Wild Crop Relatives: Genomic and Breeding Resources: Legume Crops and Forages; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2011.
- Takahashi, Y.; Somta, P.; Muto, C.; Iseki, K.; Naito, K.; Pandiyan, M.; Natesan, S.; Tomooka, N. Novel Genetic Resources in the Genus Vigna Unveiled from Gene Bank Accessions. PLoS ONE 2016, 11, e0147568.
- Yoshida, J.; Tomooka, N.; Yee Khaing, T.; Shantha, P.S.; Naito, H.; Matsuda, Y.; Ehara, H. Unique Responses of Three Highly Salt-Tolerant Wild Vigna Species against Salt Stress. Plant Prod. Sci. 2020, 23, 114–128.
- van Zonneveld, M.; Rakha, M.; Tan, S.y.; Chou, Y.-Y.; Chang, C.-H.; Yen, J.-Y.; Schafleitner, R.; Nair, R.; Naito, K.; Solberg, S.Ø. Mapping Patterns of Abiotic and Biotic Stress Resilience Uncovers Conservation Gaps and Breeding Potential of Vigna Wild Relatives. Sci. Rep. 2020, 10, 1–11.
- Carvalho, M.d.; Halecki, W. Modeling of Cowpea (Vigna unguiculata) Yield and Control Insecticide Exposure in a Semi-Arid Region. Plants 2021, 10, 1074.
- Peña-Valdivia, C.B.; García-Nava, J.R.; Aguirre R, J.R.; Ybarra-Moncada, M.C.; López H, M. Variation in Physical and Chemical Characteristics of Common Bean (Phaseolus vulgaris L.) Grain along a Domestication Gradient. Chem. Biodivers. 2011, 8, 2211–2225.
- Fernández-Marín, B.; Milla, R.; Martín-Robles, N.; Arc, E.; Kranner, I.; Becerril, J.M.; García-Plazaola, J.I. Side-Effects of Domestication: Cultivated Legume Seeds Contain Similar Tocopherols and Fatty Acids but Less Carotenoids than Their Wild Counterparts. BMC Plant Biol. 2014, 14, 1–11.
- Larson, G.; Piperno, D.R.; Allaby, R.G.; Purugganan, M.D.; Andersson, L.; Arroyo-Kalin, M.; Barton, L.; Vigueira, C.C.; Denham, T.; Dobney, K. Current Perspectives and the Future of Domestication Studies. Proc. Natl. Acad. Sci. USA 2014, 111, 6139–6146.
- D’Andrea, A.C.; Kahlheber, S.; Logan, A.L.; Watson, D.J. Early Domesticated Cowpea (Vigna unguiculata) from Central Ghana. Antiquity 2007, 81, 686–698.
- Allaby, R.G.; Fuller, D.Q.; Brown, T.A. The Genetic Expectations of a Protracted Model for the Origins of Domesticated Crops. Proc. Natl. Acad. Sci. USA 2008, 105, 13982–13986.
- Purugganan, M.D.; Fuller, D.Q. Archaeological Data Reveal Slow Rates of Evolution during Plant Domestication. Evol. Int. J. Org. Evol. 2011, 65, 171–183.
- Engels, J.M.M.; Ebert, A.W.; Thormann, I.; De Vicente, M.C. Centres of Crop Diversity and/or Origin, Genetically Modified Crops and Implications for Plant Genetic Resources Conservation. Genet. Resour. Crop. Evol. 2006, 53, 1675–1688.
- Huynh, B.; Close, T.J.; Roberts, P.A.; Hu, Z.; Wanamaker, S.; Lucas, M.R.; Chiulele, R.; Cissé, N.; David, A.; Hearne, S. Gene Pools and the Genetic Architecture of Domesticated Cowpea. Plant Genome 2013, 6, 1–8.
- Herniter, I.A.; Muñoz-Amatriaín, M.; Close, T.J. Genetic, Textual, and Archeological Evidence of the Historical Global Spread of Cowpea (Vigna unguiculata Walp.). Legume Sci. 2020, 2, e57.
- Vijaykumar, A.; Saini, A.; Jawali, N. Assessment of Hybridization among Wild and Cultivated Vigna unguiculata Subspecies Revealed by Arbitrarily Primed Polymerase Chain Reaction Analysis. AoB Plants 2012, 2012, pls012.
- Small, E. Top 100 Food Plants: The World’s Most Important Culinary Crops; NRC Research Press: Ottawa, ON, Canada, 2009.
- Timko, M.P.; Ehlers, J.D.; Roberts, P.A. Cowpea. In Pulses, Sugar and Tuber Crops; Springer: Berlin/Heidelberg, Germany, 2007; pp. 49–67.
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 17 January 2022).
- Smith, M.R.; Veneklaas, E.; Polania, J.; Rao, I.M.; Beebe, S.E.; Merchant, A. Field Drought Conditions Impact Yield but Not Nutritional Quality of the Seed in Common Bean (Phaseolus vulgaris L.). PLoS ONE 2019, 14, e0217099.
- Adjei-Nsiah, S.; Kuyper, T.W.; Leeuwis, C.; Abekoe, M.K.; Cobbinah, J.; Sakyi-Dawson, O.; Giller, K.E. Farmers’ Agronomic and Social Evaluation of Productivity, Yield and N 2-Fixation in Different Cowpea Varieties and Their Subsequent Residual N Effects on a Succeeding Maize Crop. Nutr. Cycl. Agroecosyst. 2008, 80, 199–209.
- Chikowo, R.; Mapfumo, P.; Nyamugafata, P.; Giller, K.E. Woody Legume Fallow Productivity, Biological N2-Fixation and Residual Benefits to Two Successive Maize Crops in Zimbabwe. Plant Soil 2004, 262, 303–315.
- Singh, B.B.; Sharma, B. Restructuring Cowpea for Higher Yield. Indian J. Genet. 1996, 56, 389–405.
- Boukar, O.; Fatokun, C.A.; Huynh, B.-L.; Roberts, P.A.; Close, T.J. Genomic Tools in Cowpea Breeding Programs: Status and Perspectives. Front. Plant Sci. 2016, 7, 757.
- Horn, L.; Shimelis, H.; Laing, M. Participatory Appraisal of Production Constraints, Preferred Traits and Farming System of Cowpea in the Northern Namibia: Implications for Breeding. Legume Res. Int. J. 2015, 38, 691–700.
- Boukar, O.; Massawe, F.; Muranaka, S.; Franco, J.; Maziya-Dixon, B.; Singh, B.; Fatokun, C. Evaluation of Cowpea Germplasm Lines for Protein and Mineral Concentrations in Grains. Plant Genet. Resour. 2011, 9, 515–522.
- Abadassi, J. Cowpea (Vigna unguiculata (L.) Walp.) Agronomic Traits Needed in Tropical Zone. Int. J. Pure Appl. Biosci. 2015, 3, 158–165.
- Dakora, F.D.; Belane, A.K. Evaluation of Protein and Micronutrient Levels in Edible Cowpea (Vigna unguiculata L. Walp.) Leaves and Seeds. Front. Sustain. Food Syst. 2019, 3, 70.
- Weng, Y.; Shi, A.; Ravelombola, W.S.; Yang, W.; Qin, J.; Motes, D.; Moseley, D.O.; Chen, P. A Rapid Method for Measuring Seed Protein Content in Cowpea (Vigna unguiculata (L.) Walp.). Am. J. Plant Sci. 2017, 8, 2387.
- Zuluaga, D.L.; Lioi, L.; Delvento, C.; Pavan, S.; Sonnante, G. Genotyping-by-Sequencing in Vigna unguiculata Landraces and Its Utility for Assessing Taxonomic Relationships. Plants 2021, 10, 509.
- Padulosi, S.; Ng, N.Q. Origin, Taxonomy, and Morphology of Vigna unguiculata (L.) Walp. Adv. Cowpea Res. 1997, 1–12.
- Padulosi, S. Genetic Diversity, Taxonomy and Ecogeographic Survey of the Wild Relatives of Cowpea (Vigna unguiculata (L.) Walpers). Ph.D. Thesis, University of Louvain La Neuve, Ottignies-Louvain-la-Neuve, Belgium, 1993.
- Pasquet, R.S. Classification Infraspécifique des Formes Spontanées de Vigna unguiculata (L.) Walp. (Fabaceae) à Partir de Données Morphologiques. Bull. Du Jard. Bot. Natl. Belg. Bull. Natl. Plantentuin Belg. 1993, 62, 127–173.
- Pasquet, R.S. Wild Cowpea (Vigna unguiculata) Evolution. Adv. Legume Syst. 1996, 8, 95–100.
- Pasquet, R. A New Subspecies of Vigna unguiculata (Leguminosae: Papilionoideae). Kew Bull. 1997, 52, 840.
- Pasquet, R.S. Genetic Relationships among Subspecies of Vigna unguiculata (L.) Walp. Based on Allozyme Variation. Theor. Appl. Genet. 1999, 98, 1104–1119.
- Coulibaly, S.; Pasquet, R.S.; Papa, R.; Gepts, P. AFLP Analysis of the Phenetic Organization and Genetic Diversity of Vigna unguiculata L. Walp. Reveals Extensive Gene Flow between Wild and Domesticated Types. Theor. Appl. Genet. 2002, 104, 358–366.
- Pasquet, R. Genus Vigna and Cowpea (V. unguiculata Walp.) Taxonomy: Current Status and Prospects. In Proceedings of the Fifth World Cowpea Conference on Improving Livelihoods in the Cowpea Value Chain through Advancement in Science, Dakar, Senegal, 27 September–1 October 2010.
- Pasquet, R.S.; Feleke, Y.; Gepts, P. Cowpea Maternal Lineages, Chloroplast Captures, and Wild Cowpea Evolution. Genet. Resour. Crop. Evol. 2021, 68, 2799–2812.
- Lush, W.M.; Evans, L.T.; Wien, H.C. Environmental Adaptation of Wild and Domesticated Cowpeas (Vigna unguiculata (L.) Walp.). Field Crop. Res. 1980, 3, 173–187.
- Faris, D.G. The Origin and Evolution of the Cultivated Forms of Vigna sinensis. Can. J. Genet. Cytol. 1965, 7, 433–452.
- Vaillancourt, R.E.; Weeden, N.F. Chloroplast DNA Polymorphism Suggests Nigerian Center of Domestication for the Cowpea, Vigna unguiculata (Leguminosae). Am. J. Bot. 1992, 79, 1194–1199.
- Lush, W.M.; Evans, L.T. The Domestication and Improvement of Cowpeas (Vigna unguiculata (L.) W Alp.). Euphytica 1981, 30, 579–587.
- Champion, L.; Fuller, D.Q.; Ozainne, S.; Huysecom, É.; Mayor, A. Agricultural Diversification in West Africa: An Archaeobotanical Study of the Site of Sadia (Dogon Country, Mali). Archaeol. Anthropol. Sci. 2021, 13, 60.
- Vavilov, N.I. Centers of Origin of Cultivated Plants. In Origin and Geography of Cultivated Plants; Cambridge University Press: Cambridge, UK, 1926.
- Faris, D.G. Evidence for the West African Origin of Vigna sinensis (L.) Savi; University of California: Davis, CA, USA, 1963.
- Rawal, K.M. Natural Hybridization among Wild, Weedy and Cultivated Vigna unguiculata (L.) Walp. Euphytica 1975, 24, 699–707.
- Ba, F.S.; Pasquet, R.S.; Gepts, P. Genetic Diversity in Cowpea as Revealed by RAPD Markers. Genet. Resour. Crop. Evol. 2004, 51, 539–550.
- Fang, J.; Chao, C.-C.T.; Roberts, P.A.; Ehlers, J.D. Genetic Diversity of Cowpea in Four West African and USA Breeding Programs as Determined by AFLP Analysis. Genet. Resour. Crop. Evol. 2007, 54, 1197–1209.
- Xiong, H.; Shi, A.; Mou, B.; Qin, J.; Motes, D.; Lu, W.; Ma, J.; Weng, Y.; Yang, W.; Wu, D. Genetic Diversity and Population Structure of Cowpea (Vigna unguiculata L. Walp.). PLoS ONE 2016, 11, e0160941.
- Muñoz-Amatriaín, M.; Lo, S.; Herniter, I.; Boukar, O.; Fatokun, C.; Carvalho, E.; Castro, I.; Guo, Y.; Huynh, B.; Roberts, P.A. The UCR Minicore: A Valuable Resource for Cowpea Research and Breeding. Legume Sci. 2021, 3, e95.
- Sarr, A.; Bodian, A.; Gbedevi, K.M.; Ndir, K.N.; Ajewole, O.O.; Gueye, B.; Foncéka, D.; Diop, E.A.; Diop, B.M.; Cissé, N. Genetic Diversity and Population Structure Analyses of Wild Relatives and Cultivated Cowpea (Vigna unguiculata (L.) Walp.) from Senegal Using Simple Sequence Repeat Markers. Plant Mol. Biol. Rep. 2021, 39, 112–124.
- Kongjaimun, A.; Kaga, A.; Tomooka, N.; Somta, P.; Vaughan, D.A.; Srinives, P. The Genetics of Domestication of Yardlong Bean, Vigna unguiculata (L.) Walp. ssp. unguiculata Cv.-Gr. sesquipedalis. Ann. Bot. 2012, 109, 1185–1200.
- Xu, P.; Wu, X.; Wang, B.; Luo, J.; Liu, Y.; Ehlers, J.D.; Close, T.J.; Roberts, P.A.; Lu, Z.; Wang, S. Genome Wide Linkage Disequilibrium in Chinese Asparagus Bean (Vigna unguiculata ssp. sesquipedialis) Germplasm: Implications for Domestication History and Genome Wide Association Studies. Heredity 2012, 109, 34–40.
- Xu, P.; Wu, X.; Wang, B.; Liu, Y.; Qin, D.; Ehlers, J.D.; Close, T.J.; Hu, T.; Lu, Z.; Li, G. Development and Polymorphism of Vigna Unguiculata ssp. Unguiculata Microsatellite Markers Used for Phylogenetic Analysis in Asparagus Bean (Vigna unguiculata ssp. sesquipedialis (L.) Verdc.). Mol. Breed. 2010, 25, 675–684.
- Pasquet, R.S. Morphological Study of Cultivated Cowpea Vigna unguiculata (L.) Walp. Importance of Ovule Number and Definition of Cv Gr Melanophthalmus. Agronomie 1998, 18, 61–70.
- Pasquet, R.S. Allozyme Diversity of Cultivated Cowpea Vigna unguiculata (L.) Walp. Theor. Appl. Genet. 2000, 101, 211–219.
- Kongjaimun, A.; Kaga, A.; Tomooka, N.; Somta, P.; Shimizu, T.; Shu, Y.; Isemura, T.; Vaughan, D.A.; Srinives, P. An SSR-Based Linkage Map of Yardlong Bean (Vigna unguiculata (L.) Walp. subsp. unguiculata Sesquipedalis Group) and QTL Analysis of Pod Length. Genome 2012, 55, 81–92.
- Mubaiwa, J.; Fogliano, V.; Chidewe, C.; Linnemann, A.R. Hard-to-Cook Phenomenon in Bambara Groundnut (Vigna subterranea (L.) Verdc.) Processing: Options to Improve Its Role in Providing Food Security. Food Rev. Int. 2017, 33, 167–194.
- Aliyu, S.; Massawe, F.; Mayes, S. Genetic Diversity and Population Structure of Bambara Groundnut (Vigna subterranea (L.) Verdc.): Synopsis of the Past Two Decades of Analysis and Implications for Crop Improvement Programmes. Genet. Resour. Crop. Evol. 2016, 63, 925–943.
- Olayide, O.E.; Donkoh, S.A.; Ansah, I.G.K.; Adzawla, W.; O’Reilly, P.J.; Mayes, S.; Feldman, A.; Halimi, R.A.; Nyarko, G.; Ilori, C.O. Assessing Socioeconomic Factors Influencing Production and Commercialization of Bambara Groundnut as an Indigenous Climate Resilient Crop in Nigeria; Springer Nature: Cham, Switzerland, 2018.
- Azam-Ali, S.; Azam-Ali, S.N.; Aguilar-Manjarrez, J.; Bannayan-Avval, M. A Global Mapping System for Bambara Groundnut Production; Food and Agriculture Organization: Rome, Italy, 2001; Volume 1.
- Pasquet, R.S.; Schwedes, S.; Gepts, P. Isozyme Diversity in Bambara Groundnut. Crop. Sci. 1999, 39, 1228–1236.
- Doku, E.V.; Karikari, S.K. Operational Selection in Wild Bambara Groundnuts. Ghana J. Sci. 1971, 11, 45–56.
- Majola, N.G.; Gerrano, A.S.; Shimelis, H. Bambara Groundnut (Vigna subterranea Verdc.) Production, Utilisation and Genetic Improvement in Sub-Saharan Africa. Agronomy 2021, 11, 1345.
- Mohammed, S.M. Pre-Breeding of Bambara Groundnut (Vigna subterranea Verdc.). Ph.D. Thesis, Abubakar Tafawa Balewa University, Bauchi, Nigeria, 2014.
- Dalziel, J.M. The Useful Plants of West Tropical Africa; Royal Botanic Gardens: London, UK, 1937.
- Begemann, F. Ecogeographic Differentiation of Bambarra Groundnut (Vigna subterranea) in the Collection of the International Institute of Tropical Agriculture (IITA); Wissenschaftlicher Fachverlag; Wissenschaftlicher Fachverlag: Berlin, Germany, 1988.
- Goli, A.E.; Begemann, F.; Ng, N.Q. Characterisation and Evaluation of IITA’S Bambara Groundnut (Vigna subterranea (L) Verdc). Promoting the Conservation and Use of Underutilized and Neglected Crops; International Plant Genetic Resources Institute: Rome, Italy, 1997; Volume 9, pp. 101–118.
- Somta, P.; Chankaew, S.; Rungnoi, O.; Srinives, P. Genetic Diversity of the Bambara Groundnut (Vigna subterranea (L.) Verdc.) as Assessed by SSR Markers. Genome 2011, 54, 898–910.
- Olukolu, B.A.; Mayes, S.; Stadler, F.; Ng, N.Q.; Fawole, I.; Dominique, D.; Azam-Ali, S.N.; Abbott, A.G.; Kole, C. Genetic Diversity in Bambara Groundnut (Vigna subterranea (L.) Verdc.) as Revealed by Phenotypic Descriptors and DArT Marker Analysis. Genet. Resour. Crop. Evol. 2012, 59, 347–358.
- Molosiwa, O.O.; Aliyu, S.; Stadler, F.; Mayes, K.; Massawe, F.; Kilian, A.; Mayes, S. SSR Marker Development, Genetic Diversity and Population Structure Analysis of Bambara Groundnut Landraces. Genet. Resour. Crop. Evol. 2015, 62, 1225–1243.
- Tanimu, B.; Aliyu, L. Bambara Groundnut, Vigna subterranea (L.) verdc. In Proceedings of the Workshop on Conservation and Improvement of Bambara Groundnut, Harare, Zimbabwe, 14–16 November 1995; Country Report: Northern Nigeria; Promoting the Conservation and Use of Underutilized and Neglected Crops. International Plant Genetic Resources Institute: Rome, Italy, 1997.
- Muhammad, I.; Rafii, M.Y.; Ramlee, S.I.; Nazli, M.H.; Harun, A.R.; Oladosu, Y.; Musa, I.; Arolu, F.; Chukwu, S.C.; Sani Haliru, B. Exploration of Bambara Groundnut (Vigna subterranea (L.) Verdc.), an Underutilized Crop, to Aid Global Food Security: Varietal Improvement, Genetic Diversity and Processing. Agronomy 2020, 10, 766.
- Molosiwa, O.; Basu, S.M.; Stadler, F.; Azam-Ali, S.; Mayes, S. Assessment of Genetic Variability of Bambara Groundnut (Vigna subterranean (L.) Verdc.) Accessions Using Morphological Traits and Molecular Markers. In Proceedings of the II International Symposium on Underutilized Plant Species: Crops for the Future-Beyond Food Security 979, Kuala Lumpur, Malaysia, 27 June1 July 2011; pp. 779–790.
- Molosiwa, O.O. Genetic Diversity and Population Structure Analysis of Bambara Groundnuts (Vigna subterranea (L.) Verdc.) Landraces Using Morpho-Agronomic Characters and SSR Markers. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2012.
- Verdcourt, B. Studies in the Leguminosae-Papilionoïdeae for the’Flora of Tropical East Africa’: III. Kew Bull. 1970, 24, 379–447.
- Vanderborght, T. Some Observations on Seedlings of Vigna vexillata (L.) A. Rich. (Fabaceae). Bull. Du Jard. Bot. Natl. Belg. Bull. Natl. Plantentuin Belg. 1989, 59, 179–187.
- Damayanti, F.; Lawn, R.J.; Bielig, L.M. Genotypic Variation in Domesticated and Wild Accessions of the Tropical Tuberous Legume Vigna vexillata (L.) A. Rich. Crop. Pasture Sci. 2010, 61, 771–784.
- Dachapak, S.; Somta, P.; Poonchaivilaisak, S.; Yimram, T.; Srinives, P. Genetic Diversity and Structure of the Zombi Pea (Vigna vexillata (L.) A. Rich.) Gene Pool Based on SSR Marker Analysis. Genetica 2017, 145, 189–200.
- Cosmas, P.; Agathar, K.; Ronald, M.; John, C.T.; Simon, M. Preliminary Evaluation of Different Seed Dormancy Breaking Methods In Wild Tuber Cowpea (Vigna vexillata). Can. J. Agric. Crop. 2019, 4, 33–40.
- Tripathi, K.; Gore, P.G.; Pandey, A.; Nayar, E.R.; Gayacharan, C.; Pamarthi, R.K.; Bhardwaj, R.; Kumar, A. Morphological and Nutritional Assessment of Vigna Vexillata (L.) A. Rich.: A Potential Tuberous Legume of India. Genet. Resour. Crop. Evol. 2021, 68, 397–408.
- Ferguson, H. The Food Crops of the Sudan and Their Relationship to Environment; McCorquodale & Co., Ltd.: London, UK, 1954.
- Bhattacharyya, P.K.; Ghosh, A.K.; Sanyal, B.; Deb Ray, G. Grow Vigna vexillata for Protein-Rich Tuber-Cum-Pulse Crop in North-Eastern Hill Region. Seeds Farms 1984, 10, 33–36.
- Wong, K.C. Vigna vexillata (L.) A. Richard. PROSEA, Plant Resources of South-East Asia. Aux. Plants 1997, 11, 261–263.
- Asati, B.S.; Yadav, D.S. Diversity of Horticultural Crops in North Eastern Region. ENVIS Bull. Himal. Ecol. 2004, 12, 1.
- Dachapak, S.; Tomooka, N.; Somta, P.; Naito, K.; Kaga, A.; Srinives, P. QTL Analysis of Domestication Syndrome in Zombi Pea (Vigna vexillata), an Underutilized Legume Crop. PLoS ONE 2018, 13, e0200116.
- Garba, M.; Pasquet, R.S. Isozyme Diversity in Vigna vexillata (L.) A. Rich. (Fabaceae) Complex. S. Afr. J. Bot. 1998, 64, 163–175.
- Lawn, R.J.; Watkinson, A.R. Habitats, Morphological Diversity, and Distribution of the Genus Vigna Savi in Australia. Aust. J. Agric. Res. 2002, 53, 1305–1316.
- Karuniawan, A.; Iswandi, A.; Kale, P.R.; Heinzemann, J.; Grüneberg, W.J. Vigna vexillata (L.) A. Rich. Cultivated as a Root Crop in Bali and Timor. Genet. Resour. Crop. Evol. 2006, 53, 213–217.
- Butsayawarapat, P.; Juntawong, P.; Khamsuk, O.; Somta, P. Comparative Transcriptome Analysis of Waterlogging-Sensitive and Tolerant Zombi Pea (Vigna vexillata) Reveals Energy Conservation and Root Plasticity Controlling Waterlogging Tolerance. Plants 2019, 8, 264.
- Chiang, H.S.; Singh, S.R. Pod Hairs as a Factor in Vigna vexillata Resistance to the Pod-Sucking Bug, Clavigralla tomentosicollis. Entomol. Exp. Et Appl. 1988, 47, 195–199.
- Jackai, L.E.N.; Oghiakhe, S. Pod Wall Trichomes and Resistance of Two Wild Cowpea, Vigna vexillata, Accessions to Maruca testualis (Geyer) (Lepidoptera: Pyralidae) and Clavigralla tomentosicollis Stål (Hemiptera: Coreidae). Bull. Entomol. Res. 1989, 79, 595–605.
- Thottappilly, G.; Ng, N.Q.; Rossel, H.W. Screening Germplasm of Vigna vexillata for Resistance to Cowpea Mottle Virus. Int. J. Trop. Plant Dis. 1994, 12, 75–80.
- Ogundiwin, E.A.; Thottappilly, G.; AkenOva, M.E.; Ekpo, E.J.A.; Fatokun, C.A. Resistance to Cowpea Mottle Carmovirus in Vigna vexillata. Plant Breed. 2002, 121, 517–520.
- Amkul, K.; Wang, L.; Somta, P.; Wang, S.; Cheng, X. Construction of a High Density Linkage Map and Genome Dissection of Bruchid Resistance in Zombi Pea (Vigna vexillata (L.) A. Rich.). Sci. Rep. 2019, 9, 1–10.
- Sodedji, F.A.K.; Agbahoungba, S.; Nguetta, S.-P.A.; Agoyi, E.E.; Ayenan, M.A.T.; Sossou, S.H.; Mamadou, C.; Assogbadjo, A.E.; Kone, D. Resistance to Legume Pod Borer (Maruca vitrata Fabricius) in Cowpea: Genetic Advances, Challenges, and Future Prospects. J. Crop. Improv. 2020, 34, 238–267.
More
Information
Subjects:
Biodiversity Conservation
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
4 times
(View History)
Update Date:
04 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




